Abstract
The current studies demonstrate complex and seemingly contradictory effects by gamma interferon (IFN-γ) on Friend virus (FV) infection. Both temporal and tissue-specific effects were observed. During the first week of infection, IFN-γ-deficiency caused increased levels of FV infection in multiple tissues. Surprisingly, however, by 2 weeks postinfection, IFN-γ-deficient mice had significantly lower levels of infection in both the spleen and bone marrow compared to wild-type mice. The rapid reduction of virus in the IFN-γ-deficient mice correlated with a more rapid virus-neutralizing antibody response than was observed in the wild-type mice. Furthermore, the virus-neutralizing antibody response in wild-type mice could be accelerated by ablation of their IFN-γ response. Although the IFN-γ-deficient mice developed an accelerated virus-neutralizing antibody response, they did not class-switch to immunoglobulin G class immunoglobulins nor could they maintain long-term virus-neutralizing antibody titers. Eventually, all of the IFN-γ-deficient mice failed to keep persistent virus in check and developed fatal FV-induced erythroleukemia.
Understanding the basic immunological mechanisms that facilitate resistance to retroviral infections is vital for the rational development of preventative and therapeutic treatments against retrovirus-induced diseases. An important immunological mechanism influencing host defense against pathogens is the release of cytokines. One pivotal cytokine involved in resistance to viral infections is gamma interferon (IFN-γ), which can render cells resistant to virus infection (11) and inhibit virus replication in infected cells (20, 21). IFN-γ also promotes protective T helper type 1 (Th1) immune responses (10, 40) dominated by cell-mediated immunity and virus-neutralizing antibodies of the immunoglobulin G2a (IgG2a) isotype (48). Recent studies in the Friend virus (FV) model of retroviral infection in mice have shown an association between T-cell production of IFN-γ in vitro and recovery from acute infection in vivo (45). In addition, IFN-γ has been shown to play an important role in controlling persistent FV infection (30). However, we still do not understand the role of IFN-γ in virus spread and pathogenesis during the course of FV infection.
FV is a retroviral complex comprised of nonpathogenic, replication-competent Friend murine leukemia helper virus (F-MuLV), and pathogenic but replication-defective, spleen focus-forming virus (SFFV). The SFFV genome is spread by coinfection of cells with F-MuLV helper virus, which encodes the proteins necessary for virus particle formation and infectivity. Early Friend disease is characterized by gross splenomegaly due to proliferation of erythroid precursors stimulated by SFFV gp55 envelope binding to erythropoietin receptors (15, 28, 33). In the absence of protective immune responses, SFFV eventually integrates into the Spi-1 oncogene and into the p53 tumor suppressor gene to induce erythroleukemia (39, 41).
In the current study, we use mice with genetic inactivation of the IFN-γ gene (B6.IFN-γ−/−) to examine how a lack of IFN-γ affects virus spread and pathogenesis during FV infection. Surprisingly, results from kinetic analysis of viral infection indicated that mice deficient in IFN-γ production were initially more susceptible to FV infection, but then reduced virus levels faster than wild-type mice. This decrease in acute infection correlated with an accelerated virus-neutralizing antibody response. Despite the faster antibody response, the IFN-γ-deficient mice could not class-switch to IgG class immunoglobulins or maintain IgM virus-neutralizing antibodies during long-term infection such as wild-type mice. The absence of long-term virus-neutralizing antibody responses correlated with a loss of virus control, FV-induced splenomegaly, and a recurrence of viremia in the IFN-γ-deficient mice.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were obtained from Jackson Laboratory, Bar Harbor, Maine. B6.129S7-Ifngtm1Ts (B6.IFN-γ−/−) mice were N8 generation backcrosses to B6 and were obtained from Genentech, San Francisco, Calif. (9). All animals were treated in accordance with the National Institutes of Health regulations and the guidelines of the Animal Care and Use Committee of Rocky Mountain Laboratories.
Virus and virus infection.
The B-cell-tropic, polycythemia-inducing FV complex (35) was used for all virus challenge experiments and was from an uncloned virus stock obtained from a 10% spleen cell homogenate as previously described (8). Mice were inoculated by intravenous injection with 3,000 spleen focus-forming units of FV complex diluted in 0.5 ml of phosphate-buffered balanced salt solution (PBBS) containing 2% fetal bovine serum. The progression of Friend disease was monitored by weekly spleen palpations for splenomegaly in a blinded fashion as described elsewhere (23) and by virus assays as indicated.
Infectious center and virus-neutralizing antibody assays.
Single-cell suspensions from spleens of infected mice were titrated onto susceptible Mus dunni cells (32) for detection of infectious centers as described elsewhere (12). Heat-inactivated (56°C, 30 min) plasma samples from infected mice were tested for virus-neutralizing antibodies in the presence of complement as previously described (12).
Flow cytometric analyses.
Single cell suspensions of nucleated, live cells were analyzed by flow cytometry with a FACSCalibur instrument (Becton Dickinson, San Jose, Calif.). To detect FV infection, cells were stained with tissue culture supernatant containing monoclonal antibody (MAb) 34 (7), which is specific for F-MuLV glycosylated Gag protein. MAb 34 binding was detected with allophycocyanin-labeled goat anti-mouse IgG2b-specific antiserum (Caltag Laboratories, Burlingame, Calif.) that was preabsorbed with naive mouse spleen cells to remove background activity (13). Directly labeled fluorescent antibodies specific for Ter119 (Ly-76), CD4, CD8, CD19, and CD43 were obtained from Pharmingen (San Diego, Calif.). A total of 1 million cells were analyzed per sample, and propidium iodide staining was used to gate out the dead cells.
Tetramers and tetramer staining.
