Abstract
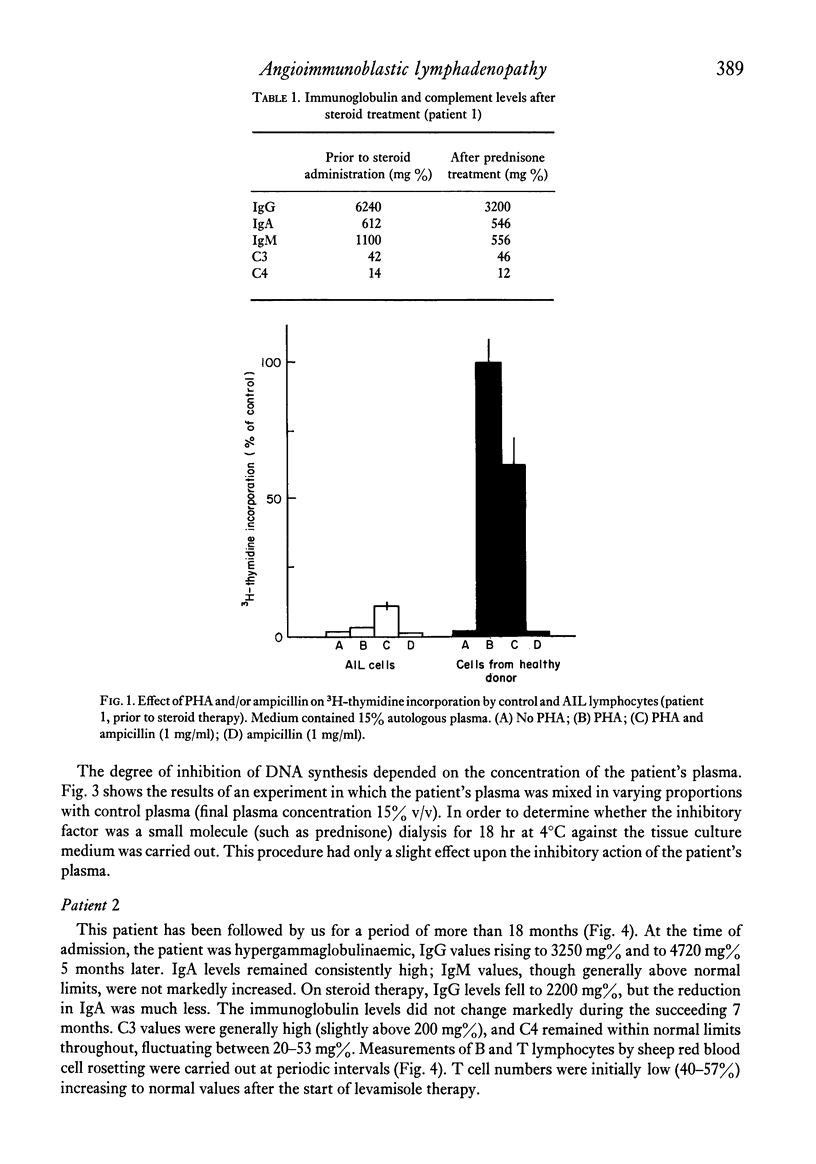
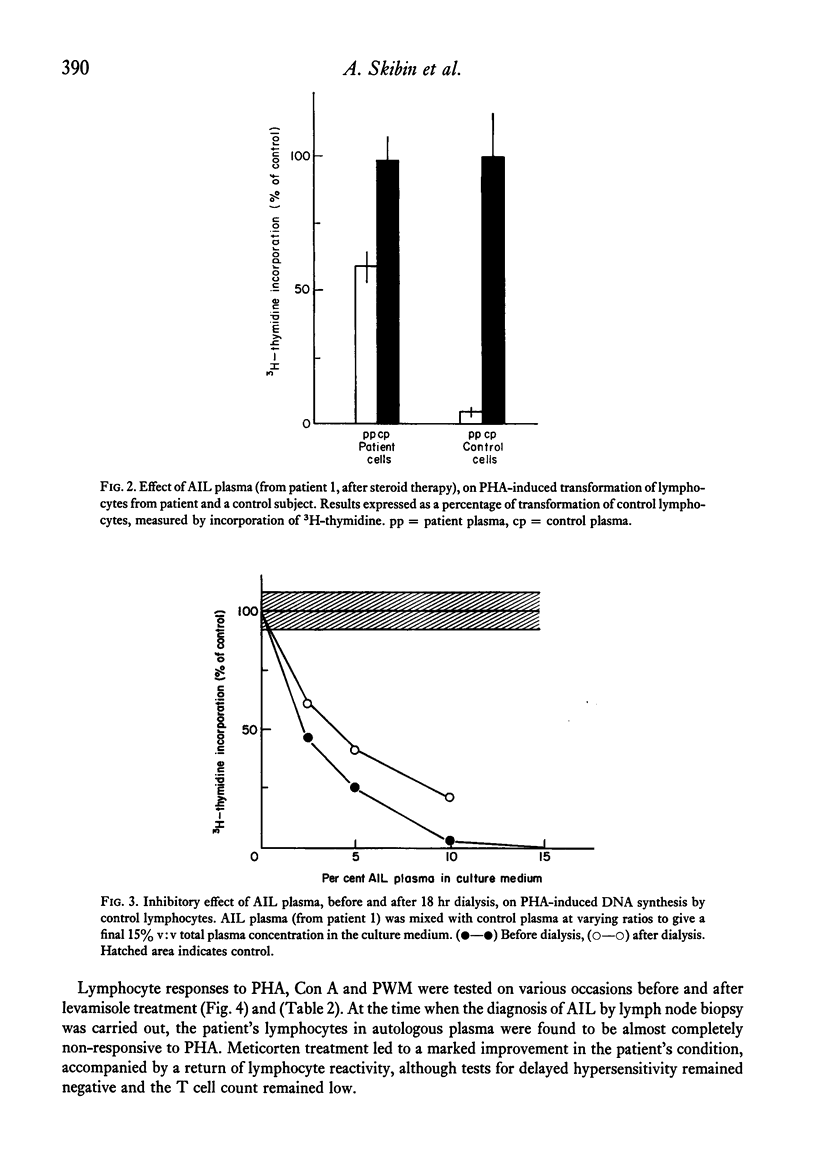
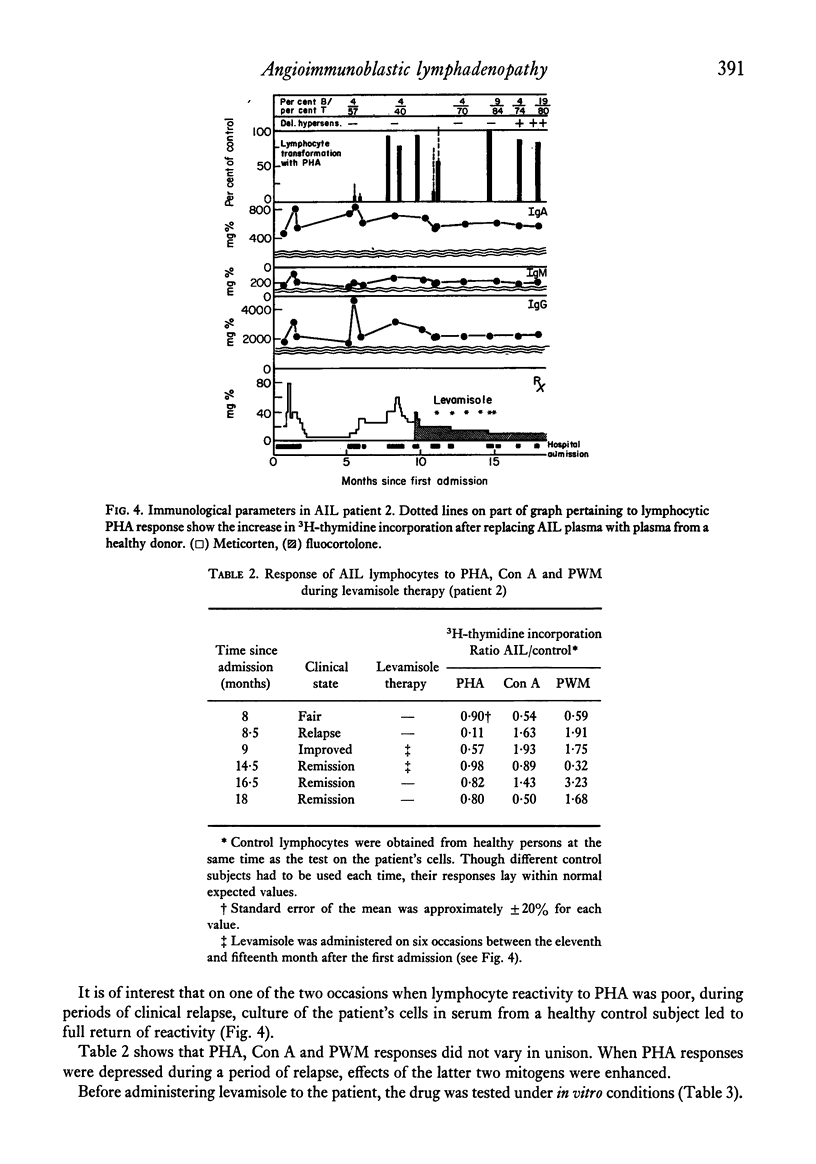
Immunological studies were carried out on two female patients with angioimmunoblastic lymphadenopathy (AIL). Both presented with fever, lymphadenopathy, hepatosplenomegaly, rash and apparent ampicillin hypersensitivity. During the active phase of the disease, cellular immunity was depressed and T cell blastogenesis induced by lectins was abnormal. In the first patient, a non-dialysable plasma factor was found that inhibited normal lymphocyte blastogenesis, the removal of which enhanced the activation of AIL lymphocytes. This inhibitory plasma factor was also observed in the second patient during relapse of the disease. The latter patient responded well to steroid and levamisole therapy, showing clinical remission and a return of in vivo and in vitro parameters of cellular immunity. Defective B cell regulation due to impaired suppressor function, followed by immunoglobulin overproduction, is suggested to occur in AIL.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensa J., Faure J., Martin H., Sotto J., Schaerer R. Letter: Levamisole in angio-immunoblastic lymphadenopathy. Lancet. 1976 May 15;1(7968):1081–1081. doi: 10.1016/s0140-6736(76)92262-5. [DOI] [PubMed] [Google Scholar]
- Bentwich Z., Douglas S. D., Siegal F. P., Kunkel H. G. Human lymphocyte-sheep erythrocyte rosette formation: some characteristics of the interaction. Clin Immunol Immunopathol. 1973 Jul;1(4):511–522. doi: 10.1016/0090-1229(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Bullock W. W., Möller E. "Spontaneous" B cell activation due to loss of normal mouse serum suppressor. Eur J Immunol. 