Abstract
Measles virus (MV) is typically spread by aerosol droplets and enters via the respiratory tract. The progression of MV infection has been widely studied; yet, the pathway for virus entry in polarized human airway epithelia has not been investigated. Herein we report the use of a replication-competent Edmonston vaccine strain of MV expressing enhanced green fluorescent protein (MV-eGFP) to infect primary cultures of well-differentiated human airway epithelia. Previous studies with polarized Caco-2 cells (intestine-derived human epithelia) and MDCK cells (kidney-derived canine epithelia) demonstrated that MV primarily infected and exited the apical surface. In striking contrast, our results indicate that MV preferentially transduces human airway cells from the basolateral surface; however, virus release remains in an apical direction. When MV-eGFP was applied apically or basolaterally to primary cultures of airway epithelia, discrete foci of eGFP expression appeared and grew; however, the cell layer integrity was maintained for the duration of the study (7 days). Interestingly, utilizing immunohistochemistry and confocal microscopy, we observed widespread expression of the receptor for the vaccine strain of MV (CD46) at greatest abundance on the apical surface of the differentiated human airway epithelia as well as in human tracheal tissue sections. These data suggest that the progression of MV infection through the respiratory epithelium may involve pathways other than direct binding and entry through the apical surface of airway epithelia.
Measles virus (MV) exemplifies the Morbillivirus genus in the Paramyxoviridae family. Fever, cough, conjunctivitis, and the appearance of a generalized rash characterize this highly contagious disease (12). The primary infection with MV begins in the respiratory tract; specifically, studies of experimentally infected primates and human autopsy tissue show virus propagation in tracheal and bronchial epithelial cells (22). Initiation of infection occurs more readily in the conducting airways than in the nasopharynx (10). The virus replicates locally in respiratory epithelia and subsequently spreads to the lymphatic system, possibly transported by phagocytic cells such as pulmonary macrophages (17, 21). Although many human and monkey cell types are permissive for MV infection in vitro, virus replication is restricted to a few cell types in vivo (12).
Previous studies using monkey kidney cells (Vero C1008) and human colon carcinoma cells (Caco-2) suggested that MV preferentially enters polarized epithelial cells across the apical surface (2, 3). Additionally, studies using Vero C1008, Caco-2, and Madin-Darby canine kidney (MDCK) cells demonstrated that MV is released from the apical membrane of polarized epithelia (2, 3, 15, 18). Indeed, because MV is spread via an aerosol route, it would also be hypothesized to enter the apical surface of polarized airway epithelia. However, not all polarized epithelia behave in the same way, and the airways in particular have evolved barriers to viral infection (25).
The apical surface of airway epithelia presents a formidable barrier to many viral vector systems currently under investigation for pulmonary gene therapy applications (29). To develop a lentivirus vector capable of transducing the apical surface of airway epithelia, we are investigating the pseudotyping of lentivirus vectors with envelope glycoproteins from respiratory viruses that naturally enter via the apical surface of airway epithelia. We hypothesized that the MV envelope glycoproteins (H and F) were excellent pseudotyping candidates for multiple reasons. As mentioned above, previous in vitro evidence demonstrated that MV preferentially enters polarized epithelia across the apical surface. In addition, the MV viral receptors are known and reagents to localize those receptors are readily available (7, 24). However, before pseudotyping experiments were performed, we sought to verify that MV would transduce efficiently when applied to the apical surface of primary cultures of well-differentiated human airway epithelia.
To evaluate the polarity of MV entry in human airway epithelia, we utilized a recombinant MV vaccine strain expressing the enhanced green fluorescent protein (MV-eGFP) (8). Previously this recombinant, replication-competent virus was used to monitor viral replication, in real time, in animal tissues (9). In this study, we report the unexpected observation that MV enters more efficiently from the basolateral surface than from the apical surface of differentiated primary cultures of human airway epithelial cells. In addition, the receptor for the vaccine strain of MV, CD46, was abundantly expressed in the airway cells and largely polarized to the apical surface as determined by confocal microscopy. These data suggest that the MV may require access to the basolateral surface of airway epithelia in vivo for efficient infection to occur. In addition, receptor localization may not be the limiting barrier to MV infection.
MATERIALS AND METHODS
Cell culture.
