Abstract
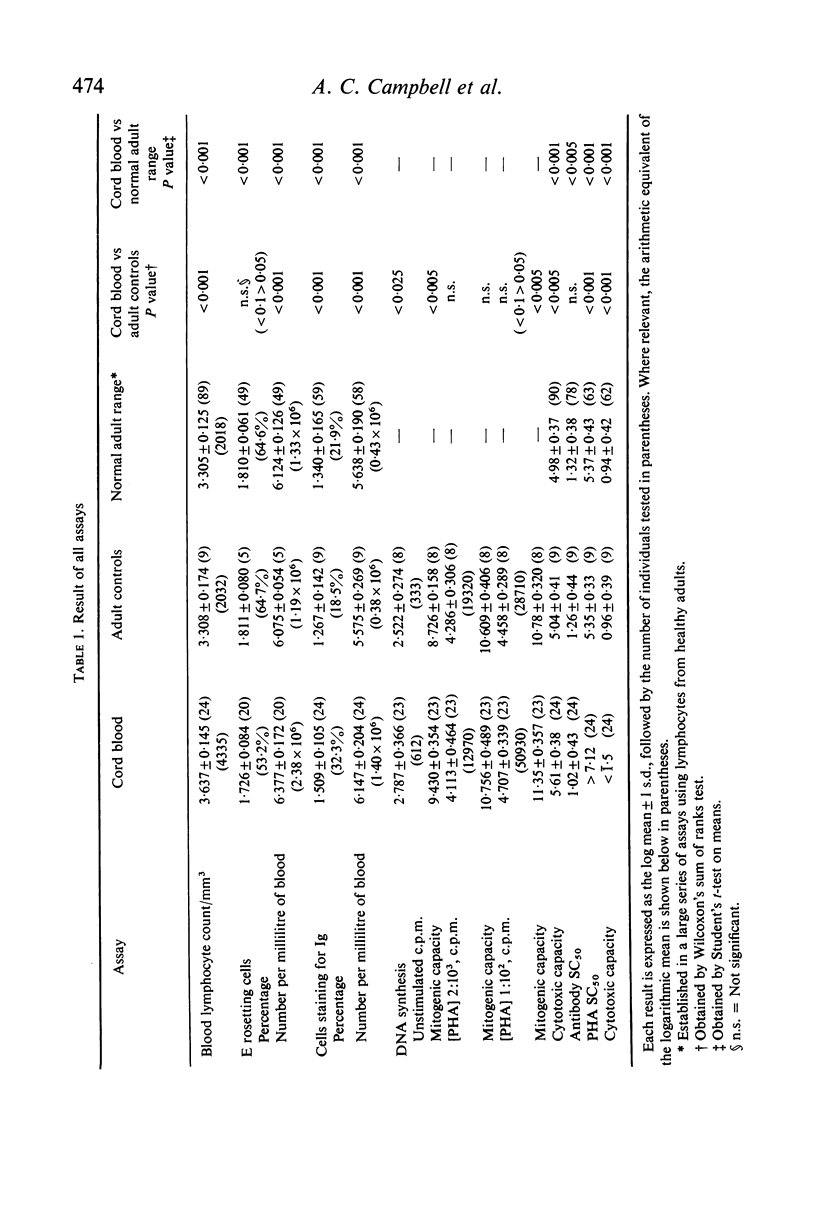
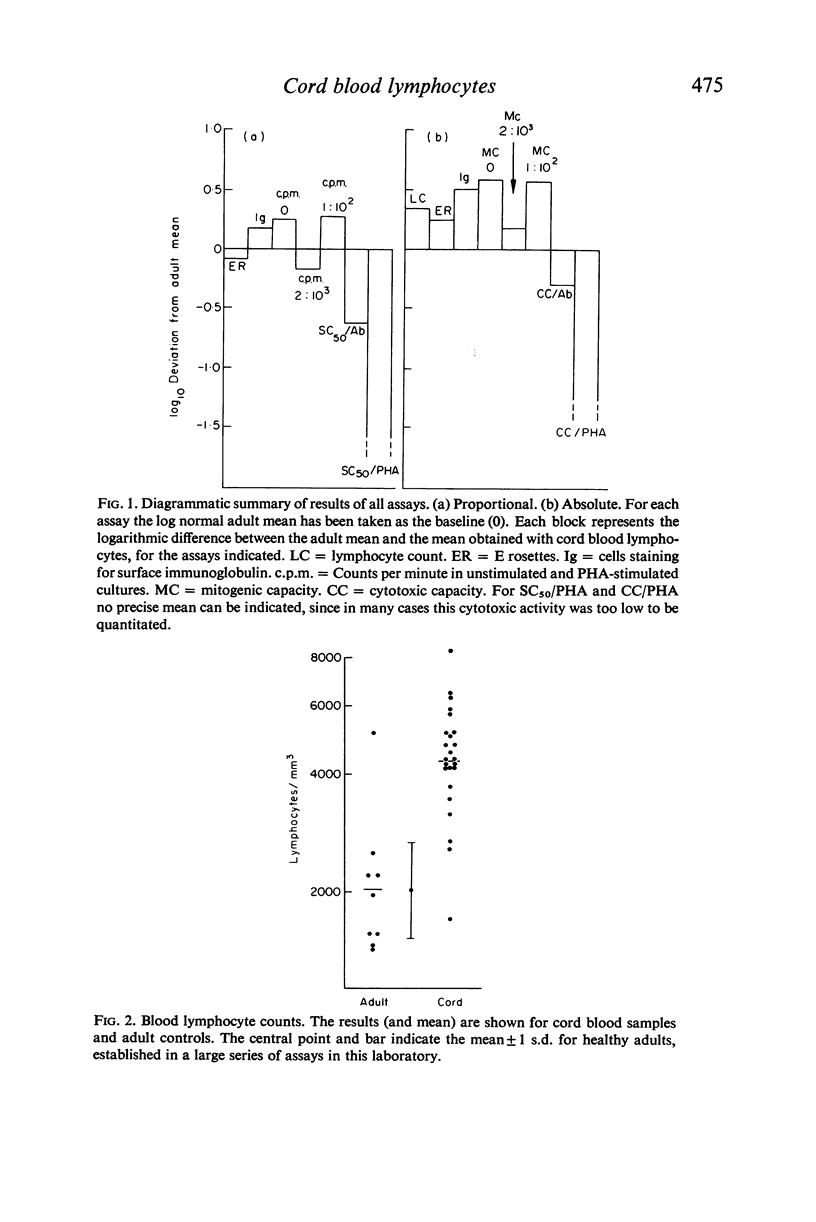
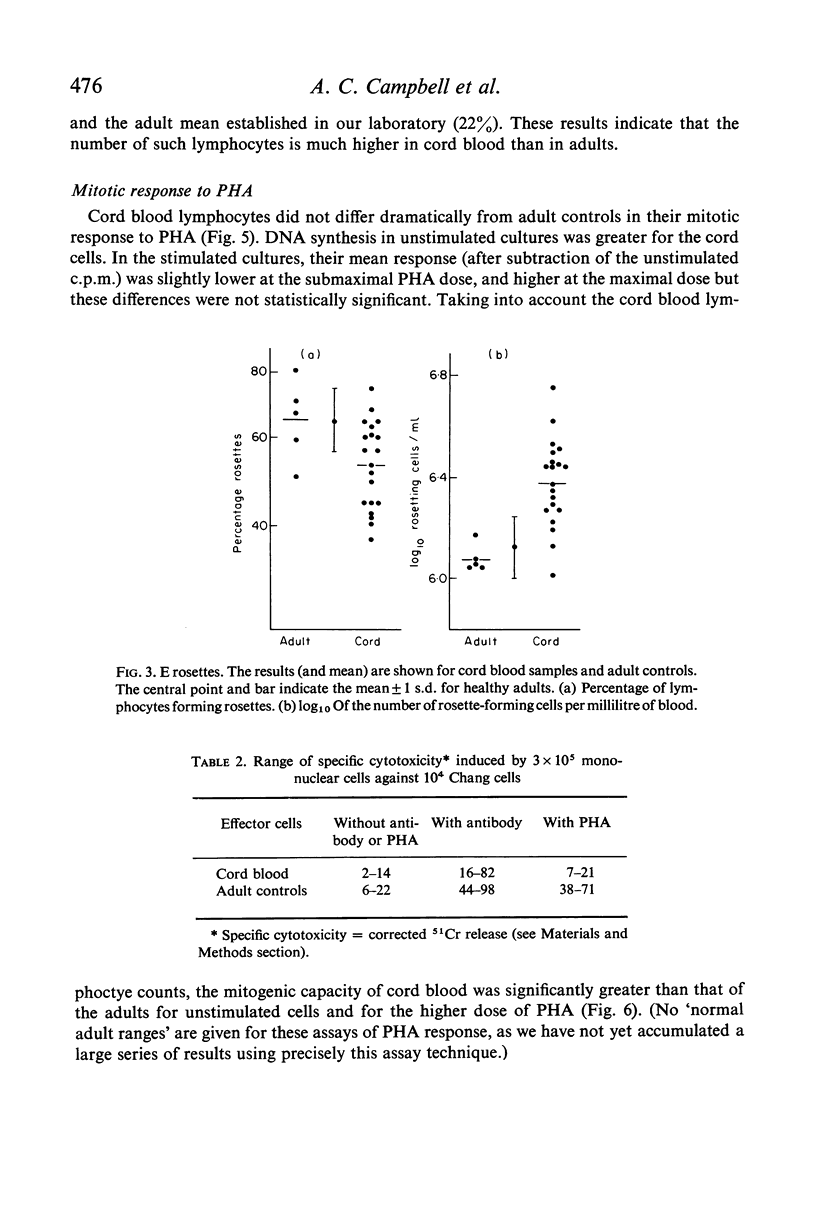
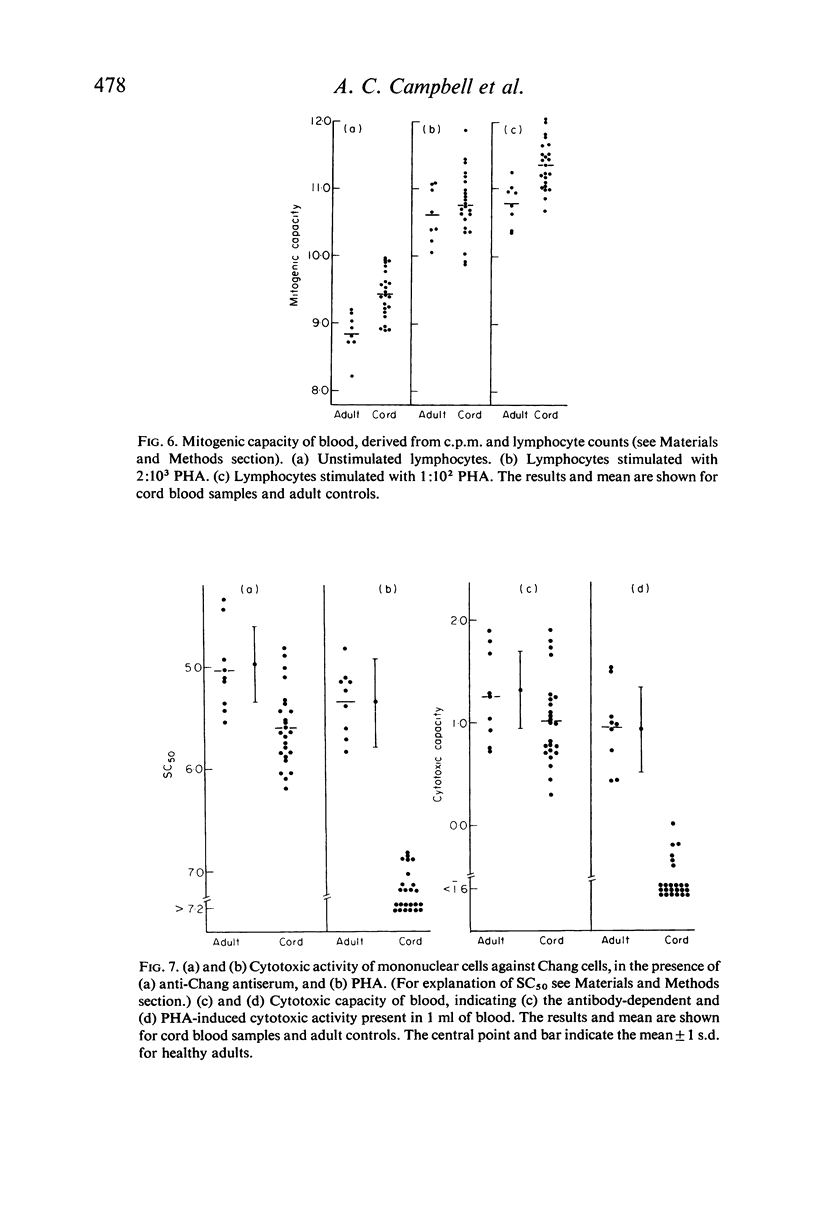
Assays of lymphocyte subpopulations and function have been applied to cells from the cord blood of twenty-four infants. The results are compared with those obtained in healthy adults. T cells, assayed by spontaneous rosette formation with sheep red blood cells (E rosettes) were