Abstract
The Ebola virus envelope glycoprotein (GP) derived from the pathogenic Zaire subtype mediates cell rounding and detachment from the extracellular matrix in 293T cells. In this study we provide evidence that GPs from the other pathogenic subtypes, Sudan and Côte d'Ivoire, as well as from Reston, a strain thought to be nonpathogenic in humans, also induced cell rounding, albeit at lower levels than Zaire GP. Sequential removal of regions of potential O-linked glycosylation at the C terminus of GP1 led to a step-wise reduction in cell detachment without obviously affecting GP function, suggesting that such modifications are involved in inducing the detachment phenotype. While causing cell rounding and detachment in 293T cells, Ebola virus GP did not cause an increase in cell death. Indeed, following transient expression of GP, cells were able to readhere and continue to divide. Also, the rounding effect was not limited to 293T cells. Replication-deficient adenovirus vectors expressing Ebola virus GP induced the loss of cell adhesion in a range of cell lines and primary cell types, including those with proposed relevance to Ebola virus infection in vivo, such as endothelial cells and macrophages. In both transfected 293T and adenovirus-infected Vero cells, a reduction in cell surface expression of adhesion molecules such as integrin β1 concurrent with the loss of cell adhesion was observed. A number of other cell surface molecules, however, including major histocompatibility complex class I and the epidermal growth factor receptor, were also down-modulated, suggesting a global mechanism for surface molecule down-regulation.
Ebola virus and Marburg virus form the Filoviridae, a family of negative-strand RNA viruses capable of causing severe hemorrhagic fever in humans (32). Ebola virus subspecies Zaire (EboZ) and Sudan (EboS), as well as Marburg, are highly pathogenic in human and nonhuman primates, with mortality rates of up to 90% described for EboZ. The virulence of Ebola virus Côte d'Ivoire (EboC) in humans and laboratory primates is unknown, although it is pathogenic in wild chimpanzees (16). Ebola virus Reston (EboR) is also pathogenic in nonhuman primates, although it has reduced pathogenicity compared to other strains (14). EboR appears not to cause disease in humans, although there have been a limited number of known exposures.
Transcription of the EboZ envelope glycoprotein (GP) gene results in the production of a 364-residue soluble GP, sGP. A transcriptional editing event is required to produce full-length, membrane-anchored GP, which shares its first 295 residues with sGP (35). This requirement for transcriptional editing may be a mechanism to limit the amount of GP produced (44); however, a firm function for sGP has yet to be established. Membrane-bound GP consists of a single 160-kDa precursor, which is subsequently cleaved by host proteases to form two disulfide-linked subunits, GP1 and GP2 (43), although this proteolytic processing is not required for infectivity (24, 49).
The pathogenic determinants of Ebola virus have yet to be fully described, though recent reports have suggested that GP may play an important role (10, 39, 50). Transient expression of GP (but not sGP) derived from EboZ in human embryonic kidney 293T cells has been shown to cause rounding and detachment of cells from the extracellular matrix (10, 39, 50). In addition, Yang et al. reported that cell death subsequently occurred and that this GP-mediated effect may play a role in hemorrhage following endothelial cell infection (50). While filovirus replicates both in vitro and in vivo in endothelial cells, direct damage to the microvascular endothelium is minimal and occurs only during the terminal stages of disease (5, 25, 26, 31, 34, 36). An alternative mechanism for hemorrhage is the elaboration of cytokines by virus-infected cells. Inflammatory cytokines such as tumor necrosis factor alpha are secreted by macrophages infected with Marburg virus in vitro, and supernatant from such cells mediates increased permeability of endothelial cell monolayers (13). Indeed, levels of inflammatory cytokines in serum are markedly elevated in cases of fatal filovirus infection compared to those in survivors (3, 41). Thus, Ebola virus may mediate endothelial cell damage both directly via GP-mediated effects and indirectly via cytokines. Loss of adherence mediated by GP expression may also play a role in modulating immune responses to Ebola virus, since firm cell-to-cell contact is essential for many aspects of the immune system.
The effects of membrane GP expression in a range of cell lines and primary cell types was studied both by transient transfection and transduction using replication-incompetent adenovirus vectors. GP caused rounding and detachment of a number of cell types including those relevant to pathogenic Ebola virus infection such as endothelial cells and macrophages. Furthermore, Ebola virus GP down-regulated a range of cell surface markers including several involved in cell adhesion and immune surveillance.
MATERIALS AND METHODS
Cell lines and primary cell types.
Human embryonic kidney 293 and 293T cells, baby hamster kidney (BHK) cells, and murine MC57 cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% bovine calf serum. Stable MC57 cell lines bearing the human coxsackievirus/adenovirus receptor (hCAR) (7) were made following transfection of the hCAR coding sequence (kindly provided by J. Bergelson) in pcDNA3(+)neo using Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer's instructions and selection with Geneticin G-418 sulfate (Invitrogen). Human microglial (U87) cells, African green monkey kidney cells (Vero), and cat kidney cells (CCC) were maintained in DMEM supplemented with 10% fetal calf serum (FCS). Human promonocyte cell line U937 was maintained in RPMI 1640 medium supplemented with 10% FCS. Serum-free, nonadherent 293H cells (Invitrogen) were maintained in 293 SFM II (Invitrogen). Human umbilical vein endothelial cells (HUVEC; Clonetics) were maintained in EGM medium (Clonetics). Human pulmonary artery endothelial cells (HPAEC; Clonetics) were maintained in EGM-2 medium (Clonetics). Coronary artery smooth muscle cells (CASMC; Clonetics) were maintained in SmGM-2 medium (Clonetics). Blood monocyte-derived macrophages were prepared by plastic adherence as described previously (38) and were maintained in RPMI 1640 supplemented with 10% human serum.
Plasmids and expression.
cDNAs encoding the EboZ, EboS, EboC, and EboR GPs were kindly provided by Anthony Sanchez (Centers for Disease Control and Prevention) and their coding sequences were subcloned together with that of the avian sarcoma and leukosis virus type A (ASLV-A) envelope, EnvA, into mammalian expression plasmid pCB6 as well as pAD-TrackCMV (23). EboZ, EboS, and EboR GP cDNAs lacking a stop codon were also subcloned via PCR amplification into pCDNA6/V5-His (Invitrogen) to produce full-length GP bearing a V5-His tag at the C terminus. EboZ GP mutants lacking various lengths of the mucin-like domain at the C terminus of GP1 (see Fig. 3A) were made by overlapping extension PCR using flanking primers to the 5" and 3" ends of cDNA for EboZ GP (with the addition of a V5-His tag) as well as a pair of internal primers. The internal primers consisted of 311-5"GTCTTTCACAGTTGTTAACTCCTCTGCAATGG3"-346 and its reverse complement to produce mutΔ1, 311-5"GTCTTTCACAGTTGTTAACGACAGCACAGCCTC3"-412 and its reverse complement to produce mutΔ12, 311-5"GTCTTTCACAGTTGTTAACACGAGCAAGAGCAC3"-436 and its reverse complement to produce mutΔ123, 311-5"GTCTTTCACAGTTGTTAACACTCATCACCAAG3"-463 and its reverse complement to produce mutΔ1234, 341-5"CTGAAGACCACAAAATTAACACGAGCAAGATCAG3"-436 and its reverse complement to produce mutΔ23, and 341-5"CTGAAGACCACAAAATTAACACTCATCACCAAG3"-463 and its reverse complement to produce mutΔ234. Regions of O-linked glycosylation were predicted (see Fig. 3A) based on sequence context and surface accessibility using NetOGlyc,version 2.0 (http://www.cbs.dtu.dk/services/NetOGlyc/) (18).