Primary cultures of human airway epithelia were prepared from trachea and bronchi by enzymatic dispersion by established methods (30). Briefly, epithelial cells were dissociated and seeded onto collagen-coated, semipermeable membranes with a 0.4-μm pore size (Millicell-HA; surface area, 0.6 cm2; Millipore Corp., Bedford, Mass.). Human airway epithelial cultures were maintained in Ultraser G (USG) medium at 37°C with 5% CO2. Millicell inserts were placed into 24-well plastic cell culture plates (Costar, Cambridge, Mass.). Twenty-four hours after seeding, the mucosal medium was removed and the cells were allowed to grow at the air-liquid interface as reported previously (30). Only well-differentiated cultures (>2 weeks old) were used in these studies. The presence of tight junctions was confirmed by transepithelial resistance using a volt-ohm meter (World Precision Instruments, Sarasota, Fla.; resistance, >500 Ω·cm2). This study was approved by the institutional review board of the University of Iowa.
Caco-2 cells were seeded onto the same semipermeable membranes as were the airway epithelial cells. Caco-2 cells were maintained with USG medium applied to both the apical and basolateral surfaces and incubated at 37°C with 5% CO2. The transepithelial resistance of all Caco-2 samples was >400 Ω·cm2 prior to infection with MV-eGFP.
Virus production and titer determination.
MV-eGFP production was conducted as previously described (8). Briefly, MV-eGFP was produced in Vero cells cultured in Dulbecco's modified Eagle's medium (Gibco) containing 8% newborn calf serum (Gibco) and penicillin-streptomycin (100 μg/ml). MV-eGFP titers of approximately 107 50% tissue culture infective doses/ml were obtained. To determine the polarity of viral release from airway epithelia, 500-μl washings were collected from both the apical and basolateral surfaces of airway epithelia at progressing time points. The time points included preinfection and 1, 3, 5, and 7 days post-MV-eGFP infection. Transepithelial resistances were concurrently measured at the time points of wash collection. Washings were stored at −80°C until they were collectively thawed and used to infect Vero cells at a limiting dilution. Titers were calculated by counting eGFP-positive cells after 30 h.
Infection of polarized airway epithelial cells.
MV-eGFP viral preparations were diluted in USG medium, and 100 μl of the solution was applied to the apical surface of airway epithelial cells (multiplicity of infection [MOI] of 1). After incubation for 4 h at 37°C, the virus was removed and cells were further incubated at 37°C, for indicated time periods. To infect airway epithelia with MV-eGFP from the basolateral side, the Millicell culture insert containing the airway epithelial culture was turned over and the virus was applied to the basolateral surface for 4 h in 100 μl of USG medium. Following the 4-h infection, the virus was removed and the culture was turned upright and allowed to incubate at 37°C with 5% CO2 for the indicated time periods. A recombinant adenovirus vector expressing eGFP (Ad5-eGFP), produced as previously described (1), was used as a positive control.
Western blot analysis.
Western blot analysis for verifying CD46 protein expression was conducted using standard techniques. Briefly, cell lysates were denatured for 5 min at 100°C in Laemmli sample buffer, electrophoresed on previously prepared 10% polyacrylamide gels (Bio-Rad; catalog no. 161-1155) at 125 V, and transferred to pure nitrocellulose (Bio-Rad; catalog no. 162-0145) overnight at 200 mA. The membrane was probed with a rabbit anti-human CD46 primary antibody (courtesy of J. Atkinson, St. Louis, Mo.) at a 1:4,000 dilution and detected using goat anti-rabbit immunoglobulin G conjugated to alkaline phosphatase at a 1:1,000 dilution (Sigma; catalog no. A-3812).
Immunohistochemistry and confocal microscopy.
Epithelial cells were washed with 1× phosphate-buffered saline (PBS), fixed in 2% paraformaldehyde for 5 to 10 min, and rinsed with 1× PBS. The epithelial cells were then incubated for 30 min at 37°C with a rabbit anti-human CD46 antibody (tail2) (4) diluted 1:100 in Hanks' buffer (Gibco). The cells were washed with 1× PBS and incubated with a tetramethyl rhodamine isocyanate (TRITC)-conjugated anti-rabbit 2° antibody (Sigma; T6778) diluted 1:100 in 1× PBS for 30 min at 37°C. The primary and secondary antibodies were always applied to both the apical and basolateral surfaces. Tracheal tissues were paraffin embedded using standard techniques, sectioned, and deparaffinized prior to immunostaining. Immunohistochemistry assays of sectioned tracheal specimens were performed using the same protocol as previously described for the epithelial cells. Images were captured with a Bio-Rad MRC-1024 Hercules laser scanning confocal microscope equipped with a Kr-Ar laser.
RESULTS
MV infection of human airway epithelia.
