Abstract
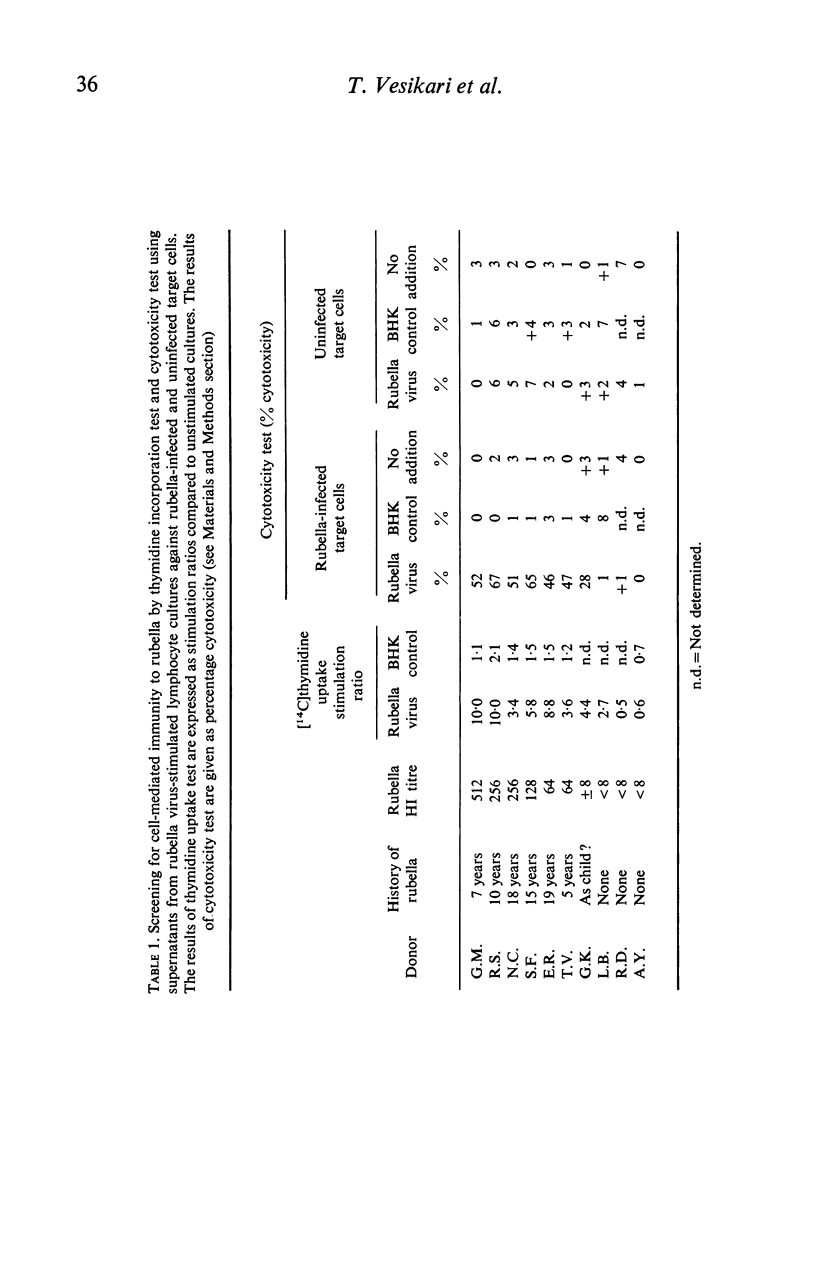
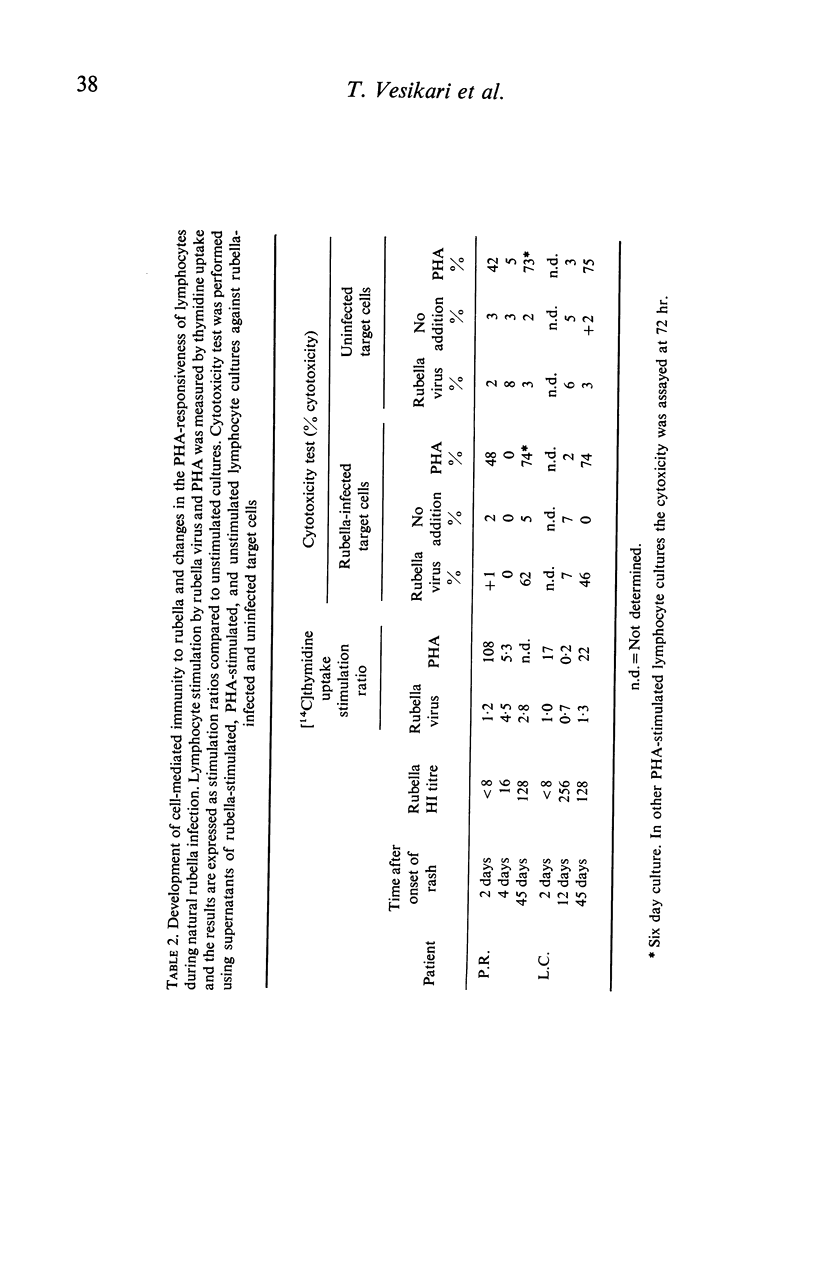
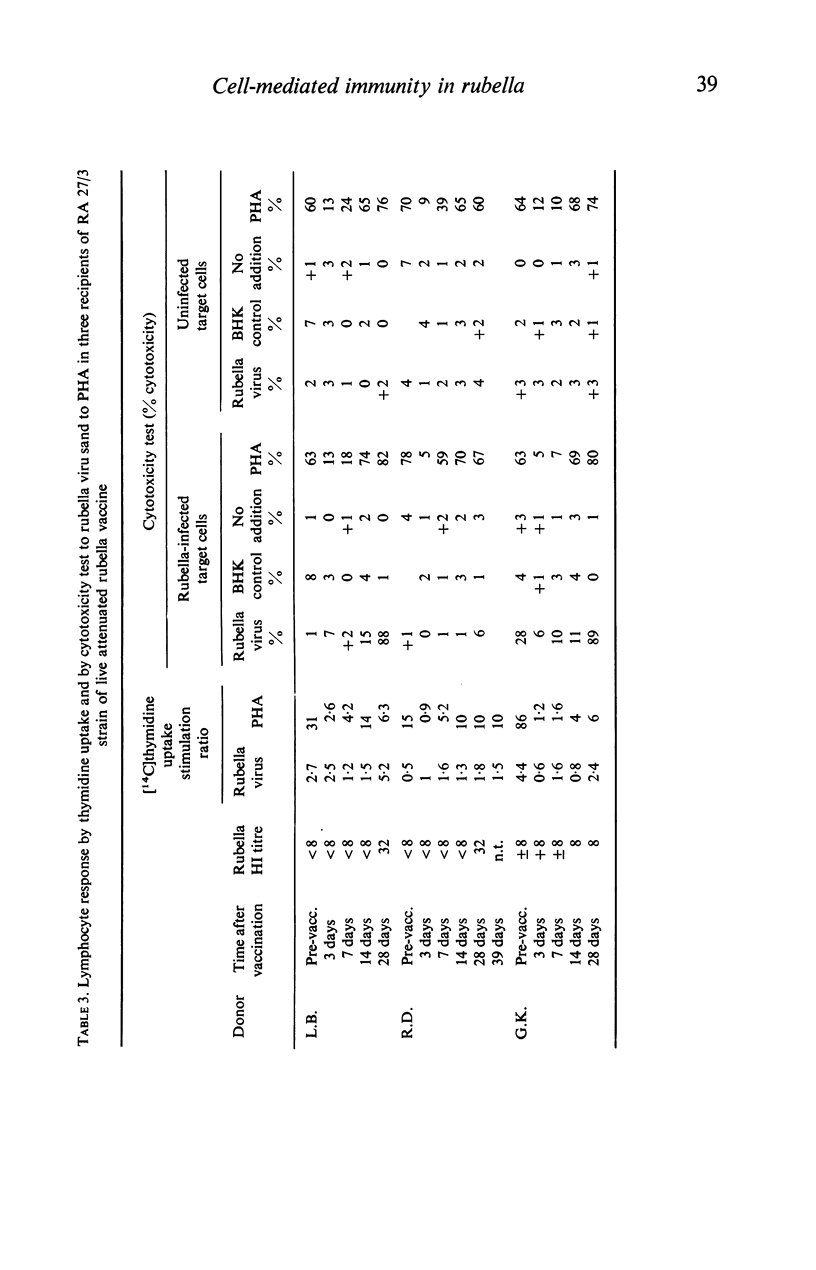
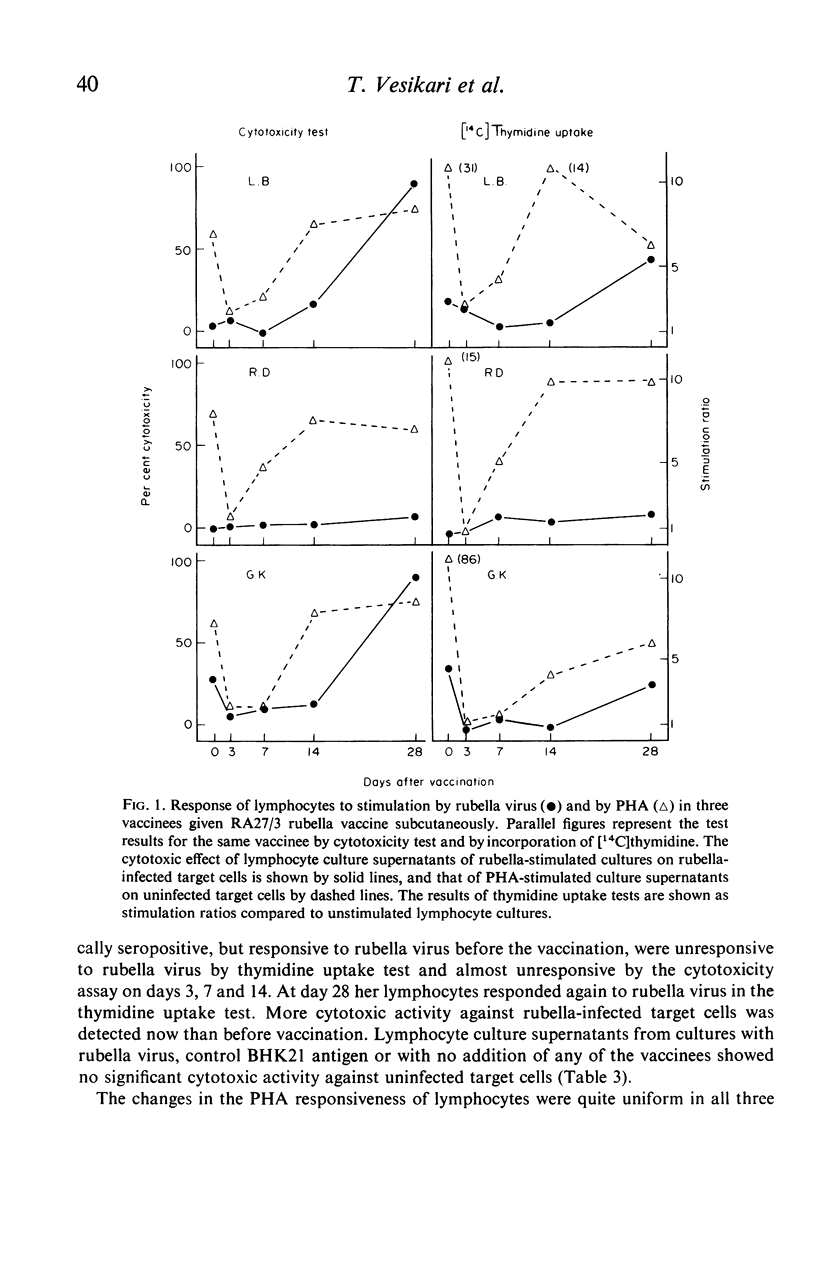
Rubella virus-stimulated lymphocytes from rubella-seropositive donors produced in the culture medium cytotoxic activity with preferential action against rubella-infected over uninfected target cells. The ability of lymphocytes to produce the cytotoxic activity upon stimulation by rubella virus correlated with the humoral rubella-immunity status, i.e. no such cytotoxic activity developed in the supernatants of lymphocyte cultures of rubella-seronegative donors. Stimulation of lymphocytes from seropositive donors by rubella virus was also detected by thymidine incorporation, but the correlation of lymphocyte responsiveness to the humoral rubella antibody status was not so clear as in the cytotoxicity assay. Conversion of lymphocytes from unresponsive to responsive to rubella virus following natural rubella infection and after rubella vaccination was demonstrated using both methods. Following vaccination rubella-specific cell-mediated immunity first became demonstrable at 14 days. The responsiveness of lymphocytes to phytohaemagglutinin (PHA) after rubella vaccination was followed by studying thymidine uptake and the ability of lymphocytes to produce lymphootoxin. By both tests marked suppression of PHA response occurred at days 3 and 7 after vaccination.