FIG. 3.
Cell rounding is dependent on O-linked glycosylation-rich domains in GP1. (A) Serial deletions in the C-terminal mucin-like domain of GP1 were made by overlapping extension PCR. Potential O-linked glycosylations (T) were predicted using NetOGlyc, version 2.0. (B) 293T cells were transfected with 30 μg of pcDNA6/V5-His containing the coding sequences for EboZ GP or EboZ GP mucin domain mutants. Floating cells were counted, and values are numbers of floating cells above background levels (2.4 × 105 cells), calculated from mock-transfected cells. Cell lysates from 5 × 106 cells were subjected to SDS-4 to 15% PAGE, and mature-GP2 levels were quantified by phosphorimager analysis following immunoblotting for the presence of the V5 epitope using a mouse anti-V5 monoclonal antibody followed by I125-labeled protein A. GP2 levels are presented as a percentage of EboZ GP2 wild-type expression. Data are representative of three independent experiments.
Ebola virus GP was transiently expressed in 293T cells by CaPO4 transfection, and, 48 h posttransfection, cells were monitored for rounding and/or fluorescence. Medium containing floating cells was carefully collected and pooled with 2 ml of phosphate-buffered saline (PBS), used to very gently wash the monolayer. An aliquot of cells was taken to estimate floating cell numbers using an improved Neubauer hemacytometer.
Cell viability assays.
Floating 293T cells were collected 48 h after transfection with pCB6-EboZ-GP as described above and replated at 3 × 105 cells per well on a six-well plate. Daily, floating cells were carefully harvested and quantified, while adherent cells were removed by EDTA treatment, pooled with floating cells, and stained with trypan blue (Sigma), and numbers of viable and nonviable cells were estimated. Cells were then adhered for 1 h to poly-d-lysine-coated plates (Costar), and live-cell immunofluorescence was conducted using KZ52, a human Fab fragment directed to EboZ GP (28, 29), at 0.5 μg/ml, followed by a fluorescein isothiocyanate-conjugated anti-human immunoglobulin. Numbers of GP-positive cells were then quantified.
Determination of Ebola virus GP expression levels.
For Western blot analysis cell monolayers were lysed in Triton lysis buffer (50 mM Tris [pH 8.0], 5 mM EDTA, 150 mM NaCl, 1% Triton X-100) containing a protease inhibitor cocktail (Sigma). This lysate was added to pelleted floating cells harvested prior to lysis. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Ebola virus GP expression was detected by Western blot analysis using an anti-V5 antibody (Invitrogen) and I125 conjugated to protein A (NEN).
Production and titration of murine leukemia virus (MLV) pseudotypes.
Pseudotypes were prepared as previously described (48). EboZ GP or EboZ GP mutΔ1234 plasmids were cotransfected into 293T cells together with pHIT60 and pHIT111. Forty-eight hours posttransfection, supernatant was collected and clarified by filtration through a 0.45-μm-pore-size filter. Serial dilutions of the pseudotypes were made, and 200 μl of each dilution was added to 293T cells seeded at 7.5 × 104 cells/well in a 24-well dish for 6 h of incubation at 37°C. Forty-eight hours postinfection, cells were washed with PBS and then fixed in 2% paraformaldehyde and stained for β-galactosidase activity as described previously (48). Viral titers were determined by enumeration of β-galactosidase-positive foci and expressed as focus-forming units per milliliter.
Production and assaying of adenovirus vectors.
Bacterial recombination to produce adenovirus vectors with E1 and E3 deleted was carried out as previously described (23). Briefly, pAD-TrackCMV containing the coding sequences of EboZ GP, EboR GP, and ASLV-A EnvA and an empty vector were cotransformed with pADEasy-1 into BJ5183 bacteria (Quantum Biotech) by electroporation, and pADTrack/pADEasy recombinants were amplified and transfected into 293 cells. Spread of adenovirus in these E1A-positive cells was assayed by measuring green fluorescent protein (GFP) expression, and virus was harvested by multiple rounds of freezing and thawing. Stocks for assaying transgene expression were made by two further rounds of amplification in 293 cells. Following titration, 400 μl of virus at the correct multiplicity of infection (MOI) was used to challenge between 5 × 104 and 4 × 105 cells in a six-well plate for 6 h at 37°C. For macrophages this was done in serum-free RPMI 1640. Then 2 ml of medium was added to the cells, and they were monitored at regular intervals for GFP expression and cell rounding. Forty-eight hours before infection with adenovirus vectors, U937 cells were induced to differentiate to adherent, macrophage-like cells by the addition of 1.6 × 10−7 M phorbol myristate acetate (PMA) (12).
Flow-cytometric analysis.
293T and Vero cells was collected at 48 h after transfection or after challenge with adenovirus (MOI of 10) by using EDTA to remove adherent cells, which were pooled with harvested floating cells. HUVEC were collected 24 h after transduction with adenovirus at an MOI of 10 as described above except that rapid exposure to trypsin-EDTA was used to remove cells. Cells were preincubated at 4°C in PBS containing 5% FCS for 30 min. Following incubations with primary antibodies to cell surface markers (Chemicon) and fluorescein isothiocyanate- or Cy5-conjugated anti-mouse immunoglobulin (Chemicon), cells were assayed on a FACalibur (Becton Dickinson). Cells were gated on forward and side scatter, and 10,000 gated events were accumulated and analyzed.
RESULTS
GP-induced cell rounding is common to all subspecies of Ebola virus.