1972 Dec;2(6):514–517. doi: 10.1002/eji.1830020609. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J Clin Invest. 1974 Jan;53(1):240–246. doi: 10.1172/JCI107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. G., Hosking C. S. Plasma inhibitors of lymphocyte response to phytohaemagglutinin in children with recurrent infections. Immunology. 1976 Jan;30(1):33–42. [PMC free article] [PubMed] [Google Scholar]
- Frizzera G., Moran E. M., Rappaport H. Angio-immunoblastic lymphadenopathy with dysproteinaemia. Lancet. 1974 Jun 1;1(7866):1070–1073. doi: 10.1016/s0140-6736(74)90553-4. [DOI] [PubMed] [Google Scholar]
- Howarth C. B., Bird C. C. Immunoblastic sarcoma arising in child with immunoblastic lymphadenopathy. Lancet. 1976 Oct 2;2(7988):747–748. doi: 10.1016/s0140-6736(76)90049-0. [DOI] [PubMed] [Google Scholar]
- Kreisler J. M., Moreno E., Moneo I., Bootello A., Bouza E., De Letona J. M., Anaya A. Immunological findings in immunoblastic lymphadenopathy. A detailed case study. Clin Exp Immunol. 1977 Mar;27(3):497–501. [PMC free article] [PubMed] [Google Scholar]
- Lapes M. J., Vivacqua R. J., Antoniades K. Letter: Immunoblactic lymphadenopathy associated with phenytoin (diphenylhydantoin). Lancet. 1976 Jan 24;1(7952):198–198. doi: 10.1016/s0140-6736(76)91307-6. [DOI] [PubMed] [Google Scholar]
- Lawrence E. C., Broder S., Jaffe E. S., Braylan R. C., Dobbins W. O., Young R. C., Waldmann T. A. Evolution of a lymphoma with helper T cell characteristics in Sézary syndrome. Blood. 1978 Sep;52(3):481–492. [PubMed] [Google Scholar]