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODY J. A., OVERFIELD T., HAMMES L. M. DEPRESSION OF THE TUBERCULIN REACTION BY VIRAL VACCINES. N Engl J Med. 1964 Dec 17;271:1294–1296. doi: 10.1056/NEJM196412172712505. [DOI] [PubMed] [Google Scholar]
- Berkovich S., Steiner P., Steiner M. Live rubella virus vaccine in tuberculous children. Am J Dis Child. 1969 Aug;118(2):252–257. [PubMed] [Google Scholar]
- Feinstone S. M., Beachey E. H., Rytel M. W. Induction of delayed hypersensitivity to influenza and mumps viruses in mice. J Immunol. 1969 Oct;103(4):844–849. [PubMed] [Google Scholar]
- Kanra G. Y., Vesikari T. Cytotoxic activity against rubella-infected cells in the supernatants of human lymphocyte cultures stimulated by rubella virus. Clin Exp Immunol. 1975 Jan;19(1):17–32. [PMC free article] [PubMed] [Google Scholar]
- Lalla M., Vesikari T., Virolainen M. Lymphoblast proliferation and humoral antibody response after rubella vaccination. Clin Exp Immunol. 1973 Oct;15(2):193–202. [PMC free article] [PubMed] [Google Scholar]
- Lamon E. W., Wigzel H., Klein E., Andersson B., Skurzak H. M. The lymphocyte response to primary Moloney sarcoma virus tumors in BALB-c mice. Definition of the active subpopulations at different times after infection. J Exp Med. 1973 Jun 1;137(6):1472–1493. doi: 10.1084/jem.137.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midulla M., Businco L., Moschini L. Some effects of rubella vaccination of immunologic responsiveness. Acta Paediatr Scand. 1972 Sep;61(5):609–611. doi: 10.1111/j.1651-2227.1972.tb15954.x. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Tissue injury in lymphocytic choriomeningitis viral infection: virus-induced immunologically specific release of a cytotoxic factor from immune lymphoid cells. Virology. 1970 Dec;42(4):805–813. doi: 10.1016/0042-6822(70)90330-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg G. L., Farber P. A., Notkins A. L. In vitro stimulation of sensitized lymphocytes by herpes simplex virus and vaccinia virus. Proc Natl Acad Sci U S A. 1972 Mar;69(3):756–760. doi: 10.1073/pnas.69.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M. J., Fitzgerald M. G. Rubella virus and human lymphocytes in culture. Lancet. 1968 Nov 2;2(7575):937–940. doi: 10.1016/s0140-6736(68)91167-7. [DOI] [PubMed] [Google Scholar]