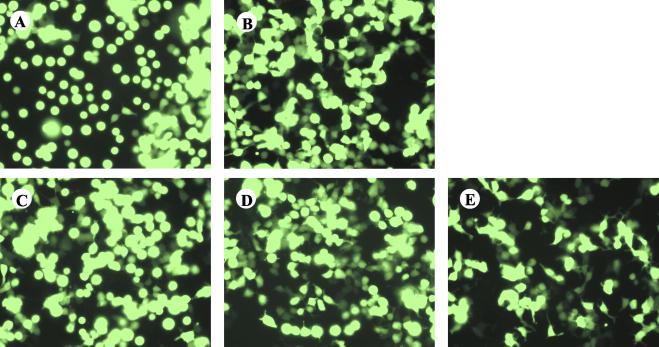
293T cells were transiently transfected with the EboZ GP coding sequence in pAD-TrackCMV coexpressing GFP under the control of a separate cytomegalovirus (CMV) promoter. GP-transfected cells underwent cell rounding and detachment from the tissue culture plastic as previously described (Fig. 1) (10, 39, 50). In addition, reduced adherence to plates coated with extracellular matrix components (e.g., fibronectin and vitronectin) was seen (data not shown). Rounding was observed within 18 h posttransfection and reached a peak at 48 h. GPs from the other African strains, EboS and EboC, were also found to cause 293T cell rounding, although less dramatically than EboZ GP (Fig. 1). A less defined effect was observed with the “nonpathogenic” strain, EboR (Fig. 2A). Mock-transfected cells or cells transfected with a control viral envelope GP, the ASLV-A envelope (EnvA), did not demonstrate the rounding phenotype. EboS has a lower fatality rate than EboZ, while EboR appears to be nonpathogenic in humans (2); thus the extent of cell rounding appears to correlate with pathogenesis. Although variations in the processing efficiency of GP are apparent, the differences in cell rounding were not merely a reflection of levels of expression, as EboZ, EboS, and EboR GPs bearing V5 epitope tags on their C termini were expressed at broadly similar levels (Fig. 2B), despite dramatic differences in the numbers of floating cells induced (Fig. 2A).
FIG. 1.
Ebola virus GP expression induces cell rounding. 293T cells were transfected with 20 μg of pAD-TrackCMV containing the coding sequence for EboZ GP (A), EboS GP (B), EboR GP (C), EboC-GP (D), or ASLV-A EnvA (E). GFP expression was observed at 48 h posttransfection using a Nikon TE300 inverted microscope. Fields represent findings from multiple experiments.
FIG. 2.
The cell rounding phenotype correlates with the level of GP expression. (A) 293T cells were transfected with 5, 10, or 15 μg of pcDNA6/V5-His containing the coding sequence for EboZ GP (♦), EboS GP (▪), or EboR GP (▴). The floating cells in the cultures were counted 48 h posttransfection, and results are presented as numbers of floating cells above background levels (8 × 104 cells), calculated from mock-transfected cells. (B) Lysates from cells harvested 48 h posttransfection were subjected to SDS-4 to 15% PAGE, transferred to nitrocellulose, and immunoblotted for the presence of the C-terminal V5 epitope on both the uncleaved precursor (GP0) and processed GP2 using a mouse anti-V5 monoclonal antibody followed by I125-labeled protein A. Data are representative of three independent experiments.
The rounding phenotype maps to multiple O-linked glycosylation sites in GP1.
Deletion of an O-linked glycosylation-rich, mucin-like domain in the C-terminal region of GP1 results in the loss of the rounding phenotype (50). Tellingly, insertion of the mucin domain into the envelope GP from MLV also produces a protein capable of inducing cell rounding (50). It is not clear, however, if the carbohydrate moieties or the protein itself is responsible for the phenotype. Interestingly, the membrane-bound mucin GP, episialin, induces a cell rounding and detachment phenotype very similar to that induced by Ebola virus GP despite little primary sequence homology between the two proteins (40, 46). Thus, to further map the determinants involved in the rounding phenotype, EboZ GP mutants containing serial deletions in the mucin-like domain were made (Fig. 3A); the associated mutations were based on four clusters of potential O-link glycosylated serines/threonines in the C-terminal portion of GP. Forty-eight hours posttransfection, levels of total cellular expression of EboZ GP and the mutants varied by less than twofold, as judged by SDS-PAGE and immunoblotting for the V5 epitope tag on the C terminus of GP2 (Fig. 3B). These levels correlated with cell surface expression, as determined by flow cytometry using KZ52, a human Fab fragment directed against GP (data not shown). Protein folding of the mutants was likely unaffected by the deletions, as coexpression of mutΔ1234, lacking all of the predicted C-terminal O-linked glycosylation sites, with plasmids encoding MLV Gag/Pol and LacZ produced infectious MLV pseudotype particles with titers equivalent to those of wild-type EboZ GP (titers of <5, 9.2 × 104, 5.3 × 104, and 1.2 × 105 FFU/ml for particles with no envelope and particles with envelopes consisting of EboZ GP, EboZ GP with a C-terminal V5-His tag, and mutΔ1234 with a C-terminal V5-His tag, respectively). Thus, the regions of EboZ GP involved in binding to cellular receptors expressed on 293T cells, as well as those mediating membrane fusion, lie outside the region removed in mutΔ1234.
Sequential removal of segments of the mucin-like domain led to a successive reduction in cell detachment induced in 293T cells. Removal of two predicted glycosylation sites in mutΔ1 reduced the levels of floating cells by approximately 50%. The loss of a further five potential sites reduced rounding by another 50% and so forth until removal of all of the predicted type O glycosylation sites from the C terminus of GP1 virtually restored the numbers of floating cells to levels characteristic of mock-transfected cells. Retention of the first two O-linked glycosylation sites in mutΔ23 and mutΔ234 led to reductions of approximately 65 and 90%, respectively. Thus, the overall length of the domain in EboZ GP rather than specific determinants appears to contribute to the rounding-and-detachment phenotype (Fig. 3B). EboR GP also contains a mucin-like domain; thus, the distribution or geometry of the O-linked sugars may affect the loss of adhesion observed.
GP-induced cell rounding is not confined to 293T cells.
Adenovirus vectors expressing Ebola virus GP together with GFP under the control of a separate CMV promoter were used to efficiently transduce a range of cell lines and primary cell types. In 293T cells transduced with EboZ GP encoded by adenovirus (Ad/EboZ-GP), levels of GP surface expression were approximately fivefold higher than those obtained by transient transfection, as determined by flow cytometry (data not shown). Ad/EboZ-GP caused cell rounding and detachment in glioma cell line U87 (Fig. 4A), as well as several other human adherent cell lines, including HeLa, NP2, HOS, and 293T (data not shown). Interestingly, introduction of GP into a nonadherent variant of 293 cells (293H) caused the cells to lose their ability to form large aggregates (Fig. 4A). Thus, Ebola virus GP appears to be able to affect cell-to-cell interactions as well as cell-to-substrate attachment. In each case EboR GP also demonstrated a lesser effect than EboZ GP but one that was visible, with later time points giving increased cell detachment for EboR GP. Although Ad/EboZ-GP was able to mediate cell rounding in many human and nonhuman cell types, little effect was noted in promonocytic cell line U937, which had been differentiated to macrophage-like cells by 2 days of prior treatment with phorbol ester PMA (Fig. 4A). Interestingly, in PMA-differentiated U937 cells, but not in any other cell type tested, large multinucleated syncytia were noted upon GP expression, particularly in Ad/EboR-GP-infected cells.