- Lukes R. J., Tindle B. H. Immunoblastic lymphadenopathy. A hyperimmune entity resembling Hodgkin's disease. N Engl J Med. 1975 Jan 2;292(1):1–8. doi: 10.1056/NEJM197501022920101. [DOI] [PubMed] [Google Scholar]
- Matz L. R., Papadimitriou J. M., Carroll J. R., Barr A. L., Dawkins R. L., Jackson J. M., Herrmann R. P., Armstrong B. K. Angioimmunoblastic lymphadenopathy with dysproteinemia. Cancer. 1977 Nov;40(5):2152–2160. doi: 10.1002/1097-0142(197711)40:5<2152::aid-cncr2820400525>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Miller J. F. T-cell control of B-cell responsiveness. Int Arch Allergy Appl Immunol. 1975;49(1-2):230–240. doi: 10.1159/000231402. [DOI] [PubMed] [Google Scholar]
- Moor S. B., Harrison E. G., Jr, Weiland L. H. Angioimmunoblastic lymphadenopathy. Mayo Clin Proc. 1976 May;51(5):273–280. [PubMed] [Google Scholar]
- Neiman R. S., Dervan P., Haudenschild C., Jaffe R. Angioimmunoblastic lymphadenopathy: an ultrastructural and immunologic study with review of the literature. Cancer. 1978 Feb;41(2):507–518. doi: 10.1002/1097-0142(197802)41:2<507::aid-cncr2820410218>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Newberry W. M., Shorey J. W., Sanford J. P., Combes B. Depression of lymphocyte reactivity to phytohemagglutinin by serum from patients with liver disease. Cell Immunol. 1973 Jan;6(1):87–97. doi: 10.1016/0008-8749(73)90009-9. [DOI] [PubMed] [Google Scholar]
- Pangalis G. A., Moran E. M., Rappaport H. Blood and bone marrow findings in angioimmunoblastic lymphadenopathy. Blood. 1978 Jan;51(1):71–83. [PubMed] [Google Scholar]