For the detection of virus-specific CD8+ T lymphocytes, 5 × 105 nucleated spleen cells were dually stained with fluorescein isothiocyanate-labeled anti-CD8 (Ly-2) (Pharmingen) and phycoerythrin-labeled major histocompatibility complex (MHC) class I H2-Db tetramers specific for FV GagL peptide (Db-GagL tetramers) (K. Schepers, M. Toebes, C. J. M. Melief, F. Ossendorp, and T. N. M. Schumacher, unpublished data) for 15 min at room temperature. The Db-GagL tetramers were constructed with a variant of the gagL epitope (5) in which all three cysteine residues were replaced with amino-butyric acid to allow MHC tetramer production. This variant peptide is recognized by polyclonal GagL-specific CD8+ T cells as determined by intracellular IFN-γ staining (Schepers et al., unpublished). Cells were washed two times, resuspended in buffer with propidium iodide, and analyzed by flow cytometry.
Sample preparation and real-time quantitative PCR.
An F-MuLV env-specific fluorogenic PCR probe [5"-(6FAM) ACTCCCACATTGATTTCCCCGTCC (TAMRA)-3", where 6FAM is 6-carboxy-fluorescin and TAMRA is 6-carboxy-tetramethyl-rhodamine; Applied Biosystems, Foster City, Calif.] was used for FV quantitation. The upstream and downstream primers were 5"-AAGTCTCCCCCCGCCTCTA-3" and 5"-AGTGCCTGGTAAGCTCCCTGT-3", respectively. Real-time PCR and reverse transcription-PCR (RT-PCR) amplifications (25) were performed in a 25-μl reaction mixture with either Taqman Universal PCR Master Mix or Taqman One-Step RT-PCR Master Mix Reagants Kit (Applied Biosystems), respectively, with an ABI 7700 sequence detector system (Perkin-Elmer). Each sample was run in duplicate. A standard curve was obtained by using serial dilutions of a plasmid containing the FV genome that allowed us to determine an average copy number per cell in infected mice. The housekeeping gene, GAPDH (glyceraldehyde-3-phosphate dehydrogenase; Taqman Rodent GAPDH Control Reagants, Applied Biosystems), was amplified from each sample to normalize the template concentrations.
In vivo administration of anti-IFN-γ MAb.
Neutralization of IFN-γ in B6 mice was done by intraperitoneal (i.p.) injections of 0.5 ml of artificial capillary culture supernatants containing ca. 0.25 mg of XMG1.2 antibody (6). Treatments began 1 day prior to infection and were continued three times a week for 2 weeks postinfection.
In vivo administration of rIFN-γ.
Mice were injected i.p. with 105 U (10 μg) of recombinant mouse IFN-γ (rIFN-γ; Leinco Technologies, St. Louis, Mo.) diluted in PBBS with 2% normal mouse serum. Treatments began 1 day prior to infection and continued every other day for 4 weeks postinfection. Control mice were injected with PBBS containing 2% normal mouse serum.
RESULTS
Effects of IFN-γ deficiency on acute FV infection.
To investigate the role of IFN-γ during acute virus infection, we used flow cytometric analysis of viral antigen expression to compare infection levels in spleen, bone marrow, and blood cells from FV-infected B6 and B6.IFN-γ−/− mice. At 1 week postinfection, the IFN-γ-deficient mice had slightly, but not significantly higher, levels of infected spleen cells (Table 1). However, both the bone marrow and the blood from the IFN-γ-deficient mice had significantly higher percentages of infected cells compared to B6 mice at this time point. Thus, the importance of IFN-γ in limiting early virus infection varied depending on the tissue. Paradoxically, by 2 weeks postinfection, the IFN-γ-deficient mice had fewer infected cells in both the spleen and the bone marrow than did the wild-type mice. These results suggested that IFN-γ helped limit infection levels during the first week but had detrimental effects on the ability of the mice to reduce FV infection in the spleen and bone marrow between 1 and 2 weeks. None of the wild-type B6 mice had detectable infection in their blood cells at either time point.
TABLE 1.
Tissue-specific viral antigen expression during acute FV infectiona
| Mouse strain | Mean % antigen-positive cells ± SD at:
|
||||||
|---|---|---|---|---|---|---|---|
| 1 wk postinfectionb in:
|
2 wks postinfectionc in:
|
||||||
| Spleen | Bone marrow | Blood cells | Spleen | Bone marrow | Blood cells | ||
| B6 | 7.9 ± 2.6 | 11.6 ± 6.6 | 0.5 ± 0.3d | 12.8 ± 8.9 | 20.4 ± 6.8 | 0.5 ± 0.3d | |
| B6.IFN-γ−/− | 11.4 ± 5.3 | 22.1 ± 9.4 | 6.0 ± 3.8 | 1.5 ± 2.1 | 4.3 ± 8.4 | 1.5 ± 2.0 | |
All tissues were examined by flow cytometry as single-cell suspensions of nucleated, live cells. The numbers of mice in each group were as follows at 1 week postinfection: B6.IFN-γ−/− (n = 6) and B6 (n = 6). The numbers of mice in each group were as follows at 2 weeks postinfection: B6.IFN-γ−/− (n = 11) and B6 (n = 15).
P = 0.3095, 0.0256, and 0.0002 for spleen, bone marrow, and blood cells, respectively.
P = <0.0001, 0.0006, and 0.6070 for spleen, bone marrow, and blood cells, respectively.
The percentage of cells that stained positive for antigen in the infected B6 mice was below or equivalent to the background staining in naive mice.
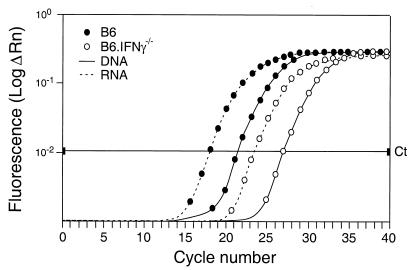
To confirm that the IFN-γ-deficient mice had actually reduced the number of virus-infected cells rather than converting to a latent infection, we developed a real-time PCR assay to quantify viral DNA and RNA. An amplification plot from a representative B6 and B6.IFN-γ−/− mouse at 2 weeks postinfection (Fig. 1) shows ca. 20 times more viral env DNA in the B6 mouse compared to the B6.IFN-γ−/− mouse. Similar results were obtained from five mice per strain (data not shown). For both strains, the levels of viral env RNA were approximately eight times higher than the viral env DNA levels. Therefore, the reduced levels of antigen-positive cells in the IFN-γ-deficient mice at 2 weeks postinfection were due to reduced levels of infection rather than to reduced levels of transcription.