FIG. 4.
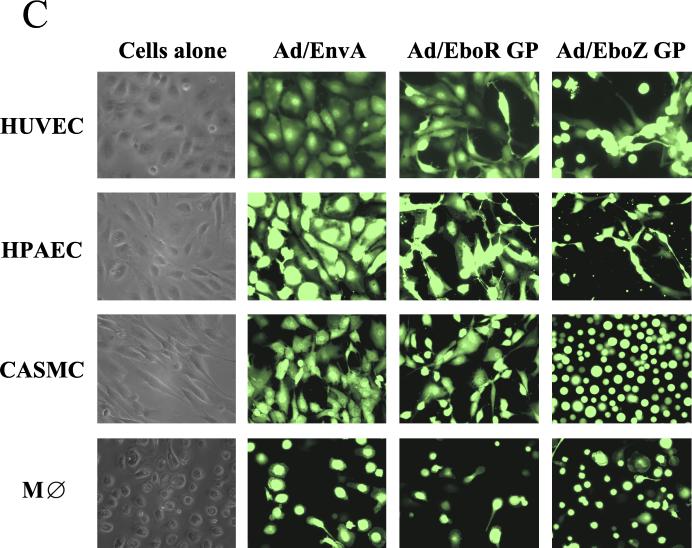
Ebola virus GP transduction of human and nonhuman cell lines and primary cell types using adenovirus vectors. (A) Human cell lines U87, 293H, and PMA-pretreated U937 were transduced with the minimum inoculum of adenovirus vectors expressing ASLV-A EnvA, EboR GP, or EboZ GP required to give the highest achievable level of GFP-positive cells (MOIs of 30, 10, and 50, respectively). Cells were monitored at regular intervals for evidence of cell rounding, and representative photographs were taken at 48 h posttransduction. (B) Adenovirus vectors expressing ASLV-A EnvA, EboR GP, or EboZ GP were used to transduce a simian cell line, Vero (MOI, 10), a cat kidney cell line, CCC (MOI, 10), baby hamster kidney cells (BHK; MOI, 50), and a murine cell line expressing the human coxsackievirus/adenovirus receptor, MC57/hCAR (MOI, 50). Cells were monitored for rounding as described for panel A. AGM, African green monkey. (C) Adenovirus vectors expressing ASLV-A EnvA, EboR GP, or EboZ GP were used to transduce low-passage primary HUVEC, HPAEC, CASMC as well as day 8 human primary blood monocyte-derived macrophages (MDM). Similar results were obtained for MDM from three separate donors. An MOI of 10 was used for all primary cell types, apart from MDM, where an MOI of 50 was required. Cells were monitored for rounding as described for panel A. In all cases, control vectors expressing just GFP gave results indistinguishable from those for EnvA (data not shown). M⊘, macrophage.
While experimental infection with EboZ is almost invariably fatal in nonhuman primates, it is initially nonpathogenic in adult rodents such as mice and guinea pigs, although Ebola virus rapidly becomes pathogenic upon serial passage in these hosts (34). The cell detachment phenotype, however, was also observed in a range of nonhuman cell types, including African green monkey kidney cells (Vero), as well as feline (CCC), hamster (BHK), and murine cells (MC57) (Fig. 4B). Although Ad/EboZ-GP was the most potent, EboR GP also induced rounding in all cell types tested. While EboZ GP-induced cell rounding and detachment were clearly visible after 18 h in some cell types, including CCC cells, other cell lines such as Vero required 48 h to fully display the phenotype. These differences may reflect the intrinsic adherent properties of different cell types, as cells such as Vero require more-stringent protocols to remove them from the extracellular matrix using trypsin-EDTA.
Ad/Ebo-GP induced rounding in primary cell types.
The finding that Ad/Ebo-GP did not mediate cell rounding in PMA-treated U937 cells (Fig. 4A), a model of macrophages, which represent the major cell target for filoviruses in vivo (5, 15, 33), prompted us to investigate the effects of Ebola virus GP in primary macrophages. In contrast to what was found in the experiments with U937 cells, transduction by Ad/EboZ-GP but not ASLV EnvA encoded by adenovirus (Ad/ASLV-EnvA) induced cell detachment of primary blood monocyte-derived macrophages from three independent donors in at least 50% of transduced cells as judged by GFP coexpression (Fig. 4C). Induction of cellular detachment in macrophages by GP may thus play a major role in viral pathogenesis.
In addition to macrophages, several other primary cell types, including HUVEC and HPAEC, as well as a smooth muscle cell type (CASMC), also demonstrated the rounding phenotype very strongly upon transduction with Ad/EboZ-GP (Fig. 4C). The levels of endothelial cell infection in vivo and the extent to which direct viral cytopathic damage of the endothelium impacts Ebola virus pathogenesis are at present controversial (4). The efficiency with which Ad/EboZ-GP induced rounding in endothelial cells, however, suggested that even if only a very low percentage of cells within an intact endothelial monolayer were infected with Ebola virus and hence expressing GP, cell detachment may impact greatly endothelium integrity and thus may play a role in the massive hemorrhage associated with filovirus infection. Indeed, transduction of intact endothelial cell monolayers with Ad/EboZ-GP at low MOIs resulting in ≤1% GFP+ cells led to an increase in monolayer permeability (data not shown).
GP cytoxicity.
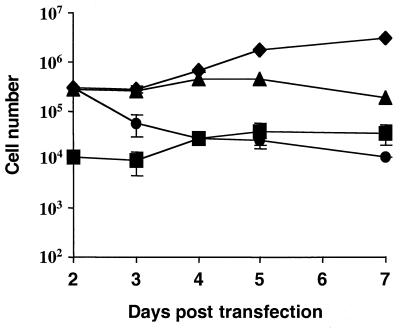
Over 90% of floating cells taken from a transiently transfected culture were strongly positive for EboZ GP by indirect immunofluorescence (Fig. 5), while trypan blue exclusion indicated that over 95% of these cells were viable. Upon being replated in a fresh culture dish, the cells adhered to the surface within 1 to 2 days (Fig. 5). Throughout the readherence over 95% of the cells remained viable. Likewise, cell viability remained constant at around 95% for 5 to 80 h, as assayed by 7AAD staining (data not shown). In addition, early apoptosis markers such as annexin V were absent from the 7AAD-negative population, suggesting no enhancement of apoptosis in these cells (data not shown). Thus, GP does not appear to be acutely toxic nor does it irreversibly trigger cell death. This does not rule out, however, the possibility that long-term expression of GP may indeed induce cytotoxicity. Indeed, loss of cell-to-cell contact can lead to induction of apoptosis in a mechanism termed anoikis (17). Stable 293 cell lines expressing the Ebola virus GPs, however, could be easily established.
FIG. 5.