present in lower proportion in cord (53%) than in adult blood (65%). There was a higher proportion of lymphocytes bearing stainable immunoglobulin in cord (32%) than in adult blood (22%). From the blood lymphocyte counts it was calculated that both T and B lymphocytes are present in greater numbers in the newborn infants' blood than in adults. Comparison of DNA synthesis showed that cord blood leucocytes had a higher spontaneous rate, but there were only minor differences in the lymphocyte mitotic response to phytohaemagglutinin (PHA). The response of cord blood lymphocytes was slightly lower to a submaximal stimulus and higher to a maximal stimulus. There was a correlation between the submaximal response and the proportion of E rosetting cells.
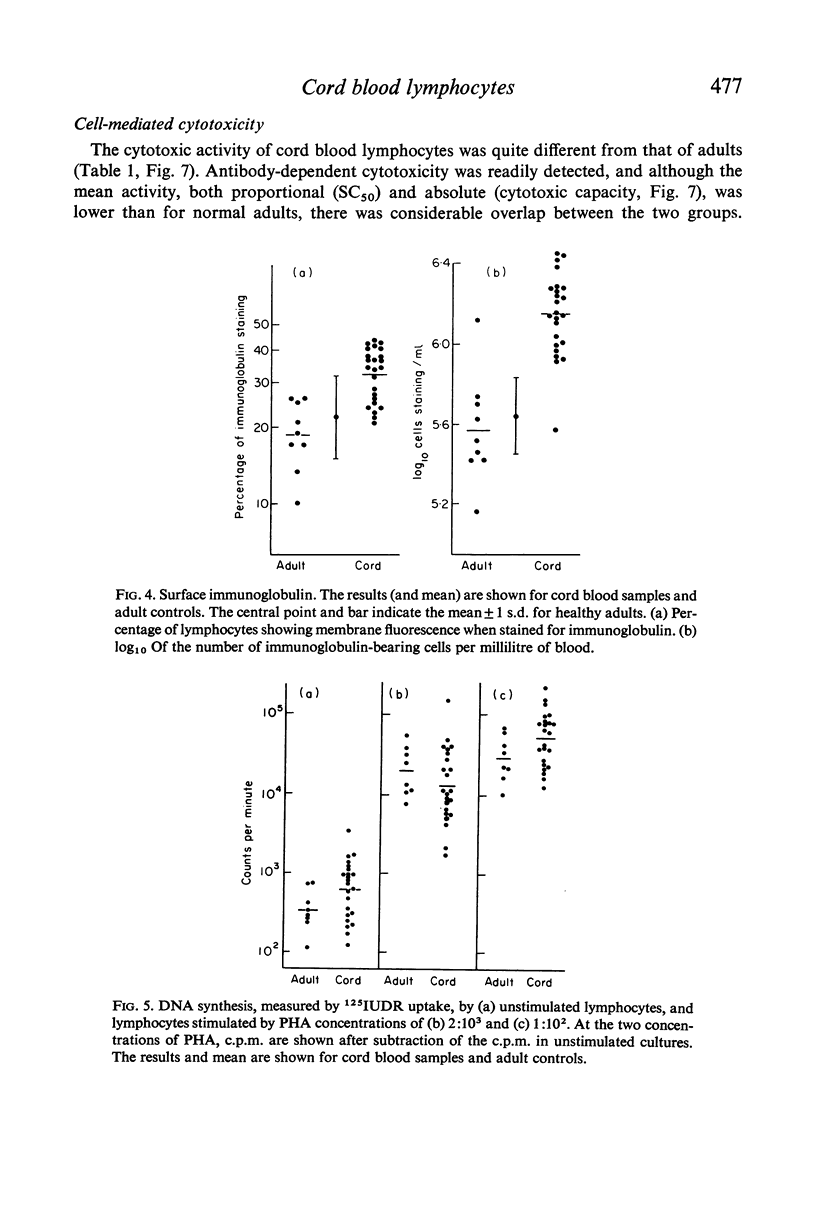
The most striking differences between infant and adult blood lymphocytes were in their cytotoxic activity against homologous target cells (Chang cells). Antibody-dependent cytotoxicity (K-cell activity) was readily detected using cord blood leucocytes, though it was lower than that of adult cells. PHA-induced cytotoxicity was very low in all cord blood samples, and in many cases was almost unmeasurable. This dissociation between the two types of cytotoxic activity is consistent with other evidence that they may be mediated by different cell types.