FIG. 1.
Real-time PCR amplification plot of F-MuLV envelope sequence from B6 and B6.IFN-γ−/− mice at 2 weeks postinfection. ΔRn represents the magnitude of the fluorescence signal generated. The threshold cycle (Ct) is an arbitrary cycle number set above background on the linear portion of the curve that is used to make relative comparisons. Each sample was run in duplicate, and the Ct values were averaged: B6 DNA = 21.5, B6.IFN-γ−/− DNA = 26.9, B6 RNA = 17.8, and B6.IFN-γ−/− RNA = 23.2. A standard curve was generated by serial fivefold dilutions ranging from 106 to 102 copies per reaction of a plasmid containing the FV genome (data not shown). From the standard curve, we calculated the copy numbers of viral DNA per cell (B6 = 2.0 and B6.IFN-γ−/− = 0.085) and viral RNA (B6 = 16.8 and B6.IFN-γ−/− = 0.8), yielding ca. 20-fold more viral DNA and RNA in wild-type mice compared to the IFN-γ-deficient mice.
Effects of IFN-γ deficiency on immune responses during acute infection.
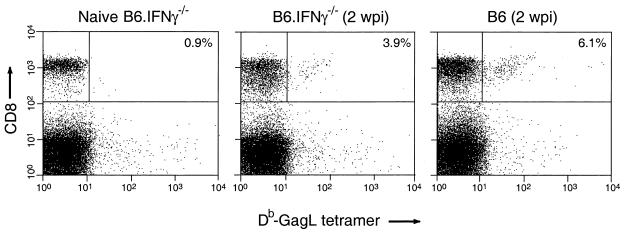
CD8+ T lymphocytes are important immunological effectors in the control of acute FV infection (24) and can be influenced by IFN-γ (18, 20, 51). To determine whether the lower virus infection in the IFN-γ-deficient mice at 2 weeks postinfection correlated with an increase in virus-specific CD8+ T cells, we stained splenic CD8+ T cells with MHC class I (H-2Db) tetramers containing an immunodominant peptide from the FV Gag protein (Db-GagL tetramers) (Schepers et al., unpublished). There was mouse-to-mouse variability in tetramer staining within both groups, but typical stains are shown in Fig. 2. Analysis of eight wild-type mice showed an average of 3.9% Db-GagL-specific CD8+ T cells in the spleen at 2 weeks postinfection and an average of 3.4% in seven IFN-γ-deficient mice. There was no statistical difference between the two groups as determined by Student's t test. The spleens of both groups were slightly enlarged due to FV infection, but the absolute numbers of CD8+ T cells were not significantly different between the two groups. Thus, the lower infection levels in the B6.IFN-γ−/− mice compared to B6 mice at 2 weeks postinfection did not correlate with improved Db-GagL-specific CD8+ T-cell responses. However, we did observe a significant difference (P = 0.0449 [Student's t test]) in the level of activated (CD43+) CD8+ T cells between the two groups at 2 weeks postinfection. An average of 31% of the CD8+ T cells in the IFN-γ-deficient mice were CD43 positive, while the wild-type mice had an average of only 15%. These results reflect a broader activation of CD8+ T cells in the IFN-γ-deficient mice than the wild-type mice at 2 weeks postinfection, but it is currently not known whether this was a virus-specific response.
FIG. 2.
Representative fluorescence-activated cell sorting profiles of FV-specific CD8+ T cells from B6 and B6.IFN-γ−/− mice at 2 weeks postinfection. Live, nucleated spleen cells were stained with anti-CD8 antibody and DbGagL tetramers at 2 weeks postinfection (wpi) to detect FV-specific CD8+ T cells. The percentage shown in each dot plot is the percentage of CD8+ T cells that stained positive for Db-GagL tetramers by flow cytometry.
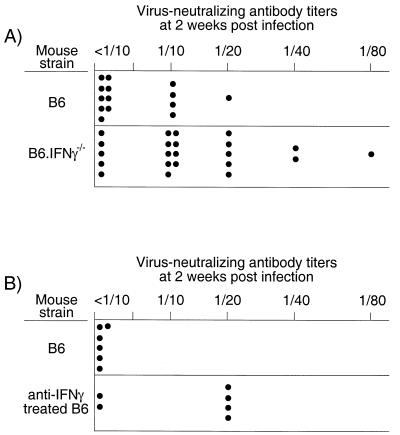
Virus-neutralizing antibodies have also been shown to be important in reducing FV infection (3, 12), so we tested for a correlation between virus-neutralizing antibody responses and infection levels at 2 weeks postinfection. Interestingly, 77% of the IFN-γ-deficient mice had detectable virus-neutralizing antibody titers, while only 35% of the wild-type mice had detectable titers at the 2-week time point (Fig. 3A). To confirm that the heightened specific antibody responses were due to the lack of IFN-γ, we ablated the IFN-γ response of wild-type mice by treatments with an IFN-γ-neutralizing MAb for the first 2 weeks after infection. The IFN-γ-depleted mice also had increased virus-neutralizing antibody titers at the 2-week time point (Fig. 3B). Thus, lack of IFN-γ resulted in an accelerated virus-neutralizing antibody response that correlated with decreased virus infection in both the spleen and bone marrow at 2 weeks postinfection (Table 1).
FIG. 3.