- Skurzak H. M., Klein E., Yoshida T. O., Lamon E. W. Synergistic or antgonistic effect of different antibody concentrations on in vitro lymphocyte cytotoxicity in the Moloney sarcoma virus system. J Exp Med. 1972 Apr 1;135(4):997–1002. doi: 10.1084/jem.135.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Chess L., Mardiney M. R., Jr The relationship between rubella hemagglutination inhibition antibody (HIA) and rubella induced in vitro lymphocyte tritiated thymidine incorporation. Cell Immunol. 1973 Aug;8(2):321–327. doi: 10.1016/0008-8749(73)90121-4. [DOI] [PubMed] [Google Scholar]
- Speel L. F., Osborn J. E., Walker D. L. An immuno-cytopathogenic interaction between sensitized leukocytes and epithelial cells carrying a persistent noncytocidal myxovirus infection. J Immunol. 1968 Sep;101(3):409–417. [PubMed] [Google Scholar]
- Takasugi M., Klein E. A microassay for cell-mediated immunity. Transplantation. 1970 Mar;9(3):219–227. doi: 10.1097/00007890-197003000-00005. [DOI] [PubMed] [Google Scholar]
- Willems F. T., Melnick J. L., Rawls W. E. Viral inhibition of the phytohemagglutinin response of human lymphocytes and application to viral hepatitis. Proc Soc Exp Biol Med. 1969 Feb;130(2):652–661. doi: 10.3181/00379727-130-33628. [DOI] [PubMed] [Google Scholar]