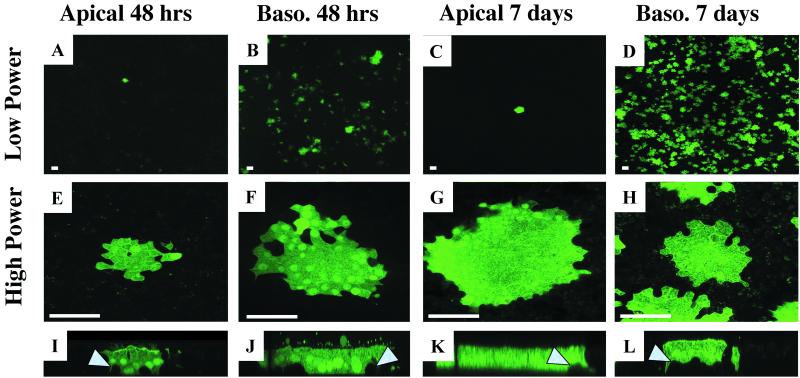
We utilized a replication-competent vaccine strain of MV (Edmonston), which expresses eGFP. MV-eGFP was applied at an MOI of 1 to the apical or basolateral surface as described in Materials and Methods. Surprisingly, we found that MV-eGFP preferentially transduced the basolateral surface of human airway epithelia (Fig. 1). When the virus was applied to the apical surface, we observed very few foci of eGFP expression (1 to 10 foci/cm2) after 48 h (Fig. 1A). Furthermore, the number of foci changed little after 7 days (Fig. 1C). This observation is in striking contrast to the results from applying the virus to the basolateral surface. Forty-eight hours following basolateral infection, more than 1,000 foci/cm2 were detected (Fig. 1B). Seven days following a basolateral infection, the density of eGFP-positive foci visually increased (Fig. 1D); however, this augmentation is primarily a result of increased focus fluorescent intensity rather than increased focus number. Individual foci resulting from apical or basolateral infections were indistinguishable from either en face (Fig. 1E to H) or vertical section (Fig. 1I to L) views. These observations were replicated with six independent human primary culture specimens and demonstrate that MV more readily transduces the basolateral surface than the apical surface of tracheally or bronchially derived primary cultures of airway epithelia. Further, these data suggest that the apical surface of airway epithelia may present barriers to MV infection in vivo. Interestingly, when virus was applied to either the apical or the basolateral surface, eGFP-expressing basal cells were rarely detected, as indicated by arrowheads in Fig. 1I to L. This observation suggests that the nonciliated basal cells may be less susceptible than other cells to infection by MV.
FIG. 1.
Polarity of MV infection in well-differentiated human airway epithelia. The apical or basolateral surfaces of human airway epithelia were infected with MV-eGFP at an MOI of 1 for 4 h and allowed to incubate for 2 or 7 days as indicated. Panels I to L are representative vertical sections of panels E to H, respectively. Arrowheads indicate uninfected basal cells. Bars = 100 μm (A to D) or 50 μm (E to L). n = 6.
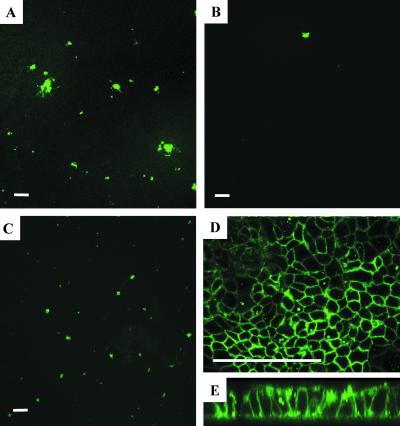
The novel observation of preferential basolateral MV infection prompted us to replicate studies of MV infection in polarized Caco-2 cells (2). Virus was applied directly to the apical surface at an MOI of 1 in a 100-μl volume (Fig. 2A) or to the basal medium at an MOI of 3 in a 300-μl volume (Fig. 2B) as described previously (2). As shown in Fig. 2, MV more readily transduced the apical surface than the basolateral surface in Caco-2 cells under these test conditions. Our results were consistent with the previous observation that MV preferentially enters the apical surface of polarized Caco-2 cells. However, we observed that, if the membrane supporting the epithelial cells was inverted and MV was applied at an MOI of 1 in a 100-μl volume directly to the basolateral surface (Fig. 2C), there was little difference between this and apical application. We suspect that the difference between the basolateral infections done upright and those done inverted is simply a gravitational effect. As the viral particles have a greater density than do the media, they will gradually settle to the bottom of the cell culture dish. Therefore, fewer viral particles will have access to the basolateral membrane when the infection is performed upright. This notion is supported by our previous observations with retrovirus infection in epithelia (27). In addition, we found abundant expression of CD46 in Caco-2 cells by immunohistochemistry assays (Fig. 2D); however, receptor expression appeared to be similarly distributed on apical and basolateral surfaces (Fig. 2E). In contrast, Maisner and coworkers demonstrated by domain-selective biotinylation that simian CD46 in Vero C1008 cells and human CD46 in stably transfected MDCK cells predominantly sort to the basolateral surface (16).