Ebola virus GP does not induce cell death. Floating cells (3 × 105 per well) from 293T cells 48 h posttransfection were replated into fresh six-well plates. At 24-h intervals floating cells were counted (•) and then pooled with versine-detached adherent cells, and viable (♦) and nonviable (▪) cell numbers were estimated using trypan blue exclusion. Cells were then adhered to poly-d-lysine-coated plates, and the percentage of Ebola virus GP-positive cells (▴) was quantified using indirect immunofluorescence staining with KZ52. Values represent the geometric means of triplicate samples ± standard errors. Data are representative of three independent experiments.
GP modulates expression of adhesion and other cell surface molecules.
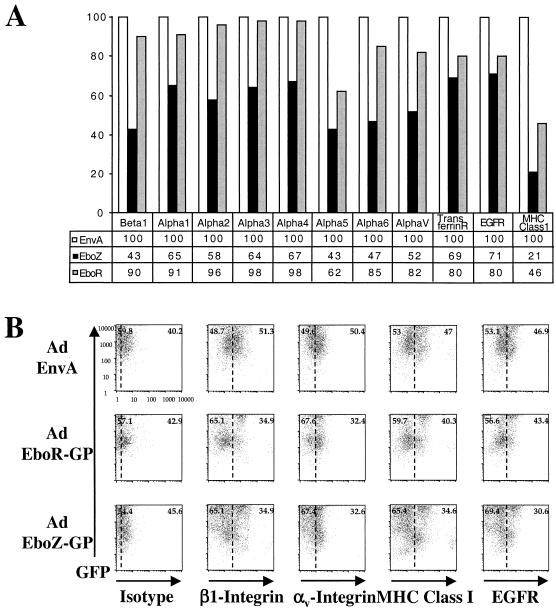
The observation that EboZ GP expression caused cells to detach from the culture dish suggested that loss of functional ligands, such as the integrins, to extracellular matrix components may be occurring. Indeed, down-regulation of a number of integrin subunits, particularly β1 (CD29) and αv (CD51) (Fig. 6A), expressed in 293T cells was observed upon EboZ GP, but not EnvA, expression. Consistent with the levels of detachment observed, EboR GP expression resulted in a detectable, but less dramatic, reduction in the cell surface presence of integrin subunits. Down-regulation of other cell surface markers, however, including the epidermal growth factor receptor (EGFR), the transferrin receptor, and major histocompatibility complex class I (MHC-I), was also observed with both EboZ GP and EboR GP, suggesting that the mechanism of loss of integrins from the cell surface may not be specific but rather global.
FIG. 6.
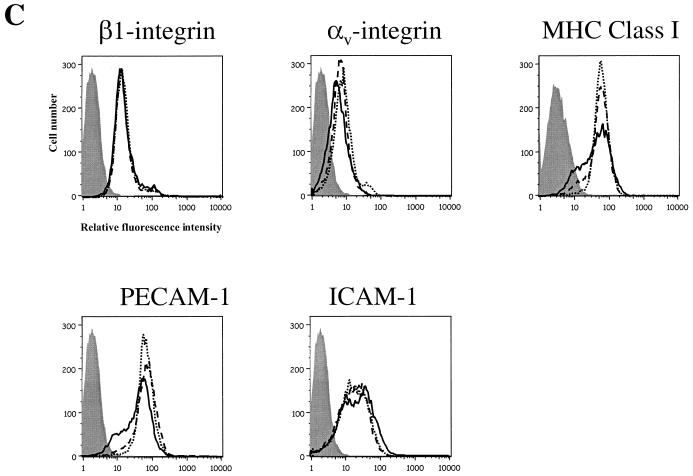
Expression of Ebola virus GP induces down-modulation of cell surface molecules. (A) At 48 h after transfection with pCB6-EboZ-GP, -EboR-GP, or -ASLV-A-EnvA, 293T cells were detached from the tissue culture plates, pooled with already-floating cells, and incubated with monoclonal antibodies directed against a number of integrin subunits as well as other common cell surface markers. Flow-cytometric analysis was then performed using secondary antibodies conjugated with fluorescein. Results are presented percentages of the mean channel fluorescence (MCF) compared to that for EnvA-transfected cells. Data are representative of multiple independent experiments. (B) Vero cells transduced at an MOI of 10 with Ad/EnvA Ad/EboR-GP, or EboZ GP were collected at 48 h postinfection. Cells were incubated with monoclonal antibodies to an isotype control, β1-integrin, αv-integrin, MHC-I, or EGFR and a secondary antibody conjugated to Cy5 and assayed by flow cytometry for GFP expression (FL1; y axis) or cell surface markers (FL4; x axis). Dotted lines, estimated MCF of expression for mock-transduced cells (C) HUVEC transduced at an MOI of 10 with Ad/EboZ-GP (solid line), Ad/EboR-GP (dashed line), or Ad/EnvA (dotted line) were collected 24 h postinfection. Cells were incubated with monoclonal antibodies to isotype controls (shaded histograms), β1-integrin, αv-integrin, MHC-I, or PECAM and a secondary antibody conjugated to Cy5 and assayed by flow cytometry. In HUVEC, unlike HPAEC, which showed up-regulation of some markers in response to adenovirus infection, there was no difference between nontransduced and Ad/EnvA-transduced cells for any of the markers (data not shown). Data are representative of three independent experiments.
A similar pattern of global cell surface marker down-regulation was seen in Ad/Ebo-GP-transduced Vero cells (Fig. 6B) and HPAEC (data not shown). In both cases down-modulation of β1 and αv-integrin was accompanied by a reduction in surface expression of EGFR and MHC-I. Interestingly, the down-regulation of surface markers on HUVEC transduced with Ebola virus GP showed a more specific pattern, with MHC-I and adhesion molecule PECAM-1 (CD31) showing strong down-modulation with EboZ GP, but not β1 (Fig. 6C). Levels of αv-integrin were modestly reduced. Ad/EboR-GP also induced strong down-regulation of MHC-I, but not of PECAM. Thus, adhesion molecules other than the integrins may be involved in GP-induced cell detachment in some cell types.
DISCUSSION
Expression of recombinant envelope GP from all four subspecies of Ebola virus induced variable degrees of rounding and detachment of human cells from the tissue culture surface, although expression via an adenovirus vector was required to observe significant rounding with GP from the nonpathogenic strain, EboR. Thus, intrinsic differences in the abilities of GPs from distinct strains to induce rounding and detachment exist, and these abilities follow the same order as mortality rates in humans and other primates, i.e., EboZ >> EboS >> EboR. The relative differences in the rounding phenotypes induced by EboZ GP and EboR GP in simian and human cells appeared to be similar (Fig. 4) despite a report that Ad/EboR-GP caused endothelial damage in cynomolgus monkey carotid arteries but not human saphenous veins (50). Simian endothelial cells were not tested in the present study; thus it is possible that the simian, but not the human, endothelium is more sensitive to the cell rounding induced by EboR GP than the simian cell lines tested. However, these results suggest the lack of a simple correlation between EboZ and EboR pathogenesis in humans and nonhuman primates, as suggested by Yang et al. (50). It is likely that GP from both subspecies can induce endothelial damage given sufficiently high GP expression. Importantly, both strains also induced detachment in primary macrophages, the major in vivo target for Ebola virus replication, particularly during the early stages of disease.