Total virus-neutralizing antibody titers in mice at 2 weeks post-FV infection. The neutralizing antibody titer was considered to be the highest dilution at which more than 75% of the input virus was neutralized. (A) The difference between the titers in B6 and the titers in B6.IFN-γ−/− mice was significant as determined by the Mann-Whitney test (P = 0.0081). Significantly more B6.IFN-γ−/− mice had detectable titers compared to wild-type B6 mice (Fisher's exact test, P = 0.0157). (B) The IFN-γ-depleted mice had significantly higher virus-neutralizing antibody titers than did the control mice (Mann-Whitney test, P = 0.0411). Also, significantly more IFN-γ-depleted mice had detectable titers compared to wild-type B6 mice (Fisher's exact test, P = 0.0303).
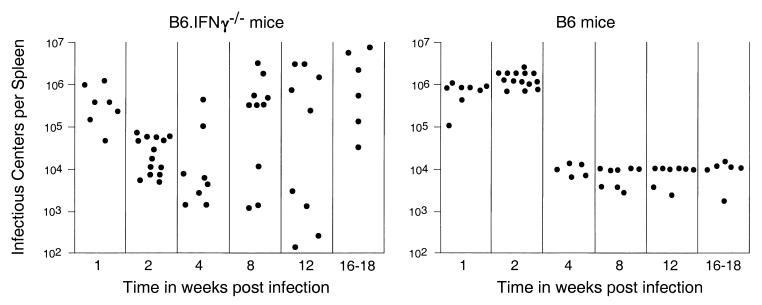
Kinetic analysis of virus infection in spleen and blood cells.
Since previous studies have shown protective roles for IFN-γ in long-term protection from FV-induced disease (30, 45), and the current results showed contrasting effects between 1 and 2 weeks postinfection, it was important to establish the temporal course of FV infection in the presence or absence of IFN-γ. The analysis was done by using an infectious center assay to compare the levels of virus-producing cells in the spleens of FV-infected B6 and B6.IFN-γ−/− mice. At 1 week postinfection, both wild-type and IFN-γ-deficient mice had comparably high virus levels in the spleen (Fig. 4). Consistent with the flow cytometric analysis (Table 1) and PCR data (Fig. 1), the mean level of virus-producing cells in the spleen at 2 weeks postinfection decreased by 16-fold in the IFN-γ-deficient mice, while infection levels remained high in B6 mice. By 4 weeks postinfection the wild-type mice had reduced spleen virus levels that remained stable over the 18-week duration of the study. In contrast, some B6.IFN-γ−/− mice began to relapse with high levels of virus in the spleen at 4 weeks postinfection, and by 18 weeks postinfection all of the B6.IFN-γ−/− mice had high levels of virus in the spleen. Although the time of relapse for individual mice was quite variable, ultimately all six IFN-γ-deficient mice developed FV-induced splenomegaly by 7 months postinfection, whereas none of seven B6 mice developed splenomegaly even 16 months after infection (data not shown). Thus, although the IFN-γ-deficient mice reduced virus infection faster than wild-type mice and in some cases had quite low levels of virus in the spleen even at 12 weeks postinfection, they were not able to maintain low virus levels and ultimately succumbed to FV-induced disease.
FIG. 4.
Infectious center assays for virus-producing cells from the spleens of infected B6 and B6.IFN-γ−/− mice. Each dot represents the results from a single mouse infected with 3,000 spleen focus-forming units of B-cell-tropic FV. The limit of detection for this assay was one F-MuLV infectious center per 3 × 107 spleen cells. The results obtained 2 weeks postinfection are combined data from two independent experiments. Because of the wide scatter of the data from the B6.IFN-γ−/− mice, the Mann-Whitney test was used for all statistical analyses. Significant differences were observed at 2 weeks postinfection (P < 0.0001) and at 16 to 18 weeks postinfection (P = 0.0022).
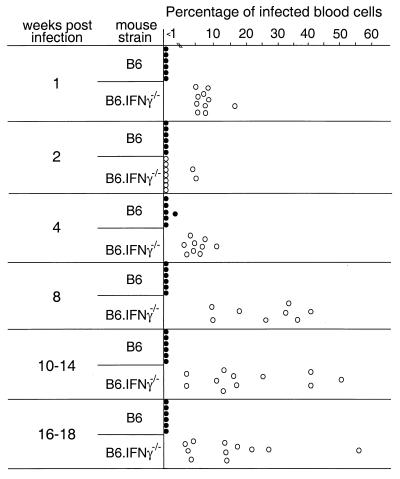
The effect of IFN-γ-deficiency on infection of blood cells was also examined. The IFN-γ-deficient mice had significantly higher percentages of viral-antigen-positive cells in the blood compared to B6 mice at 1 week postinfection (Fig. 5). As with the results from other tissues, blood cell infection also decreased in IFN-γ-deficient mice at the 2-week time point. However, by 4 weeks postinfection the percentage of antigen- positive cells increased in the IFN-γ-deficient mice and remained quite high through the 18-week time point. Thus, the data indicated that an immunological crisis occurred at between 4 and 8 weeks postinfection in the absence of IFN-γ, wherein control of virus replication and spread was lost.
FIG. 5.
Percentage of blood cells expressing cell surface viral antigen. One million live nucleated blood cells were stained for expression of glycosylated Gag protein and analyzed by flow cytometry. The IFN-γ-deficient mice had significantly higher percentages of infected blood cells compared to wild-type mice as determined by the Mann-Whitney test at all time points tested except at 2 weeks postinfection (all P < 0.0007).
Effects of IFN-γ deficiency on class-switching and maintaining virus-neutralizing antibody responses.
Although the IFN-γ-deficient mice had an accelerated total virus-neutralizing antibody response at 2 weeks postinfection (Fig. 3), by 4 weeks postinfection the virus-neutralizing antibody titers were lower compared to those of wild-type mice (Table 2). Furthermore, the IFN-γ-deficient mice failed to switch to IgG class antibodies and also failed to maintain long-term virus-neutralizing antibody titers. Thus, the IgM response in the IFN-γ-deficient mice was not durable, and the loss of the response coincided with increased FV infection levels. In contrast, wild-type mice class switched to IgG immunoglobulins and maintained detectable titers indefinitely.
TABLE 2.