FIG. 2.
Polarity of MV infection in Caco-2 cells. Polarized Caco-2 cells were infected with MV-eGFP for 4 h and allowed to incubate for 2 days. MV-eGFP was applied at an MOI of 1 in a 100-μl volume to the apical surface (A), at an MOI of 3 in a 300-μl volume to the basolateral surface via the basal medium (B), or at an MOI of 1 in a 100-μl volume directly to the basolateral surface by inverting the support membrane (C). Caco-2 cells were immunostained with a rabbit anti-human CD46 primary antibody and a goat anti-rabbit TRITC secondary antibody (D and E). Images were captured by confocal microscopy. A representative vertical section is shown (E). Bar = 50 μm. n = 3.
Spread of replication-competent MV in airway epithelia.
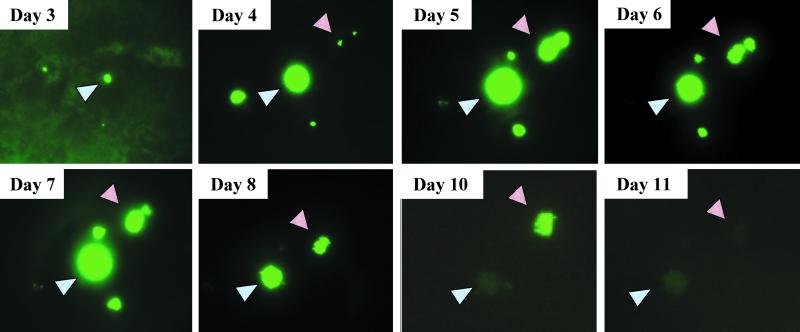
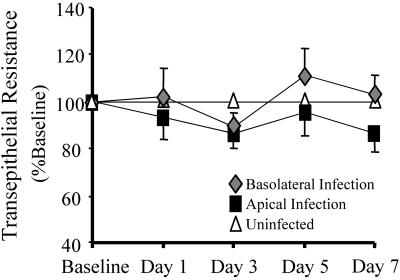
Typically, when a lytic, replication-competent virus such as MV is applied to permissive primate cells (e.g., Vero or 293 cells) at an MOI of 1 in vitro, the viral replication process ultimately destroys the cell layer within 48 h. Interestingly, this observation did not hold true for primary cultures of airway epithelia. As before, MV-eGFP was applied to the apical surface of airway epithelia at an MOI of 1. Foci began to appear 2 to 3 days postinfection (Fig. 3). Though uncommon, groupings of foci were documented daily in living cultures by use of an inverted fluorescence microscope. Foci appeared, grew in diameter, and slowly regressed over time with no corresponding change in transepithelial resistance (Fig. 4). In fact, neither basolaterally nor apically infected epithelia exhibited significant changes in their transepithelial resistances compared to those of uninfected counterparts during the 7-day experimental period. Furthermore, transepithelial resistances of MV-infected epithelia were not significantly different from those of Ad5-eGFP-transduced control epithelia (data not shown).
FIG. 3.
Time course of MV-eGFP infection. MV-eGFP was applied to the apical surface of airway epithelia at an MOI of 1 for 4 h and allowed to incubate for the indicated time periods. Focus appearance and disappearance were documented over the time interval indicated by using an inverted fluorescence microscope. Though uncommon, groupings of foci were documented to ensure that the same field was observed over time, as indicated by arrowheads. n = 3.
FIG. 4.
Maintenance of epithelial integrity following MV-eGFP infection. The epithelial integrity was determined by ohmmeter measurement of the transepithelial resistance. Values were corrected for the blank filter resistance and further standardized against baseline readings and uninfected counterparts. Neither corrected nor raw numbers resulted in a statistically significant variation from uninfected epithelia. Error bars represent the standard errors of the means. n = 6.
Polarity of MV release.