The detachment phenotype induced by a particular GP correlated with levels of GP expression, with increasing cellular levels of both EboZ and EboS GPs leading to increased detachment (Fig. 2A). Due to a transcriptional editing event required for GP expression, only 20% of transcripts are associated with GP production during virus infection (35, 42). The fact that reducing the levels of cellular GP reduces the rounding phenotype in 293T cells suggests that sGP may be a mechanism for controlling levels of GP and thus blunting its cell-rounding effects. Indeed, mutation of the transcriptional editing site to give only membrane-bound GP increases the cytopathogenicity of virus in tissue culture (44). It remains unclear, however, whether the cytotoxicity and pathogenesis seen with wild-type Ebola virus are due to the remaining levels of GP expression or other viral factors. Ad/Ebo-GP transduction of endothelial cells, however, gives levels of GP expression similar to those resulting from wild-type Ebola virus (50) infection, suggesting that the levels of membrane-bound GP expressed in the context of transcriptional editing remain sufficient to induce cell rounding. It will be interesting to see if recombinant viruses bearing envelopes, such as mutΔ1234, which do not induce cell rounding are cytotoxic in cell culture and pathogenic in animals.
The fact that Ebola virus GP did not directly cause cell death but rather induced a loss of adherence, contrary to a previous report (50), suggested that GP may modulate the surface expression or activation of cell adhesion molecules. Integrin-mediated adhesion plays many roles in cell adhesion, signaling, and immune regulation (1, 8, 9, 22, 27, 45); thus, these molecules were initially tested. Indeed, integrin molecules, especially β1- and αv-integrin subunits, were down-regulated from the surfaces of several, but not all, cell types by Ebola virus GP. Several viruses, including human CMV, down-regulate or bind to integrin subunits and can induce cell rounding and detachment (37). Moreover, similar effects can be induced by antibodies to β1-integrin (30). Thus, loss of integrins from the cell surface may play a major role in the induction of cell rounding by Ebola virus GP. EboR GP did not affect the cell surface integrin levels as strongly as EboZ GP, which correlates well with the morphological changes observed with GP expression. In addition, other adhesion molecules such as PECAM-1 (Fig. 6) and VCAM (data not shown) were down-regulated on endothelial cells by Ad/EboZ-GP while β1-integrin was not, suggesting that integrin down-regulation may not to be solely responsible for cell detachment, although loss of other integrin subunits from endothelial cells may occur. Many other nonadhesion molecules, such as MHC-I and EGFR as well as CD55, a GPI-linked protein (data not shown), are also down-modulated, suggesting that a broad mechanism is involved in surface molecule down-regulation. Thus, cell surface marker down-modulation is unlikely to be directly due to virus-cellular receptor interactions as suggested previously (39).
The mucin-like domain in Ebola virus GP is required for the rounding phenotype (Fig. 3) (50). Mucins are a diverse family of GPs epitomized by significant O-linked carbohydrate modification. Interestingly, mucins such as episialin induce strikingly similar decreases in cell attachment (11, 46). The presence of a mucin-like domain is conserved among all filoviruses, suggesting functional importance; however, this domain is unlikely to contain elements involved in binding cellular receptors required for entry as the domain is entirely dispensable for efficient infection of 293T cells. In addition, its removal does not adversely affect protein folding and conformation, suggesting that it forms an entirely separate domain. No particular motif in the mucin-like domain appears to be responsible for the rounding phenotype; rather the entire domain contributes to rounding (Fig. 3). Thus, the overall level of glycosylation and/or charge may play a role. Alternatively, the mucin-like domain may act as a nonspecific lectin, binding to many glycosylated proteins and thus causing their retention in the export machinery. Indeed, the majority of cell associated GPs appear endoglycosidase H sensitive, suggesting that they are retained in the endoplasmic reticulum or early Golgi (44). Volchkov and colleagues suggest that the large degree of N- and especially O-linked glycosylation required on GP may “exhaust” the cellular machinery for posttranslational modification of proteins (44). The expression of GP truncated before the membrane-spanning domain to produce a secreted form of GP (secGP) containing all of the glycosylation sites of full-length GP does not cause rounding, however (39). Likewise, sGP expression does not induce cell rounding, nor is coexpression of sGP together with GP able to reduce the levels of cell detachment induced by GP (data not shown). Thus, membrane-anchored expression appears to be required for the rounding phenotype. Also, over 90% of floating cells were strongly positive for GP expression (Fig. 5), suggesting that surface GP expression is unlikely to induce detachment in nontransfected neighboring cells.
The loss of cell adhesion as well as the specific loss of MHC-I and adhesion molecules involved in stabilizing cell-to-cell interactions is likely to negatively impact both the stimulation of an anti-Ebola virus CD8 T-cell response and the ability of T cells to kill infected cells. Indeed, the more-specific down-modulation of MHC-I in HUVEC may suggest that such host defense molecules are the intended target of the down-regulation. One of the most striking aspects of Ebola virus pathology is lymphocyte depletion and suppression of T-cell proliferation as well as an apparent lack of inflammatory infiltrates in target organs. T cells have been implicated as the primary mediators of clearance in nonfatal cases of filovirus infection (3), and perfusion of Ebola virus-infected nude mice with cytotoxic T cells specific for Ebola virus epitopes within 2 days of infection prevents death (47). Ad/EboZ-GP was able to induce MHC-I down-regulation in macrophages in addition to cell lines and endothelial cells (data not shown). Cells of the monocyte/macrophage lineage provide the initial target for Ebola virus replication in vivo, and many types of tissue macrophages (e.g., Kupffer cells in the liver) are infected throughout the course of disease. These cells play an important role in both innate and adaptive immune responses, particularly as antigen-presenting cells, forming conjugates to activate T cells. Thus, disruption of the ability of these cells to productively interact with T cells may adversely affect the immune response to Ebola virus, contributing to its highly pathogenic properties.
The integrins also play a critical role in the trafficking of leukocytes through the endothelium to sites of infection. Specifically, the initial tethering and rolling of leukocytes along the endothelium, followed by the arrest and final diapedesis into sites of infection, all require regulated integrin function (9). In response to direct infection by many RNA and DNA viruses or to exposure to inflammatory cytokines, endothelial cells up-regulate immunomodulatory genes such as intercellular adhesion molecule 1 and MHC genes (21). This activated state is necessary for the diapedesis of leukocytes through the endothelium and into the tissue. Infection of endothelial cells with replication-competent EboZ, however, inhibits this antiviral state from being induced, even following superinfection with measles virus (19, 20). This inhibition is at least partially due to Ebola virus protein VP35, which acts as a potent alpha interferon antagonist (6). However, the down-modulation of PECAM-1 and MHC-I in endothelial cells by GP alone indicates that GP also plays a major role in this process.