Virus-neutralizing antibody titers after infection with FVa
| Mouse strain | n | Antibody titera at:
|
|||||
|---|---|---|---|---|---|---|---|
| 4 wks postinfectionb
|
18-64 wks postinfectionc
|
||||||
| Total | IgG | Total | IgG | ||||
| B6 | 16 | 1/90 | 1/30 | 1/31 | 1/16 | ||
| B6.IFN-γ−/− | 9 | 1/23 | <1/10 | <1/10 | <1/10 | ||
F-MuLV-neutralizing antibody titers were determined as previously described (10). All values are expressed as log2 geometric mean virus-neutralizing antibody titers. The Mann-Whitney test was used for all statistical analyses.
P = 0.0442 and 0.0026 for total antibody and IgG antibody, respectively.
P = 0.0026 and 0.0019 for total antibody and IgG antibody, respectively.
Effects of rIFN-γ treatment on FV-induced erythroleukemia.
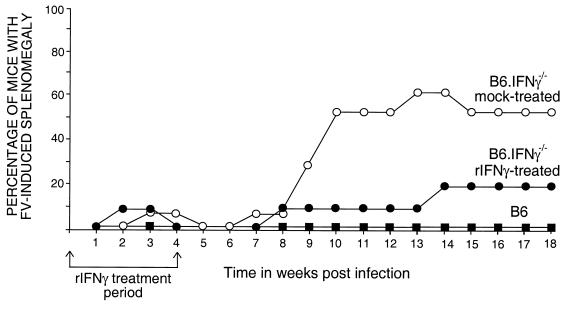
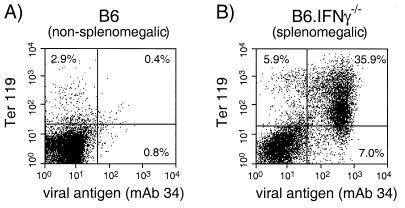
To distinguish between a requirement for IFN-γ at the time of FV infection from developmental defects that may have occurred from genetic inactivation of IFN-γ, B6.IFN-γ−/− mice were treated with rIFN-γ during the first month of FV infection. The mice were palpated weekly to monitor for FV-induced splenomegaly. Approximately 60% of the untreated B6.IFN-γ−/− control mice developed splenomegaly by 13 weeks postinfection (Fig. 6). The splenomegaly was due to FV-induced erythroleukemia, as indicated by the predominance of infected erythroid cells in the spleen (Fig. 7), and correlated with a recurrence of viremia (data not shown). Treatment with rIFN-γ delayed the onset of FV-induced splenomegaly by ca. 5 weeks and reduced the incidence of splenomegaly to 10% at 13 weeks postinfection (Fig. 6). In addition, rIFN-γ treatments significantly lowered blood infection levels in the treated mice up to 14 weeks after infection (data not shown). Thus, the rIFN-γ treatments partially restored the wild-type phenotype, indicating that the effects we observed in the IFN-γ-deficient mice during the course of FV infection were due to the role of IFN-γ during infection rather than on development.
FIG. 6.
Splenomegaly in FV-infected mice. Mice were infected with FV and monitored for induction and progression of splenomegaly as previously described (23). The arrows indicate the period of treatment by i.p. injection with either 105 U of rIFN-γ (•) or saline (○). The numbers of mice in each group were as follows: B6 mice (▪), n = 6; rIFN-γ-treated B6.IFN-γ−/− mice, n = 10; and saline-treated B6.IFN-γ−/− mice, n = 8. The numbers of splenomegalic mice in the treated versus untreated groups were significantly different between 10 and 14 weeks postinfection (all P of ≤0.0405 by Fisher's exact test). The log10 geometric means of the infectious centers per spleen between 16 to 18 weeks postinfection were as follows: B6 mice, 4.08; rIFN-γ-treated B6.IFN-γ−/− mice, 5.23; and saline-treated B6.IFN-γ−/− mice, 5.98.
FIG. 7.
Flow cytometric analysis of FV-infected spleen cells from a persistently infected B6 mouse and a splenomegalic B6.IFN-γ−/− mouse at 18 weeks postinfection. Live nucleated spleen cells were stained for cell surface expression of FV glycosylated Gag protein (MAb 34) and Ter 119, an erythroid precursor cell marker. (A) The spleen from a representative persistently infected B6 mouse (nonsplenomegalic) had normal percentages of erythroid progenitor cells with very little infection. Lymphocyte and monocyte subset levels were also normal (data not shown). (B) The spleen from a representative splenomegalic B6.IFN-γ−/− mouse contained a predominance of infected erythroid progenitor cells. Ten different splenomegalic IFN-γ-deficient mice were analyzed and had levels of FV infection in erythroid precursor cells (Ter 119+) ranging from 30 to 80% of the spleen cells (data not shown).
DISCUSSION
An antiviral immune response is a complex orchestration of multiple components of both the innate and the adaptive immune systems. The innate immune response is a rapid, nonspecific response to infection that provides an early line of defense and influences the slower-developing, but highly specific, adaptive response. IFN-γ is a key component of both systems and an important link between the two (14, 19, 42). As part of the innate response, natural killer (NK) cells play an important role in resistance against FV infection (29) and produce IFN-γ (1, 2, 31, 43), which has direct inhibitory effects on FV replication (30). The lack of this direct inhibition was a likely cause of the significantly increased levels of FV infection in the B6.IFN-γ−/− mice at 1 week postinfection (Table 1).