The assays were also applied to blood samples taken from five mothers of tested infants immediately after delivery. While some differences from normal adults were found with the mothers' lymphocytes they did not mirror those of the cord blood samples. This suggests that the pattern found for cord blood lymphocytes is not due to maternal factors crossing the placenta.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allansmith M., McClellan B. H., Butterworth M., Maloney J. R. The development of immunoglobulin levels in man. J Pediatr. 1968 Feb;72(2):276–290. doi: 10.1016/s0022-3476(68)80324-5. [DOI] [PubMed] [Google Scholar]
- Ayoub J., Kasakura S. In vitro response of foetal lymphocytes to PHA, and a factor plasma which suppresses the PHA response of adult lymphocytes. Clin Exp Immunol. 1971 Mar;8(3):427–434. [PMC free article] [PubMed] [Google Scholar]
- Britton S., Perlmann H., Perlmann P. Thymus-dependent and thymus-independent effector functions of mouse lymphoid cells. Comparison of cytotoxicity and primary antibody formation in vitro. Cell Immunol. 1973 Sep;8(3):420–434. doi: 10.1016/0008-8749(73)90133-0. [DOI] [PubMed] [Google Scholar]
- COULSON A. S., CHALMERS D. G. SEPARATION OF VIABLE LYMPHOCYTES FROM HUMAN BLOOD. Lancet. 1964 Feb 29;1(7331):468–469. doi: 10.1016/s0140-6736(64)90799-8. [DOI] [PubMed] [Google Scholar]
- Campbell A. C., Hersey P., MacLennan I. C., Kay H. E., Pike M. C. Immunosuppressive consequences of radiotherapy and chemotherapy in patients with acute lymphoblastic leukaemia. Br Med J. 1973 May 19;2(5863):385–388. doi: 10.1136/bmj.2.5863.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. C., Skinner J. M., Hersey P., Roberts-Thomson P., MacLennan I. C., Truelove S. C. Immunosuppression in the treatment of inflammatory bowel disease. I. Changes in lymphoid sub-populations in the blood and rectal mucosa following cessation of treatment with azathioprine. Clin Exp Immunol. 1974 Apr;16(4):521–533. [PMC free article] [PubMed] [Google Scholar]
- Carr M. C., Lieber E., Fudenberg H. H. In vitro cytolysis by human fetal lymphocytes. Cell Immunol. 1970 Oct;1(4):455–458. doi: 10.1016/0008-8749(70)90021-3. [DOI] [PubMed] [Google Scholar]
- Carr M. C., Stites D. P., Fudenberg H. H. Cellular immune aspects of the human fetal-maternal relationship. I. In vitro response of cord blood lymphocytes to phytohemagglutinin. Cell Immunol. 1972 Sep;5(1):21–29. doi: 10.1016/0008-8749(72)90080-9. [DOI] [PubMed] [Google Scholar]
- Craig A. W., Garrett J. V., Jackson S. M. Quantitation of lymphocyte transformation using radioactive iododeoxyuridine. J Clin Pathol. 1969 Sep;22(5):558–559. doi: 10.1136/jcp.22.5.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. G. The establishment of a normal human population dose-response curve for lymphocytes cultured with PHA (phytohaemagglutinin). Clin Exp Immunol. 1971 Mar;8(3):421–425. [PMC free article] [PubMed] [Google Scholar]
- Fröland S. S., Natvig J. B. Lymphocytes with membrane-bound immunoglobulin (B-lymphocytes) in new-born babies. Clin Exp Immunol. 1972 Aug;11(4):495–505. [PMC free article] [PubMed] [Google Scholar]