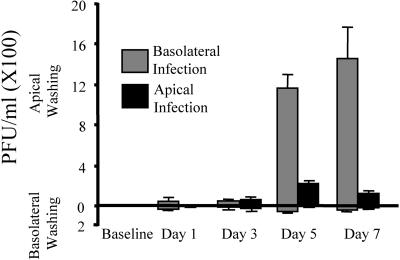
To determine the polarity of viral release, both apical and basolateral washings were collected from cells that were previously infected by MV-eGFP application to either the apical or the basolateral surface of airway epithelia. The viral titers from the washings were determined by infection of Vero cells as described in Materials and Methods. As shown in Fig. 5, only a low MV-eGFP titer (<150 PFU/ml) was detected in the basolateral washings following either an apical or a basolateral infection. The greatest viral titer was present in the apical washings following a basolateral infection (1,200 to 1,600 PFU/ml). We would expect the highest viral titer from a basolateral infection because the efficiency of infection was consistently greater from the basolateral surface than from the apical surface (Fig. 1).
FIG. 5.
Polarity of MV-eGFP release. Human airway epithelia were infected with MV-eGFP at the apical or basolateral surface as described in Materials and Methods. Both apical and basolateral washings were collected over the time interval indicated, including a preinfection baseline washing. Titers of washings were determined on Vero cells. Error bars represent the standard errors of the means. n = 6.
Localization of the ubiquitous MV receptor CD46 in human airway epithelia.
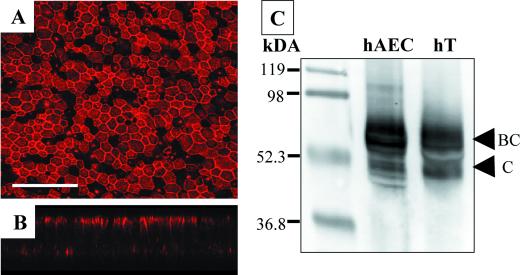
CD46, the main receptor for vaccine strains of MV, such as Edmonston, is ubiquitously expressed (7), whereas the other known receptor, SLAM (CDw150), is expressed selectively in certain immune cells (5). Using a polyclonal rabbit anti-human CD46 antibody, we investigated the expression and polarity of receptor distribution in well-differentiated airway epithelial cells (Fig. 6). Immunohistochemistry assays using an anti-CD46 antibody revealed CD46 protein expression across the epithelial sheet (Fig. 6A). Interestingly, the preponderance of the receptor was localized to the apical surface (Fig. 6B). Assays with control conditions of normal rabbit serum or omission of the secondary controls showed no CD46 expression (data not shown). Additional confirmation of CD46 expression in human airway epithelial cells was obtained by Western blotting (Fig. 6C) and fluorescence-activated cell sorting analysis (data not shown).
FIG. 6.
CD46 expression in human airway epithelia. Human airway epithelial cells were immunostained with a rabbit anti-human CD46 primary antibody and a goat anti-rabbit TRITC-conjugated secondary antibody (A). Images were captured using confocal microscopy. A representative vertical section is shown (B). Bar = 50 μm. Western blot analysis of human airway epithelial cell (hAEC) lysate and human tracheal (hT) lysate was conducted with a rabbit anti-human CD46 polyclonal antibody as described in Materials and Methods (C).
CD46 is expressed ubiquitously in human nucleated cells and is typically produced as four major isoforms that arise by alternative splicing (13, 14). Two isoforms are detected by Western analysis. The large ∼66-kDa isoform (named BC) contains a heavily O-glycosylated region that the smaller isoform (named C) lacks (14). The BC and C isoforms may each have one of two different cytoplasmic tails (named 1 and 2) which are not easily distinguishable by Western analysis. Under nonreducing conditions, we observed both BC and C isoforms in human tracheal protein extracts as well as in human airway epithelial cell protein extracts (Fig. 6C). In addition, Western analysis of CD46 expression in Vero cell lysates detected both isoforms (data not shown). These results are consistent with previous CD46 Western analysis of human lung tissue (17) and suggest that primary cultures of human airway epithelial cells express multiple isoforms of CD46.
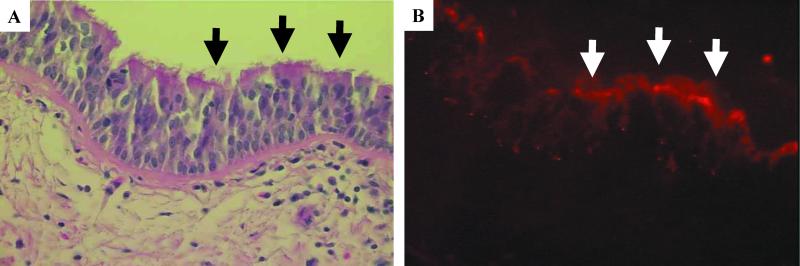
To confirm the presence and expression pattern of CD46 in vivo, human tracheal specimens were studied by immunofluorescence microscopy. Hematoxylin and eosin staining of the tracheal cross sections reveal tissue morphology and the presence of ciliated epithelial cells (Fig. 7A, black arrows). As shown in the subsequent serial section (Fig. 7B), CD46 expression was detected in tracheal epithelia and more intensely at the apical surface of ciliated cells (white arrows). When normal rabbit serum was used in place of the polyclonal CD46 primary antibody, only a diffuse nonspecific background immunofluorescence was detected (data not shown). The observation of CD46 localization in the epithelia of tracheal tissue sections closely mirrors our observation of CD46 expression and localization in vitro (Fig. 6).