- Pruzanski W., Sutton D. M., Pantalony D. Angioimmunoblastic lymphadenopathy: an immunochemical study. Clin Immunol Immunopathol. 1976 Jul;6(1):62–76. doi: 10.1016/0090-1229(76)90061-1. [DOI] [PubMed] [Google Scholar]
- Saxon A., Stevens R. H., Ramer S. J., Clements P. J., Yu D. T. Glucocorticoids administered in vivo inhibit human suppressor T lymphocyte function and diminish B lymphocyte responsiveness in in vitro immunoglobulin synthesis. J Clin Invest. 1978 Apr;61(4):922–930. doi: 10.1172/JCI109017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D. R., Yunis A. A. Immunoblastic lymphadenopathy with mixed cryoglobulinemia. A detailed case study. N Engl J Med. 1975 Jan 2;292(1):8–12. doi: 10.1056/NEJM197501022920102. [DOI] [PubMed] [Google Scholar]
- Schwartz S. A., Shou L., Good R. A., Choi Y. S. Suppression of immunoglobulin synthesis and secretion by peripheral blood lymphocytes from normal donors. Proc Natl Acad Sci U S A. 1977 May;74(5):2099–2103. doi: 10.1073/pnas.74.5.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symoens J., Rosenthal M. Levamisole in the modulation of the immune response: the current experimental and clinical state. J Reticuloendothel Soc. 1977 Mar;21(3):175–221. [PubMed] [Google Scholar]
- Waksman B. H., Namba Y. On soluble mediators of immunologic regulation. Cell Immunol. 1976 Jan;21(1):161–176. doi: 10.1016/0008-8749(76)90337-3. [DOI] [PubMed] [Google Scholar]
- Westerhausen M., Oehlert W. Chronisches pluripotentielles immunproliferatives Syndrom. Dtsch Med Wochenschr. 1972 Sep 22;97(38):1407–passim. doi: 10.1055/s-0028-1107573. [DOI] [PubMed] [Google Scholar]