In summary, expression of Ebola virus GP mediates a loss of cellular adhesion, which can be at least partially attributed to the down-modulation of many cell surface markers including adhesion molecules by an as yet unidentified mechanism. While loss of cell adhesion, particularly in endothelial cells, may contribute to the hemorrhagic disease characteristic of Ebola virus infection, it is likely that a major role of GP may be in modulating the host's ability to mount an effective immune response. Loss of adherence, as well as a reduction in MHC-I surface levels, may thus play a major role in the highly pathogenic course of Ebola virus infection. The precise role of cell rounding and cell surface marker down-regulation, however, may not be fully elucidated without the identification of the natural host(s) of filoviruses.
Acknowledgments
We thank Dennis Burton for providing KZ52 and Bert Vogelstein for pAD-Track and pAD-Easy plasmids. We also thank the members of the Bates laboratory for useful discussions, Robert Doms for critical reading of the manuscript, and Jacqueline Reeves and Stephen Eck for advice on adenovirus production.
G.S. is the recipient of a long-term EMBO fellowship. This work was supported by grants to P.B. from the National Institutes of Health.
REFERENCES
- 1.Alon, R., P. D. Kassner, M. W. Carr, E. B. Finger, M. E. Hemler, and T. A. Springer. 1995. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J. Cell Biol. 128:1243-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 1990. Update: filovirus infection in animal handlers. Morbid. Mortal. Wkly. Rep. 39:221.. [PubMed] [Google Scholar]
- 3.Baize, S., E. M. Leroy, M. C. Georges-Courbot, M. Capron, J. Lansoud-Soukate, P. Debre, S. P. Fisher-Hoch, J. B. McCormick, and A. J. Georges. 1999. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat. Med. 5:423-426. [DOI] [PubMed] [Google Scholar]
- 4.Balter, M. 2000. On the trail of Ebola and Marburg viruses. Science 290:923-924. [DOI] [PubMed] [Google Scholar]
- 5.Baskerville, A., S. P. Fisher-Hoch, G. H. Neild, and A. B. Dowsett. 1985. Ultrastructural pathology of experimental Ebola haemorrhagic fever virus infection. J. Pathol. 147:199-209. [DOI] [PubMed] [Google Scholar]
- 6.Basler, C. F., X. Wang, E. Muhlberger, V. Volchkov, J. Paragas, H. D. Klenk, A. Garcia-Sastre, and P. Palese. 2000. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc. Natl. Acad. Sci. USA 97:12289-12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 8.Berlin, C., R. F. Bargatze, J. J. Campbell, U. H. von Andrian, M. C. Szabo, S. R. Hasslen, R. D. Nelson, E. L. Berg, S. L. Erlandsen, and E. C. Butcher. 1995. Alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 80:413-422. [DOI] [PubMed] [Google Scholar]
- 9.Butcher, E. C. 1991. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 67:1033-1036. [DOI] [PubMed] [Google Scholar]
- 10.Chan, S. Y., M. C. Ma, and M. A. Goldsmith. 2000. Differential induction of cellular detachment by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J. Gen. Virol. 81:2155-2159. [DOI] [PubMed] [Google Scholar]
- 11.Chervenak, J. L., and N. P. Illsley. 2000. Episialin acts as an antiadhesive factor in an in vitro model of human endometrial-blastocyst attachment. Biol. Reprod. 63:294-300. [DOI] [PubMed] [Google Scholar]
- 12.Clapham, P. R., R. A. Weiss, A. G. Dalgleish, M. Exley, D. Whitby, and N. Hogg. 1987. Human immunodeficiency virus infection of monocytic and T-lymphocytic cells: receptor modulation and differentiation induced by phorbol ester. Virology 158:44-51. [DOI] [PubMed] [Google Scholar]
- 13.Feldmann, H., H. Bugany, F. Mahner, H. D. Klenk, D. Drenckhahn, and H. J. Schnittler. 1996. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J. Virol. 70:2208-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher-Hoch, S. P., and J. B. McCormick. 1999. Experimental filovirus infections. Curr. Top. Microbiol. Immunol. 235:117-143. [DOI] [PubMed] [Google Scholar]
- 15.Fisher-Hoch, S. P., G. I. Perez-Oronoz, E. L. Jackson, L. M. Hermann, and B. G. Brown. 1992. Filovirus clearance in non-human primates. Lancet 340:451-453. [DOI] [PubMed] [Google Scholar]
- 16.Formenty, P., C. Boesch, M. Wyers, C. Steiner, F. Donati, F. Dind, F. Walker, and B. Le Guenno. 1999. Ebola virus outbreak among wild chimpanzees living in a rain forest of Cote d'Ivoire. J. Infect. Dis. 179(Suppl. 1):S120-S126. [DOI] [PubMed] [Google Scholar]
- 17.Frisch, S. M., and H. Francis. 1994. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124:619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen, J. E., O. Lund, N. Tolstrup, A. A. Gooley, K. L. Williams, and S. Brunak. 1998. NetOglyc: prediction of mucin type O-glycosylation sites based on sequence context and surface accessibility. Glycoconj. J. 15:115-130. [DOI] [PubMed] [Google Scholar]
- 19.Harcourt, B. H., A. Sanchez, and M. K. Offermann. 1998. Ebola virus inhibits induction of genes by double-stranded RNA in endothelial cells. Virology 252:179-188. [DOI] [PubMed] [Google Scholar]
- 20.Harcourt, B. H., A. Sanchez, and M. K. Offermann. 1999. Ebola virus selectively inhibits responses to interferons, but not to interleukin-1β, in endothelial cells. J. Virol. 73:3491-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harcourt, J. L., M. K. Hagan, and M. K. Offermann. 2000. Modulation of double-stranded RNA-mediated gene induction by interferon in human umbilical vein endothelial cells. J. Interferon Cytokine Res. 20:1007-1013. [DOI] [PubMed] [Google Scholar]
- 22.Harder, R., H. Uhlig, A. Kashan, B. Schutt, A. Duijvestijn, E. C. Butcher, H. G. Thiele, and A. Hamann. 1991. Dissection of murine lymphocyte-endothelial cell interaction mechanisms by SV-40-transformed mouse endothelial cell lines: novel mechanisms mediating basal binding, and alpha 4-integrin-dependent cytokine-induced adhesion. Exp. Cell Res. 197:259-267. [DOI] [PubMed] [Google Scholar]
- 23.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito, H., S. Watanabe, A. Takada, and Y. Kawaoka. 2001. Ebola virus glycoprotein: proteolytic processing, acylation, cell tropism, and detection of neutralizing antibodies. J. Virol. 75:1576-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaax, N. K., K. J. Davis, T. J. Geisbert, P. Vogel, G. P. Jaax, M. Topper, and P. B. Jahrling. 1996. Lethal experimental infection of rhesus monkeys with Ebola-Zaire (Mayinga) virus by the oral and conjunctival route of exposure. Arch. Pathol. Lab. Med. 120:140-155. [PubMed] [Google Scholar]
