Abstract
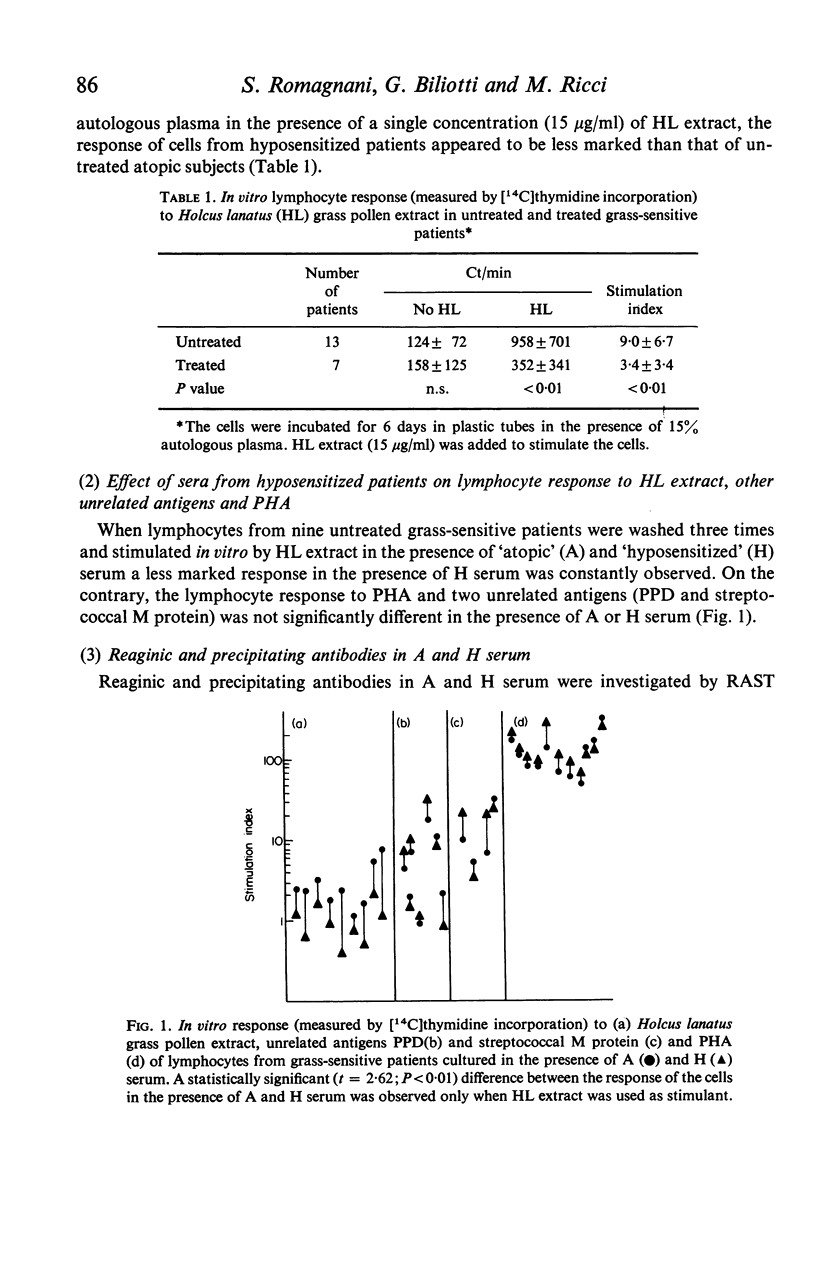
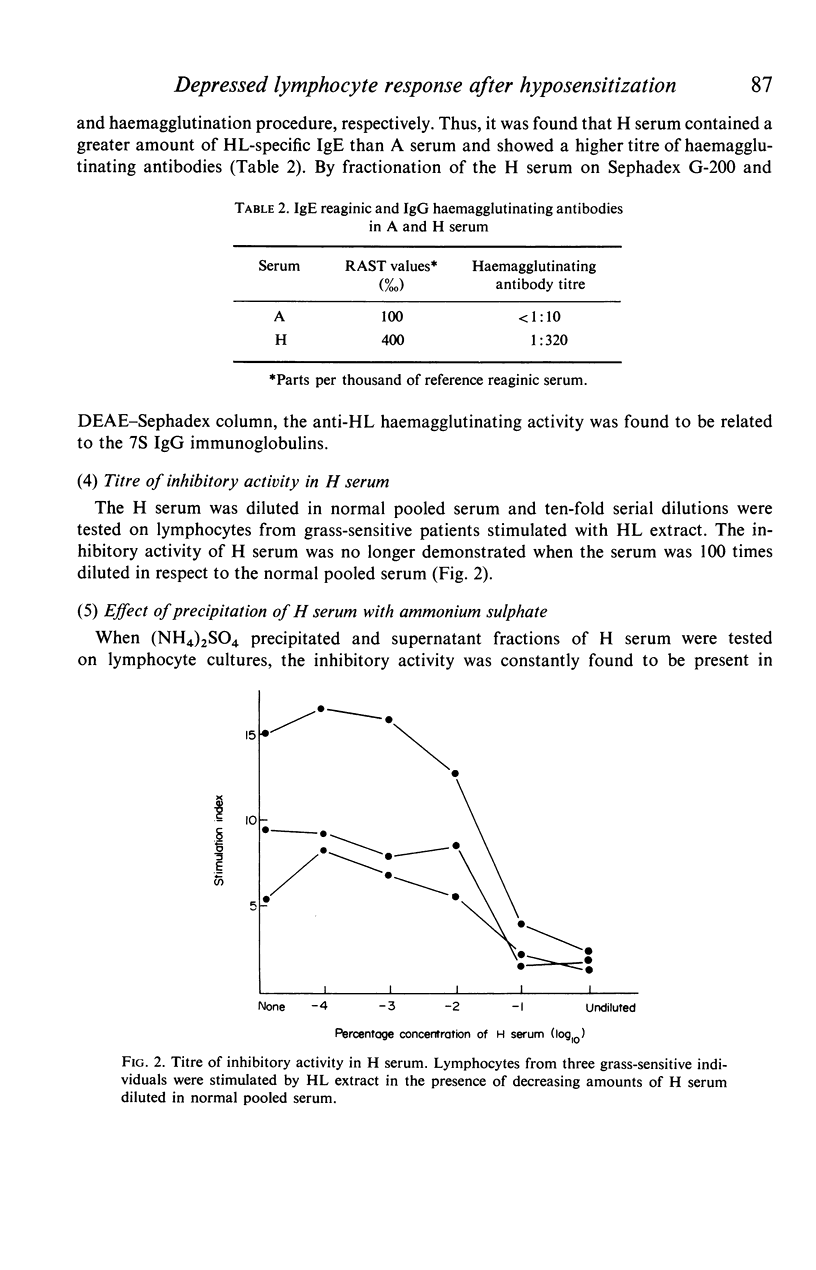
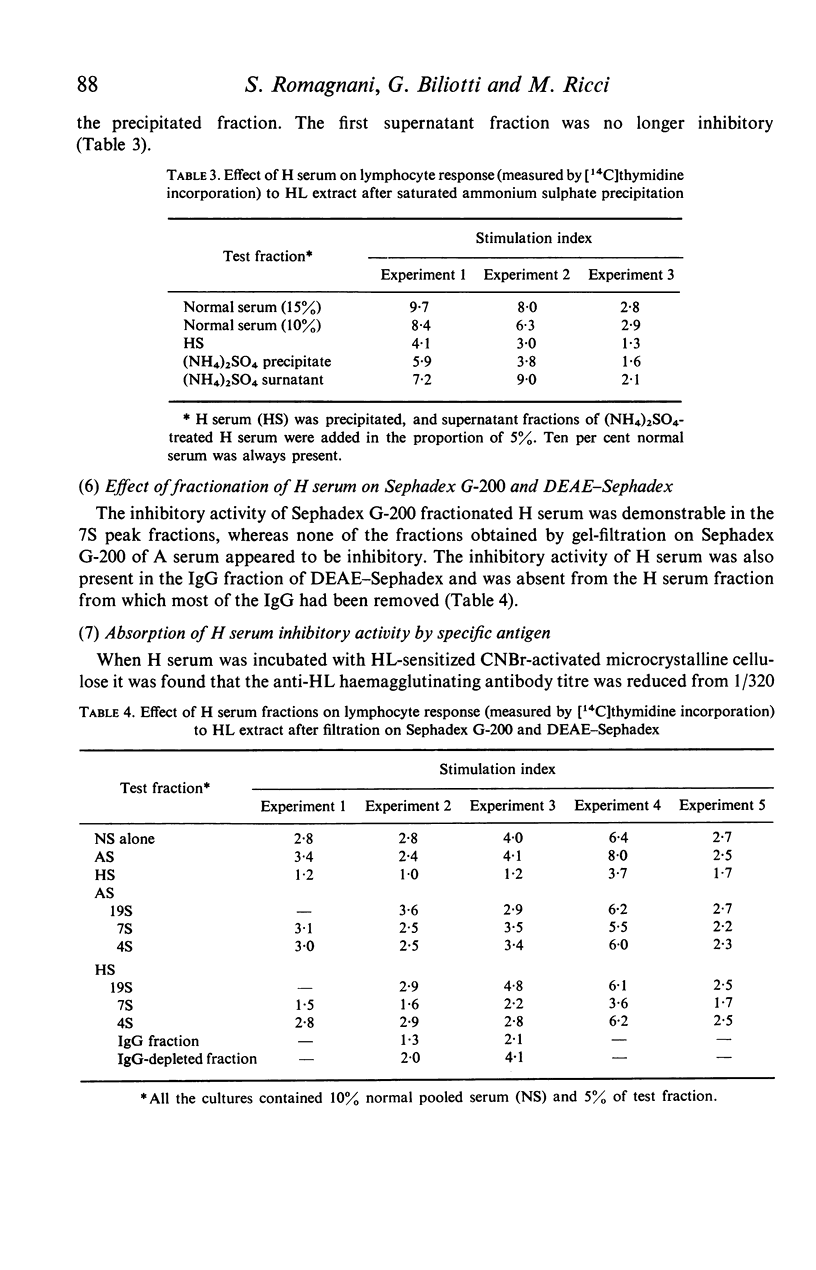
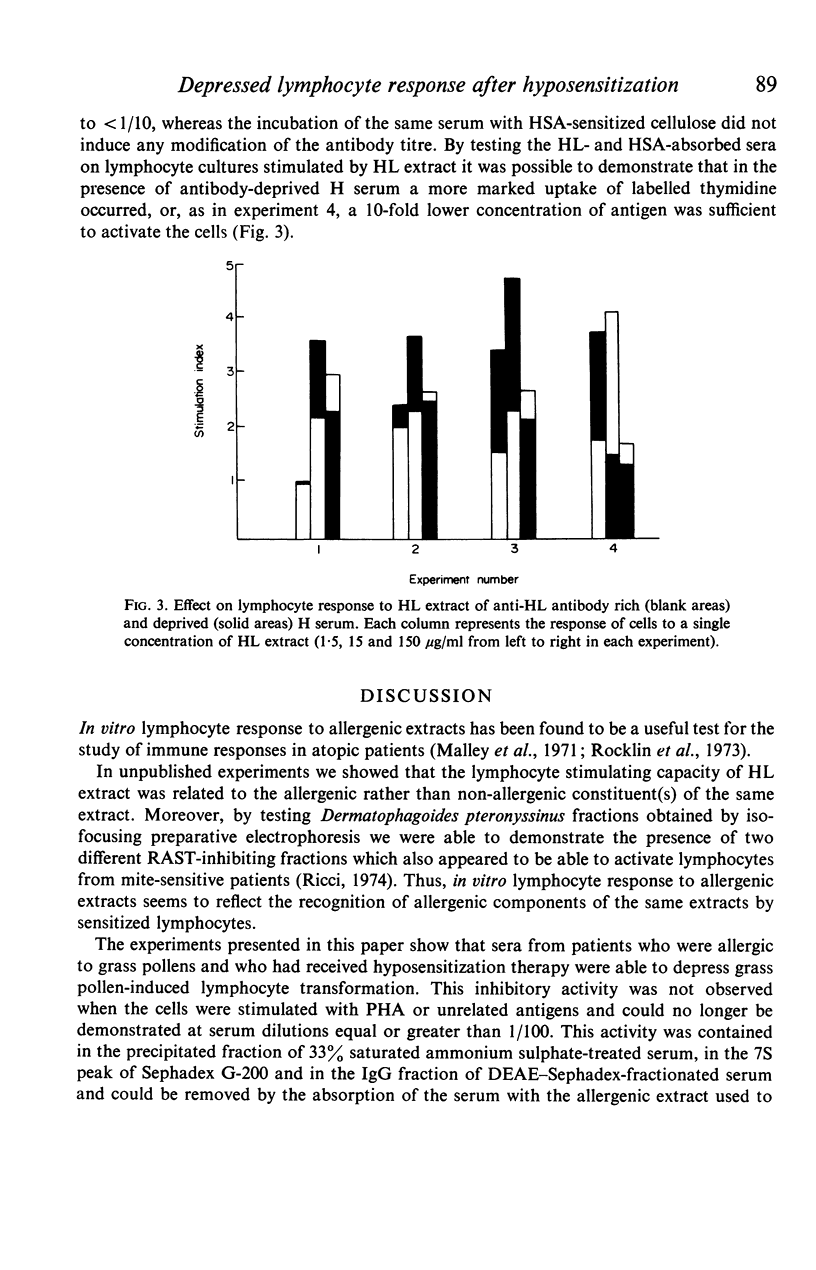
Serum from grass-sensitive patients who had received hyposensitization therapy was found able to depress the in vitro response to Holcus lanatus grass pollen extract of lymphocytes from untreated grass-sensitive individuals. The inhibitory activity could no longer be demonstrated at serum dilutions equal to or greater than 1/100 and was not seen when the cells were stimulated with PHA or unrelated antigens. It was contained in the precipitated fraction of 33% saturated ammonium sulphate-treated serum, segregated with the 7S peak of Sephadex G-200 and with the IgG fraction of DEAE-Sephadex-fractionated serum and could be removed by the absorption of the serum with the Holcus lanatus allergenic extract. These data suggest that the serum factor responsible for the depressed in vitro lymphocyte response to the allergen in hyposensitized patients was a 7S IgG antibody induced by immunotherapy.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Feldmann M., Diener E. Antibody-mediated suppression of the immune response in vitro. I. Evidence for a central effect. J Exp Med. 1970 Feb;131(2):247–274. doi: 10.1084/jem.131.