FIG. 7.
CD46 expression in human tracheal sections. Human tracheal explants were fixed, mounted in paraffin blocks, and sectioned. Hematoxylin and eosin staining reveals the cellular morphology of the airway epithelia (A). Alternating sections of the tissue were immunostained with a rabbit anti-human CD46 primary antibody and a goat anti-rabbit TRITC-conjugated secondary antibody as described in Materials and Methods (B). Arrows indicate ciliated airway epithelial cells.
DISCUSSION
Measles is classically a childhood infection of humans spread by the respiratory route. Previous evidence, which has been extrapolated to include airway epithelial cells, demonstrated that MV enters preferentially via the apical surface of polarized intestine-derived human epithelial cells (2). Indeed, our results in Caco-2 cells replicate these previously published observations. However, when an MV vaccine strain (Edmonston) is applied at an equivalent MOI to either the apical or the basolateral surface of airway cells, the virus infects with much greater efficiency from the basolateral surface than from the apical surface. These data suggest that the primary route for MV entry into airway epithelia may not be the apical membrane in vivo. Although the pathology of MV infection is known to involve inflammation of the respiratory tract from the nasal mucosa to the bronchioles (10, 12), we cannot discount the possibility that MV enters apically within cell types not represented in a primary culture of well-differentiated airway epithelium, such as alveolar epithelial cells.
Interestingly, we found that, though the virus preferentially enters across the basolateral membrane, it is released predominantly from the apical surface. We can envision a model in which MV is brought into contact with the airways via inhalation. Infection then occurs at sites of epithelial injury or to a lesser extent across the apical surface. Once replication has occurred in the epithelia, virus is released back into the lumen where it can be taken up by macrophages or released back into the environment in respiratory droplets. Macrophages may ultimately be responsible for taking the virus to the bloodstream, after which the systemic manifestations of the viral infection begin.
As discussed, our data suggest that MV preferentially enters the airway epithelium at the basolateral surface and is released at the apical surface. These data suggest a favored basolateral-to-apical movement of the virus that could help to explain the apparent resistance of airway epithelia to secondary infection as seen in Fig. 3. Interestingly, when MV is applied to the basolateral surface and the pattern of eGFP expression is visually documented over time, the foci of expression are much more numerous than, but behave in a similar manner as, those in an apical infection (data not shown). Individual foci initially become larger and more fluorescently intense but eventually wane in size and intensity; furthermore, transepithelial resistance remains relatively constant (Fig. 4).
Another potential explanation for the failure of secondary infection to progress in airway epithelia concerns the phenomenon of CD46 down-regulation following MV infection (11, 19, 23). CD46 immunostaining of airway epithelia following apical MV infection revealed prevalent CD46 expression across the epithelial sheet but not within areas of eGFP-positive cells (data not shown). These data suggest that local down-regulation of CD46 occurs within sites of infection, perhaps as a means to prevent superinfection. Down-regulation was not observed in neighboring uninfected cells; however, such conclusions require a quantitative analysis of CD46 levels before and after MV infection by fluorescence-activated cell sorting or Western analysis.
A particularly puzzling aspect of this work is the finding that the virus preferentially enters via the basolateral membrane, while CD46 expression is highest at the apical surface. One possible explanation for this observation stems from the notion that receptor accessibility is not the only requirement for successful viral infection. As in vivo, the primary cultures of human airway epithelia secrete airway surface liquid and mucus and are heavily ciliated at the apical surface (30). These features may provide barriers to infection at the apical surface. In addition, the apical surface has a dense glycocalyx that is not present at the basolateral surface and may act as a physical barrier for some viruses (20). An alternate explanation for the poor apical transduction efficiency is the absence of a putative coreceptor from the apical surface. This hypothetical coreceptor may be strictly polarized to the basolateral surface.