The paradoxical finding that the IFN-γ-deficient mice controlled infection better than wild-type mice at 2 weeks postinfection was unexpected but quite intriguing. This rapid decrease in virus infection in the IFN-γ-deficient mice suggested that IFN-γ had detrimental effects on antiviral immunity between 1 and 2 weeks postinfection. Both the genetically IFN-γ-deficient and the IFN-γ-depleted mice produced quicker virus-neutralizing antibody responses than did the wild-type mice (Fig. 3). One explanation for these findings is that the increased levels of virus in the IFN-γ-deficient mice during the first week of infection better stimulated the virus-neutralizing antibody response, which was then able to bring viral infection under control. An alternative explanation is that the lack of IFN-γ during the innate response could have polarized the T helper responses toward a Th2 response characterized by antibodies of the IgG1 subclass. However, at 2 weeks postinfection, the antibody responses were still predominated by IgM class immunoglobulins (data not shown). While our results on IFN-γ effects on antibody responses to a viral infection appear to be novel, a related finding is that an IFN-γ gene coadministered with a plasmid expressing retroviral antigens suppressed the primary antiviral antibody responses to the retroviral antigens (4). In contrast, another study showed no acceleration of the specific antibody response during influenza A virus infection in IFN-γ-deficient mice (46). Thus, the effects of IFN-γ on antibody responses may vary with the type of infection.
A compensatory mechanism in the IFN-γ-deficient mice may also have acted to reduce virus levels at 2 weeks postinfection. Previous studies have shown that IFN-γ gene-disrupted mice compensated for the lack of IFN-γ by production of tumor necrosis factor alpha (TNF-α) during Histoplasma capsulatum (53) and Leishmania donovani infections (50). We looked for such compensation by RNase protection assays comparing mRNA expression levels in the spleen cells of wild-type and IFN-γ-deficient mice at 2 weeks postinfection. However, no major differences in RNA expression of TNF-α or several other immunoregulatory genes such as interleukin-4 (IL-4), IL-6, TNF-β, transforming growth factor β, or macrophage migration inhibition factor were detected (data not shown).
Paradoxical effects from IFN-γ have also been reported in studies on infection with the LP-BM5 murine leukemia virus that induces murine AIDS (MAIDS) (16, 17, 52). However, the mechanisms of IFN-γ action during the course of LP-BM5 and FV infections are most likely different since IFN-γ appears to be involved in the pathogenesis of MAIDS by its influences on lymphoproliferation and does not appear to influence serum immunoglobulin levels (17). In contrast, IFN-γ is required for preventing splenomegaly during FV infection, and the detrimental effects of IFN-γ may be due to delaying the virus-neutralizing antibody response.
Previous studies have indicated that IgG2a isotype antibodies may be the most important of the IgG antibodies in neutralization of FV in vivo (3). Consistent with the reported role of IFN-γ in promoting class-switching to the IgG2a isotype (48), the IFN-γ-deficient mice failed to generate any detectable IgG virus-neutralizing antibodies during FV infection (Table 2). Furthermore, the IFN-γ-deficient mice failed to maintain long-term virus-neutralizing antibody titers (Table 2). Thus, although IFN-γ appeared to delay the development of the IgM virus-neutralizing antibody response, it was important for both class-switching to IgG2a virus-neutralizing antibodies, and for maintaining long-term IgM virus-neutralizing antibody titers. The lack of early virus-neutralizing antibodies in the wild-type mice correlated with higher virus infection, and the loss of virus-neutralizing antibodies in the IFN-γ-deficient mice correlated with the onset of FV-induced erythroleukemia. Thus, part of the protective effects of IFN-γ during persistent FV infection in vivo may be accomplished through influences on antibody production. Another influence might be through broader activation of CD8+ T cells, but further studies will be required to determine the function of the activated CD8+ T cells in the IFN-γ-deficient mice at 2 weeks postinfection. We did not find that the IFN-γ-deficient mice had better cytolytic activity against infected target cells than the wild-type mice at 2 weeks postinfection (data not shown).
An interesting discordance was observed between levels of infected spleen cells and blood cells during the acute phase of FV infection. Although wild-type mice had relatively high levels of spleen infection at 1 week postinfection, there was no detectable blood cell infection (Table 1). In contrast, the B6.IFN-γ−/− mice had infection in both tissues. Thus, the innate IFN-γ response appeared to be more effective at limiting infection in blood cells than in spleen cells. A previous study showed that in B6 mice, splenic NK cells exert their antiviral effects through a perforin-dependent and not an IFN-γ-dependent mechanism in response to murine cytomegalovirus (MCMV) infection, whereas IFN-γ secretion by NK cells is the predominant mechanism for MCMV control in the liver (49). Thus, one explanation for the tissue-specific effect of IFN-γ may be linked to tissue-dependent effector mechanisms of NK cells during the innate response.
Throughout this study, a high degree of variability in virus infection levels in the B6.IFN-γ−/− mice was observed. The variability in spleen virus levels in the B6.IFN-γ−/− mice compared to B6 mice was obvious at all time points (Fig. 4), and the onset of FV-induced erythroleukemia in the IFN-γ-deficient mice also ranged widely. This variability could have been due to individual differences in the ability of the mice to compensate for the lack of IFN-γ. Another possibility is that IFN-γ indirectly affected FV infection by influences on the generation of erythroid progenitors, the primary targets for FV replication (36-38). However, we did not observe any significant differences in the percentages of erythroid burst-forming units when we analyzed multiple tissues from wild-type and IFN-γ-deficient mice during acute and long-term infections (data not shown). Finally, the observed variability in the IFN-γ-deficient mice could have been due to genetic heterogeneity. The IFN-γ-deficient mice used in these studies were generation N8 backcrosses to strain C57BL/6, which theoretically results in 99.6% genetic homogeneity. Thus, there remains a remote possibility that a resistance gene from the strain 129 embryonic cells used to generate the targeted mutation is segregating in the B6.IFN-γ−/− mouse strain.
Two resistance genes, Fv2 (27, 34, 44) and Fhe (47), usually prevent FV-induced erythroleukemia in B6 strain mice. Fv2 resistance can be overcome by deletions in the viral envelope gene (26), but the viruses we isolated from erythroleukemic B6.IFN-γ−/− mice showed the same mouse strain tropism as did the original virus stock (data not shown). We conclude that the fatal FV-induced erythroleukemia in the knockout mice was due to a failure in the immunological control of virus replication and spread, similar to what has been shown in lymphocyte-deficient mice (22). While these studies clearly demonstrate a critical role for IFN-γ in resistance to FV infection, there may also be detrimental effects, especially during the acute phase of infection. A major implication of these results is that the ability to modulate the IFN-γ response in a temporal manner may lead to better control of retroviral infections.