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K., Okudaira H. Reaginic antibody formation in the mouse. I. Antibody-mediated suppression of reaginic antibody formation. J Immunol. 1972 Jul;109(1):84–89. [PubMed] [Google Scholar]
- Jacobs R. P., Blaese R. M., Oppenheim J. J. Inhibition of antigen-stimulated in vitro proliferation of thymic dependent chicken spleen cells by specific antibody. J Immunol. 1972 Aug;109(2):324–333. [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Johansson S. G., Miller A. C., Overell B. G., Wheeler A. W. Changes in serum antibody levels during treatment with grass pollen-tyrosine adsorbate. Clin Allergy. 1974 Mar;4(1):57–70. doi: 10.1111/j.1365-2222.1974.tb01363.x. [DOI] [PubMed] [Google Scholar]
- LANCEFIELD R. C., PERLMANN G. E. Preparation and properties of type-specific M antigen isolated from a group A, type 1 hemolytic streptococcus. J Exp Med. 1952 Jul;96(1):71–82. doi: 10.1084/jem.96.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. A., Lichtenstein L. M., Goldstein E. O., Ishizaka K. Immunologic and cellular changes accompanying the therapy of pollen allergy. J Clin Invest. 1971 Feb;50(2):360–369. doi: 10.1172/JCI106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malley A., Wilson B. J., Barnett M., Perlman F. The site of action of antigen D immunotherapy. J Allergy Clin Immunol. 1971 Nov;48(5):267–275. doi: 10.1016/0091-6749(71)90027-3. [DOI] [PubMed] [Google Scholar]
- Melam H., Pruzansky J., Patterson R., Singer S. Clinical and immunologic studies of ragweed immunotherapy. J Allergy. 1971 May;47(5):262–272. doi: 10.1016/s0091-6749(71)80004-0. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J. Modulation of in vitro lymphocyte transformation by antibodies: enhancement by antigen-antibody complexes and inhibition by antibody excess. Cell Immunol. 1972 Mar;3(3):341–360. doi: 10.1016/0008-8749(72)90243-2. [DOI] [PubMed] [Google Scholar]
- Perper R. J., Okimoto J. T., Cochrum K. C., Ramsey N., Najarian J. S. A rapid method for purification of large quantities of anti-lymphocytic serum. Proc Soc Exp Biol Med. 1967 Jun;125(2):575–580. doi: 10.3181/00379727-125-32150. [DOI] [PubMed] [Google Scholar]