It is important to mention that other respiratory viruses transduce the apical surface of polarized epithelia with equal or greater efficiency than they do the basolateral surface (6). For example, we previously reported that human coronavirus (serotype 229E) efficiently transduces the apical surface of well-differentiated airway epithelia (28). In addition, respiratory syncytial virus and parainfluenza virus also have the capacity to enter polarized human airway epithelial cells through the apical surface (P. L. Sinn and P. B. McCray, unpublished observations). Therefore, these cells are not inherently impenetrable to all enveloped viruses at the apical surface. Conversely, MV is not the first respiratory virus to be shown to preferentially transduce the basolateral surface. For example, adenovirus serotypes 2 and 5 preferentially transduce the basolateral surface of airway epithelia, although the original assumption was that they would cross at the apical surface (20, 26). However, unlike MV, the receptor for adenovirus serotypes 2 and 5 (coxsackievirus and adenovirus receptor) is polarized to the basolateral surface.
The pulmonary epithelium has evolved strategies to prevent the host invasion of microbes and viruses (25). Indeed, multiple factors present on the apical surface of airway epithelia may preclude viral transduction. Airway surface epithelia secrete antimicrobial proteins, peptides, and interferons; thus, innate host defenses may impact the ability of MV to infect from the apical surface of airway epithelia. Our data suggest that, in vivo, MV requires access to the basolateral surface of airway epithelia for efficient viral infection to occur. In addition, the limiting barrier to MV infection in the conducting airways of respiratory epithelia is not CD46 receptor expression on the apical surface. These results imply that pseudotyping recombinant lentivirus with Edmonston strain MV envelope glycoproteins to target the apical surface of airway epithelia is likely to be an inefficient strategy.
Acknowledgments
We thank Jennifer Nosakowski, Brent Quast, and April Hall for their excellent technical assistance. We are also grateful for the valued contributions of Kenneth Ratliff and the Central Microscopy Research Facility. Further, we thank Joe Zabner, Phil Karp, and Pary Weber for culturing the human epithelial cells. We thank Stanley Perlman for critically reviewing the manuscript.
We acknowledge the support of the Cell Culture Core and Cell Morphology Core, partially supported by the Cystic Fibrosis Foundation, NHLBI (PPG HL-51670), and the Center for Gene Therapy for Cystic Fibrosis (NIH P30 DK-54759). This work was supported by NIH grant RO1 HL-61460 (P.B.M.), PPG grant HL-51670 (P.B.M.), the Cystic Fibrosis Foundation, and the Mayo and Siebens Foundations. P.L.S. was supported by an institutional NRSA training grant (HL-07121).
REFERENCES
- 1.Anderson, R. D., R. E. Haskell, H. Xia, B. J. Roessler, and B. L. Davidson. 2000. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 7:1034-1038. [DOI] [PubMed] [Google Scholar]
- 2.Blau, D. M., and R. W. Compans. 1995. Entry and release of measles virus are polarized in epithelial cells. Virology 210:91-99. [DOI] [PubMed] [Google Scholar]
- 3.Blau, D. M., and R. W. Compans. 1997. Adaptation of measles virus to polarized epithelial cells: alterations in virus entry and release. Virology 231:281-289. [DOI] [PubMed] [Google Scholar]
- 4.Buchholz, C. J., D. Gerlier, A. Hu, T. Cathomen, M. K. Liszewski, J. P. Atkinson, and R. Cattaneo. 1996. Selective expression of a subset of measles virus receptor-competent CD46 isoforms in human brain. Virology 217:349-355. [DOI] [PubMed] [Google Scholar]
- 5.Castro, A. G., T. M. Hauser, B. G. Cocks, J. Abrams, S. Zurawski, T. Churakova, F. Zonin, D. Robinson, S. G. Tangye, G. Aversa, K. E. Nichols, J. E. de Vries, L. L. Lanier, and A. O'Garra. 1999. Molecular and functional characterization of mouse signaling lymphocytic activation molecule (SLAM): differential expression and responsiveness in Th1 and Th2 cells. J. Immunol. 163:5860-5870. [PubMed] [Google Scholar]
- 6.Compans, R. W. 1995. Virus entry and release in polarized epithelial cells. Curr. Top. Microbiol. Immunol. 202:209-219. [DOI] [PubMed] [Google Scholar]
- 7.Dorig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 8.Duprex, W. P., S. McQuaid, L. Hangartner, M. A. Billeter, and B. K. Rima. 1999. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J. Virol. 73:9568-9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duprex, W. P., S. McQuaid, B. Roscic-Mrkic, R. Cattaneo, C. McCallister, and B. K. Rima. 2000. In vitro and in vivo infection of neural cells by a recombinant measles virus expressing enhanced green fluorescent protein. J. Virol. 74:7972-7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Embree, J. E., and V. Chernick. 1998. Measles and giant cell pneumonia, p. 1005-1017. In V. Chernick and T. Boat (ed.), Kendig's disorders of the respiratory tract in children. The W. B. Saunders Co., Philadelphia, Pa.