REFERENCES
- 1.Biron, C. A. 1997. Activation and function of natural killer cell responses during viral infections. Curr. Opin. Immunol. 9:24-34. [DOI] [PubMed] [Google Scholar]
- 2.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 3.Britt, W. J., and B. Chesebro. 1983. Use of monoclonal anti-gp70 antibodies to mimic the effects of the Rfv-3 gene in mice with Friend virus-induced leukemia. J. Immunol. 130:2363-2367. [PubMed] [Google Scholar]
- 4.Cheevers, W. P., J. C. Beyer, and I. Hotzel. 2001. Plasmid DNA encoding caprine interferon gamma inhibits antibody response to caprine arthritis-encephalitis virus (CAEV) surface protein encoded by a co-administered plasmid expressing CAEV env and tat genes. Vaccine 19:3209-3215. [DOI] [PubMed] [Google Scholar]
- 5.Chen, W., H. Qin, B. Chesebro, and M. A. Cheever. 1996. Identification of a gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by Friend, Moloney, and Rauscher murine leukemia virus-induced tumors. J. Virol. 70:7773-7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherwinski, H. M., J. H. Schumacher, K. D. Brown, and T. R. Mosmann. 1987. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J. Exp. Med. 166:1229-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesebro, B., K. Wehrly, M. Cloyd, W. Britt, J. Portis, J. Collins, and J. Nishio. 1981. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: Friend-specific and FMR-specific antigens. Virology 112:131-144. [DOI] [PubMed] [Google Scholar]
- 8.Chesebro, B., K. Wehrly, and J. Stimpfling. 1974. Host genetic control of recovery from Friend leukemia virus-induced splenomegaly. Mapping of a gene within the major histocompatibility complex. J. Exp. Med. 140:1457-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 10.Desmedt, M., P. Rottiers, H. Dooms, W. Fiers, and J. Grooten. 1998. Macrophages induce cellular immunity by activating Th1 cell responses and suppressing Th2 cell responses. J. Immunol. 160:5300-5308. [PubMed] [Google Scholar]
- 11.Dhawan, S., A. Heredia, R. B. Lal, L. M. Wahl, J. S. Epstein, and I. K. Hewlett. 1994. Interferon-gamma induces resistance in primary monocytes against human immunodeficiency virus type-1 infection. Biochem. Biophys. Res. Commun. 201:756-761. [DOI] [PubMed] [Google Scholar]
- 12.Dittmer, U., D. M. Brooks, and K. J. Hasenkrug. 1998. Characterization of a live-attenuated retroviral vaccine demonstrates protection via immune mechanisms. J. Virol. 72:6554-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dittmer, U., K. E. Peterson, R. Messer, I. M. Stromnes, B. Race, and K. J. Hasenkrug. 2001. Role of interleukin-4 (IL-4), IL-12, and gamma interferon in primary and vaccine-primed immune responses to Friend retrovirus infection. J. Virol. 75:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fearon, D. T., and R. M. Locksley. 1996. The instructive role of innate immunity in the acquired immune response. Science 272:50-53. [DOI] [PubMed] [Google Scholar]
- 15.Ferro, F. E., Jr., S. L. Kozak, M. E. Hoatlin, and D. Kabat. 1993. Cell surface site for mitogenic interaction of erythropoietin receptors with the membrane glycoprotein encoded by Friend erythroleukemia virus. J. Biol. Chem. 268:5741-5747. [PubMed] [Google Scholar]
- 16.Gazzinelli, R. T., N. A. Giese, and H. C. Morse III. 1994. In vivo treatment with interleukin 12 protects mice from immune abnormalities observed during murine acquired immunodeficiency syndrome (MAIDS). J. Exp. Med. 180:2199-2208. [DOI] [PMC free article] [PubMed]
- 17.Giese, N. A., R. T. Gazzinelli, J. K. Actor, R. A. Morawetz, M. Sarzotti, and H. C. Morse III. 1996. Retrovirus-elicited interleukin-12 and tumour necrosis factor-alpha as inducers of interferon-gamma-mediated pathology in mouse AIDS. Immunology 87:467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidotti, L. G., and F. V. Chisari. 1999. Cytokine-induced viral purging: role in viral pathogenesis. Curr. Opin. Microbiol. 2:388-391. [DOI] [PubMed] [Google Scholar]
- 19.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 20.Guidotti, L. G., T. Ishikawa, M. V. Hobbs, B. Matzke, R. Schreiber, and F. V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4:25-36. [DOI] [PubMed] [Google Scholar]
- 21.Guidotti, L. G., R. Rochford, J. Chung, M. Shapiro, R. Purcell, and F. V. Chisari. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284:825-829. [DOI] [PubMed] [Google Scholar]
- 22.Hasenkrug, K. J. 1999. Lymphocyte deficiencies increase susceptibility to friend virus-induced erythroleukemia in Fv-2 genetically resistant mice. J. Virol. 73:6468-6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasenkrug, K. J., D. M. Brooks, M. N. Robertson, R. V. Srinivas, and B. Chesebro. 1998. Immunoprotective determinants in Friend murine leukemia virus envelope protein. Virology 248:66-73. [DOI] [PubMed] [Google Scholar]
- 24.Hasenkrug, K. J., and U. Dittmer. 2000. The role of CD4 and CD8 T cells in recovery and protection from retroviral infection: lessons from the Friend virus model. Virology 272:244-249. [DOI] [PubMed] [Google Scholar]
- 25.Heid, C. A., J. Stevens, K. J. Livak, and P. M. Williams. 1996. Real-time quantitative PCR. Genome Res. 6:986-994. [DOI] [PubMed] [Google Scholar]
- 26.Hoatlin, M. E., F. E. Ferro, Jr., R. W. Geib, M. T. Fox, S. L. Kozak, and D. Kabat. 1995. Deletions in one domain of the Friend virus-encoded membrane glycoprotein overcome host range restrictions for erythroleukemia. J. Virol. 69:856-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoatlin, M. E., and D. Kabat. 1995. Host-range control of a retroviral disease: Friend erythroleukemia. Trends Microbiol. 3:51-57. [DOI] [PubMed] [Google Scholar]