- GOOD R. A., PAPERMASTER B. W. ONTOGENY AND PHYLOGENY OF ADAPTIVE IMMUNITY. Adv Immunol. 1964;27:1–115. doi: 10.1016/s0065-2776(08)60706-3. [DOI] [PubMed] [Google Scholar]
- Greenberg A. H., Hudson L., Shen L., Roitt I. M. Antibody-dependent cell-mediated cytotoxicity due to a "null" lymphoid cell. Nat New Biol. 1973 Mar 28;242(117):111–113. doi: 10.1038/newbio242111a0. [DOI] [PubMed] [Google Scholar]
- Hill C. A., Finn R., Denye V. Depression of cellular immunity in pregnancy due to a serum factor. Br Med J. 1973 Sep 8;3(5879):513–514. doi: 10.1136/bmj.3.5879.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm G., Franksson C., Campbell A. C., Maclennan I. C. The cytotoxic activity of lymphocytes from human lymph in vitro. Clin Exp Immunol. 1974 Jul;17(3):361–369. [PMC free article] [PubMed] [Google Scholar]
- Hosking C. S., Fitzgerald M. G., Simons M. J. Quantified deficiency of lymphocyte response to phytohaemagglutinin in immune deficiency diseases. Clin Exp Immunol. 1971 Oct;9(4):467–476. [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. R. In vitro transformation of fetal lymphocytes. Am J Obstet Gynecol. 1969 Jun 15;104(4):586–592. doi: 10.1016/s0002-9378(16)34251-x. [DOI] [PubMed] [Google Scholar]
- Leikin S., Mochir-Fatemi F., Park K. Blast transformation of lymphocytes from newborn human infants. J Pediatr. 1968 Apr;72(4):510–517. doi: 10.1016/s0022-3476(68)80342-7. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C. Antibody in the induction and inhibition of lymphocyte cytotoxicity. Transplant Rev. 1972;13:67–90. doi: 10.1111/j.1600-065x.1972.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Papamichail M., Brown J. C., Holborow E. J. Immunoglobulins on the surface of human lymphocytes. Lancet. 1971 Oct 16;2(7729):850–852. doi: 10.1016/s0140-6736(71)90224-8. [DOI] [PubMed] [Google Scholar]
- Pentycross C. R. Lymphocyte transformation in young people. Clin Exp Immunol. 1969 Jul;5(1):213–216. [PMC free article] [PubMed] [Google Scholar]
- Perlmann P., Perlmann H., Wigzell H. Lymphocyte mediated cytotoxicity in vitro. Induction and inhibition by humoral antibody and nature of effector cells. Transplant Rev. 1972;13:91–114. doi: 10.1111/j.1600-065x.1972.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Phillips B., Roitt I. M. Evidence for transformation of human B lymphocytes by PHA. Nat New Biol. 1973 Feb 21;241(112):254–256. doi: 10.1038/newbio241254a0. [DOI] [PubMed] [Google Scholar]
- Phillips B., Weisrose E. The mitogenic response of human B lymphocytes to phytohaemagglutinin. Clin Exp Immunol. 1974 Mar;16(3):383–392. [PMC free article] [PubMed] [Google Scholar]
- Stiehm E. R., Fudenberg H. H. Serum levels of immune globulins in health and disease: a survey. Pediatrics. 1966 May;37(5):715–727. [PubMed] [Google Scholar]
- Walker J. S., Freeman C. B., Harris R. Lymphocyte reactivity in pregnancy. Br Med J. 1972 Aug 19;3(5824):469–469. doi: 10.1136/bmj.3.5824.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Schuit H. R., Hijmans W. The immunological development of the human fetus. J Exp Med. 1965 Dec 1;122(6):1173–1188. doi: 10.1084/jem.122.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]