- 26.Johnson, E., N. Jaax, J. White, and P. Jahrling. 1995. Lethal experimental infections of rhesus monkeys by aerosolized Ebola virus. Int. J. Exp. Pathol. 76:227-236. [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence, M. B., and T. A. Springer. 1991. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell 65:859-873. [DOI] [PubMed] [Google Scholar]
- 28.Maruyama, T., P. W. Parren, A. Sanchez, I. Rensink, L. L. Rodriguez, A. S. Khan, C. J. Peters, and D. R. Burton. 1999. Recombinant human monoclonal antibodies to Ebola virus. J. Infect. Dis. 179(Suppl. 1):S235-S239. [DOI] [PubMed] [Google Scholar]
- 29.Maruyama, T., L. L. Rodriguez, P. B. Jahrling, A. Sanchez, A. S. Khan, S. T. Nichol, C. J. Peters, P. W. Parren, and D. R. Burton. 1999. Ebola virus can be effectively neutralized by antibody produced in natural human infection. J. Virol. 73:6024-6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni, H., and J. A. Wilkins. 1998. Localisation of a novel adhesion blocking epitope on the human beta 1 integrin chain. Cell Adhes. Commun. 5:257-271. [DOI] [PubMed] [Google Scholar]
- 31.Pereboeva, L. A., V. K. Tkachev, L. V. Kolesnikova, L. Krendeleva, E. I. Riabchikova, and M. P. Smolina. 1993. The ultrastructural changes in guinea pig organs during the serial passage of the Ebola virus. Vopr. Virusol. 38:179-182. [PubMed] [Google Scholar]
- 32.Peters, C. J., A. Sanchez, P. E. Rollin, T. G. Ksiazek, and F. A. Murphey. 1996. Filoviridae: Marburg and Ebola viruses, p. 1161-1176. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 33.Ryabchikova, E., L. Kolesnikova, M. Smolina, V. Tkachev, L. Pereboeva, S. Baranova, A. Grazhdantseva, and Y. Rassadkin. 1996. Ebola virus infection in guinea pigs: presumable role of granulomatous inflammation in pathogenesis. Arch. Virol. 141:909-921. [DOI] [PubMed] [Google Scholar]
- 34.Ryabchikova, E. I., L. V. Kolesnikova, and S. V. Netesov. 1999. Animal pathology of filoviral infections. Curr. Top. Microbiol. Immunol. 235:145-173. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez, A., S. G. Trappier, B. W. Mahy, C. J. Peters, and S. T. Nichol. 1996. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc. Natl. Acad. Sci. USA 93:3602-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnittler, H. J., F. Mahner, D. Drenckhahn, H. D. Klenk, and H. Feldmann. 1993. Replication of Marburg virus in human endothelial cells. A possible mechanism for the development of viral hemorrhagic disease. J. Clin. Investig. 91:1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shahgasempour, S., S. B. Woodroffe, G. Sullivan-Tailyour, and H. M. Garnett. 1997. Alteration in the expression of endothelial cell integrin receptors alpha 5 beta 1 and alpha 2 beta 1 and alpha 6 beta 1 after in vitro infection with a clinical isolate of human cytomegalovirus. Arch. Virol. 142:125-138. [DOI] [PubMed] [Google Scholar]
- 38.Simmons, G., J. D. Reeves, A. McKnight, N. Dejucq, S. Hibbitts, C. A. Power, E. Aarons, D. Schols, E. De Clercq, A. E. Proudfoot, and P. R. Clapham. 1998. CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J. Virol. 72:8453-8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takada, A., S. Watanabe, H. Ito, K. Okazaki, H. Kida, and Y. Kawaoka. 2000. Downregulation of beta1 integrins by Ebola virus glycoprotein: implication for virus entry. Virology 278:20-26. [DOI] [PubMed] [Google Scholar]
- 40.Takeda, T., W. Y. Go, R. A. Orlando, and M. G. Farquhar. 2000. Expression of podocalyxin inhibits cell-cell adhesion and modifies junctional properties in Madin-Darby canine kidney cells. Mol. Biol. Cell 11:3219-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villinger, F., P. E. Rollin, S. S. Brar, N. F. Chikkala, J. Winter, J. B. Sundstrom, S. R. Zaki, R. Swanepoel, A. A. Ansari, and C. J. Peters. 1999. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J. Infect. Dis. 179(Suppl. 1):S188-S191. [DOI] [PubMed] [Google Scholar]
- 42.Volchkov, V. E., S. Becker, V. A. Volchkova, V. A. Ternovoj, A. N. Kotov, S. V. Netesov, and H. D. Klenk. 1995. GP mRNA of Ebola virus is edited by the Ebola virus polymerase and by T7 and vaccinia virus polymerases. Virology 214:421-430. [DOI] [PubMed] [Google Scholar]
- 43.Volchkov, V. E., H. Feldmann, V. A. Volchkova, and H. D. Klenk. 1998. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc. Natl. Acad. Sci. USA 95:5762-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volchkov, V. E., V. A. Volchkova, E. Muhlberger, L. V. Kolesnikova, M. Weik, O. Dolnik, and H. D. Klenk. 2001. Recovery of infectious Ebola virus from complementary DNA: RNA editing of the GP gene and viral cytotoxicity Science 291:1965-1969. [DOI] [PubMed] [Google Scholar]
- 45.von Andrian, U. H., J. D. Chambers, L. M. McEvoy, R. F. Bargatze, K. E. Arfors, and E. C. Butcher. 1991. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc. Natl. Acad. Sci. USA 88:7538-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wesseling, J., S. W. van der Valk, H. L. Vos, A. Sonnenberg, and J. Hilkens. 1995. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J. Cell Biol. 129:255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson, J. A., and M. K. Hart. 2001. Protection from Ebola virus mediated by cytotoxic T lymphocytes specific for the viral nucleoprotein. J Virol. 75:2660-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wool-Lewis, R. J., and P. Bates. 1998. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J. Virol. 72:3155-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wool-Lewis, R. J., and P. Bates. 1999. Endoproteolytic processing of the Ebola virus envelope glycoprotein: cleavage is not required for function. J. Virol. 73:1419-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, Z. Y., H. J. Duckers, N. J. Sullivan, A. Sanchez, E. G. Nabel, and G. J. Nabel. 2000. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat. Med. 6:886-889. [DOI] [PubMed] [Google Scholar]