- 11.Galbraith, S. E., A. Tiwari, M. D. Baron, B. T. Lund, T. Barrett, and S. L. Cosby. 1998. Morbillivirus downregulation of CD46. J. Virol. 72:10292-10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin, D. E. 2001. Measles virus, p. 1401-1441. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 13.Kurita, M., Y. Yanagi, T. Hara, S. Nagasawa, M. Matsumoto, and T. Seya. 1995. Human lymphocytes are more susceptible to measles virus than granulocytes, which is attributable to the phenotypic differences of their membrane cofactor protein (CD46). Immunol. Lett. 48:91-95. [DOI] [PubMed] [Google Scholar]
- 14.Liszewski, M. K., T. W. Post, and J. P. Atkinson. 1991. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 9:431-455. [DOI] [PubMed] [Google Scholar]
- 15.Maisner, A., H. Klenk, and G. Herrler. 1998. Polarized budding of measles virus is not determined by viral surface glycoproteins. J. Virol. 72:5276-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maisner, A., M. K. Liszewski, J. P. Atkinson, R. Schwartz-Albiez, and G. Herrier. 1996. Two different cytoplasmic tails direct isoforms of the membrane cofactor protein (CD46) to the basolateral surface of Madin-Darby canine kidney cells. J. Biol. Chem. 271:18853-18858. [DOI] [PubMed] [Google Scholar]
- 17.Mrkic, B., J. Pavlovic, T. Rülicke, P. Volpe, C. J. Buchholz, D. Hourcade, J. P. Atkinson, A. Aguzzi, and R. Cattaneo. 1998. Measles virus spread and pathogenesis in genetically modified mice. J. Virol. 72:7420-7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naim, H. Y., E. Ehler, and M. A. Billeter. 2000. Measles virus matrix protein specifies apical virus release and glycoprotein sorting in epithelial cells. EMBO J. 19:3576-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naniche, D., T. F. Wild, C. Rabourdin-Combe, and D. Gerlier. 1993. Measles virus haemagglutinin induces down-regulation of gp57/67, a molecule involved in virus binding. J. Gen. Virol. 74:1073-1079. [DOI] [PubMed] [Google Scholar]
- 20.Pickles, R. J., D. McCarty, H. Matsui, P. J. Hart, S. H. Randell, and R. C. Boucher. 1998. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J. Virol. 72:6014-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roscic-Mrkic, B., R. A. Schwendener, B. Odermatt, A. Zuniga, J. Pavlovic, M. A. Billeter, and R. Cattaneo. 2001. Roles of macrophages in measles virus infection of genetically modified mice. J. Virol. 75:3343-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakaguchi, M., Y. Yoshikawa, K. Yamanouchi, T. Sata, K. Nagashima, and K. Takeda. 1986. Growth of measles virus in epithelial and lymphoid tissues of cynomolgus monkeys. Microbiol. Immunol. 30:1067-1073. [DOI] [PubMed] [Google Scholar]
- 23.Schneider-Schaulies, J., L. M. Dunster, F. Kobune, B. Rima, and V. ter Meulen. 1995. Differential downregulation of CD46 by measles virus strains. J. Virol. 69:7257-7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893-897. [DOI] [PubMed] [Google Scholar]
- 25.Tucker, S. P., and R. W. Compans. 1993. Virus infection of polarized epithelial cells. Adv. Virus Res. 42:187-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walters, R. W., T. Grunst, J. M. Bergelson, R. W. Finberg, M. J. Welsh, and J. Zabner. 1999. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J. Biol. Chem. 274:10219-10226. [DOI] [PubMed] [Google Scholar]
- 27.Wang, G., B. L. Davidson, P. Melchert, V. A. Slepushkin, H. H. G. van Es, M. Bodner, D. J. Jolly, and P. B. McCray, Jr. 1998. Influence of cell polarity on retrovirus-mediated gene transfer to differentiated human airway epithelia. J. Virol. 72:9818-9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, G., C. Deering, M. Macke, J. Shao, R. Burns, D. Blau, K. Holmes, B. Davidson, S. Perlman, and P. B. McCray, Jr. 2000. Human coronavirus 229E infects differentiated airway epithelia from the apical surface. J. Virol. 74:9234-9239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, G., P. L. Sinn, and P. B. McCray, Jr. 2000. Development of retroviral vectors for gene transfer to airway epithelia. Curr. Opin. Mol. Ther. 2:497-506. [PubMed] [Google Scholar]
- 30.Zabner, J., B. G. Zeiher, E. Friedman, and M. J. Welsh. 1996. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J. Virol. 70:6994-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]