- 28.Hoatlin, M. E., S. L. Kozak, F. Lilly, A. Chakraborti, C. A. Kozak, and D. Kabat. 1990. Activation of erythropoietin receptors by Friend viral gp55 and by erythropoietin and downmodulation by the murine Fv-2r resistance gene. Proc. Natl. Acad. Sci. USA 87:9985-9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwanami, N., A. Niwa, Y. Yasutomi, N. Tabata, and M. Miyazawa. 2001. Role of natural killer cells in resistance against friend retrovirus-induced leukemia. J. Virol. 75:3152-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwashiro, M., K. Peterson, R. J. Messer, I. M. Stromnes, and K. J. Hasenkrug. 2001. CD4+ T cells and gamma interferon in the long-term control of persistent friend retrovirus infection. J. Virol. 75:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kakimi, K., L. G. Guidotti, Y. Koezuka, and F. V. Chisari. 2000. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 192:921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lander, M. R., and S. K. Chattopadhyay. 1984. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ecotropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J. Virol. 52:695-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, J.-P., A. D. D'Andrea, H. F. Lodish, and D. Baltimore. 1990. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature 343:762-764. [DOI] [PubMed] [Google Scholar]
- 34.Lilly, F. 1970. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J. Natl. Cancer Inst. 45:163-169. [PubMed] [Google Scholar]
- 35.Lilly, F., and R. A. Steeves. 1973. B-tropic Friend virus: a host-range pseudotype of spleen focus-forming virus (SFFV). Virology 55:363-370. [DOI] [PubMed] [Google Scholar]
- 36.Mamus, S. W., S. Beck-Schroeder, and E. D. Zanjani. 1985. Suppression of normal human erythropoiesis by gamma interferon in vitro. Role of monocytes and T lymphocytes. J. Clin. Investig. 75:1496-1503. [DOI] [PMC free article] [PubMed]
- 37.Means, R. T., Jr., and S. B. Krantz. 1991. Inhibition of human erythroid colony-forming units by gamma interferon can be corrected by recombinant human erythropoietin. Blood 78:2564-2567. [PubMed] [Google Scholar]
- 38.Means, R. T., Jr., S. B. Krantz, J. Luna, S. A. Marsters, and A. Ashkenazi. 1994. Inhibition of murine erythroid colony formation in vitro by interferon gamma and correction by interferon receptor immunoadhesin. Blood 83:911-915. [PubMed] [Google Scholar]
- 39.Moreau-Gachelin, F., A. Tavitian, and P. Tambourin. 1988. Spi-1 is a putative oncogene in virally induced murine erythroleukemia. Nature 331:277-280. [DOI] [PubMed] [Google Scholar]
- 40.Mosmann, T. R., and R. L. Coffman. 1989. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 41.Munroe, D. G., J. W. Peacock, and S. Benchimol. 1990. Inactivation of the cellular p53 gene is a common feature of Friend virus-induced erythroleukemia: relationship of inactivation to dominant transforming alleles. Mol. Cell. Biol. 10:3307-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orange, J. S., and C. A. Biron. 1996. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J. Immunol. 156:1138-1142. [PubMed] [Google Scholar]
- 43.Orange, J. S., B. Wang, C. Terhorst, and C. A. Biron. 1995. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 182:1045-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Persons, D. A., R. F. Paulson, M. R. Loyd, M. T. Herley, S. M. Bodner, A. Bernstein, P. H. Correll, and P. A. Ney. 1999. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat. Genet. 23:159-165. [DOI] [PubMed] [Google Scholar]
- 45.Peterson, K. E., M. Iwashiro, K. J. Hasenkrug, and B. Chesebro. 2000. Major histocompatibility complex class I gene controls the generation of gamma interferon-producing CD4+ and CD8+ T cells important for recovery from friend retrovirus-induced leukemia. J. Virol. 74:5363-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price, G. E., A. Gaszewska-Mastarlarz, and D. Moskophidis. 2000. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J. Virol. 74:3996-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silver, J. E., and T. N. Fredrickson. 1983. A new gene that controls the type of leukemia induced by Friend murine leukemia virus. J. Exp. Med. 158:493-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snapper, C. M., and W. E. Paul. 1987. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236:944-947. [DOI] [PubMed] [Google Scholar]
- 49.Tay, C. H., and R. M. Welsh. 1997. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J. Virol. 71:267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor, A. P., and H. W. Murray. 1997. Intracellular antimicrobial activity in the absence of interferon-gamma: effect of interleukin-12 in experimental visceral leishmaniasis in interferon-gamma gene-disrupted mice. J. Exp. Med. 185:1231-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tishon, A., H. Lewicki, G. Rall, M. Von Herrath, and M. B. Oldstone. 1995. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology 212:244-250. [DOI] [PubMed] [Google Scholar]
- 52.Uehara, S., Y. Hitoshi, F. Numata, M. Makino, M. Howard, T. Mizuochi, and K. Takatsu. 1994. An IFN-gamma-dependent pathway plays a critical role in the pathogenesis of murine immunodeficiency syndrome induced by LP-BM5 murine leukemia virus. Int. Immunol. 6:1937-1947. [DOI] [PubMed] [Google Scholar]
- 53.Zhou, P., G. Miller, and R. A. Seder. 1998. Factors involved in regulating primary and secondary immunity to infection with Histoplasma capsulatum: TNF-alpha plays a critical role in maintaining secondary immunity in the absence of IFN-gamma. J. Immunol. 160:1359-1368. [PubMed] [Google Scholar]