- Pierce C. W. Immune responses in vitro. II. Suppression of the immune response in vitro by specific antibody. J Exp Med. 1969 Aug 1;130(2):365–379. doi: 10.1084/jem.130.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S., Biliotti G., Passaleva A., Ricci M. In vitro lymphocyte response to pollen extract constituents in grass pollen-sensitive individuals. Int Arch Allergy Appl Immunol. 1973;44(1):40–50. doi: 10.1159/000230916. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Sarsfield J. K., Gowland G. A modified radioallergosorbent test for the in vitro detection of allergen antibodies. Clin Exp Immunol. 1973 Apr;13(4):619–624. [PMC free article] [PubMed] [Google Scholar]
- Tada T., Okumura K. Regulation of homocytotropic antibody formation in the rat. I. Feed-back regulation by passively administered antibody. J Immunol. 1971 Apr;106(4):1002–1011. [PubMed] [Google Scholar]
- Tada T., Okumura K., Taniguchi M. Regulation of homocytotropic antibody formation in the rat. 8. An antigen-specific T cell factor that regulates anti-hapten homocytotropic antibody response. J Immunol. 1973 Sep;111(3):952–961. [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]
- Wide L. Radioimmunoassays employing immunosorbents. Acta Endocrinol Suppl (Copenh) 1969;142:207–221. doi: 10.1530/acta.0.062s207. [DOI] [PubMed] [Google Scholar]


