Abstract
An acute infection with lymphocytic choriomeningitis virus (LCMV) is efficiently controlled by the cytotoxic-T-cell (CTL) response of the host, and LCMV titers in the spleen and peripheral solid organs usually fall sharply after day 4 to 6 postinfection. Surprisingly, infection of immunodeficient recombination-activating gene 2-deficient (RAG2−/−) mice with 5 × 102 PFU of LCMV-WE causes about 80-fold-lower LCMV titers in the spleen on day 4 postinfection compared with C57BL/6 control mice. This could not be attributed to NK cell activity, since common gamma-chain-deficient RAG2−/− mice lacking NK cells show low LCMV titers comparable to those for RAG2−/− mice. Furthermore, the reduced early LCMV production in spleens could not be explained by an enhanced gamma interferon production in RAG2−/− mice. Analysis of mutant mice exhibiting various defects in the splenic microarchitecture, including (i) tumor necrosis factor alpha-negative (TNF-α−/−), lymphotoxin alpha-negative (LTα−/−), B-cell-deficient μMT mice, (ii) immunoglobulin M-negative mice, and (iii) RAG−/− mice reconstituted with wild-type versus TNF-α−/− LTα−/− B cells, revealed a clear correlation between an intact splenic marginal zone, rapid early replication of LCMV in the spleen, and efficient CTL induction. These results suggest that by the preferential infection of the highly organized splenic microarchitecture, LCMV seems to successfully exploit one of the key elements in the chain of the adaptive immune system. Not only does the early tropism of LCMV for the splenic marginal zone trigger a potent immune response, but at the same time the marginal zone may also become a target of early CTL-mediated immunopathology that impairs immune responsiveness.
Coevolution of the noncytopathic arenavirus lymphocytic choriomeningitis virus (LCMV) (23) and of mice, its natural hosts, has resulted in exquisitely balanced mechanisms that ensure the survival of both the virus and the host. Intrauterine transmission and perinatal infection of the host lead to high-level persistence of the virus due to the induction of immune unresponsiveness in the host (8). Alternatively, if an immunocompetent host is infected with a low dose of LCMV, the virus proliferates and expands before the potent immune effector mechanisms of the host control LCMV; in this case the virus apparently is never cleared completely (18). Early and efficient virus elimination reduces T-cell-mediated immunopathology in the host and reduces or prevents further horizontal spread of the virus.
During generalized bacterial or viral infections, infectious agents are usually rapidly filtered out from the circulation in secondary lymphoid organs, particularly the spleen (22, 28). Within the highly organized microarchitecture of lymph nodes and spleen, cell-cell interactions between components of the innate and the adaptive immune systems generate an efficient specific immune response (19, 22). In immunocompetent mice an acute infection with LCMV is efficiently controlled by the massive induction of virus-specific CD8 T cells, which are able to control the initial burst of virus production that peaks at around day 4 to 5 after intravenous (i.v.) infection (6, 18, 27). The tropism of LCMV for cells within the immune system, including macrophages (25), marginal zone macrophages (30), dendritic cells (1, 36), and, rarely, lymphocytes (33, 39), is an important factor for this early T-cell response. Several factors influencing this tropism have been analyzed, and they include α-dystroglycan (7, 36, 38), natural antibodies (28), Fc and complement receptors (29), and perhaps even neutralizing receptor antibodies on specific B cells (33). The various mechanisms of tropism may be additive rather than mutually exclusive. Most LCMV strains can be assigned to the category of rapidly replicating, hepatotropic, immunosuppressive strains with a tendency to cause persistent infection even in immunocompetent mice (strains WE [type strain], Traub, and Cl13), in contrast to strains with lesser activity (type strain Armstrong). One probable link between tropism and these two general traits seems to be that the CD8 T-cell-mediated specific destruction of LCMV-infected cells such as macrophages and dendritic cells in secondary lymphoid organs of immunocompetent mice may cause a transient general immunosuppression; this is due both to the loss of antigen-presenting cells and to disruption of their microarchitecture (5, 42).
While monitoring several strains of mice exhibiting various mutations causing immunodeficiency, we found that the initial virus production after infection with a low dose (500 PFU) of LCMV is impaired in some mice. The analysis presented here indicates an important role of the marginal zone and of the splenic microarchitecture in early LCMV tropism and host-virus balance.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were originally obtained from the Institut für Labortierkunde, University of Zurich, Zurich, Switzerland. C57BL/6 recombination-activating gene 2-deficient (RAG2−/−) (37) mice and B6/129 tumor necrosis factor alpha-negative, lymphotoxin alpha-negative (TNF-α−/− LTα−/−) mice (13) were generously provided by E. Wagner (Basel, Switzerland) and H. P. Eugster (Zurich, Switzerland), respectively. B6/129 tnftm-GKl (TNF-α−/−) (32) and B6/129 ltatm-Dch (LTα−/−) (11) mice were purchased from Jackson Laboratory, Bar Harbor, Maine. All of these mouse strains are currently reared and bred in the specific-pathogen-free facility of the Medical School, University of Bern. μMT and RAG1−/− mice were obtained from the Institut für Labortierkunde, University of Zurich, Zurich, Switzerland. Immunoglobulin M-negative (IgM−/−) mice were provided by F. Brombacher and H. Mossmann, Max-Planck-Institute for Immunobiology, Freiburg, Germany. All of these mouse strains were kept under specific-pathogen-free conditions.
LCMV and infections.
A stock of LCMV strain WE was generously provided by S. Oehen, Zurich, Switzerland, and was subsequently propagated by infection of the L929 fibroblast cell line and harvesting of virus in the supernatant. For infections, 500 PFU of LCMV in a volume of 100 μl of minimal essential medium (MEM)-2% fetal calf serum (FCS) was injected i.v. into the tail vein. LCMV carrier mice were obtained by injecting 104 PFU of LCMV in a volume of 50 μl of MEM-2% FCS intraperitoneally into newborn C57BL/6 mice (within 24 h after birth).
L. monocytogenes culture and infection.
Listeria monocytogenes was originally obtained from B. Blanden (Canberra, Australia). It was cultured in Trypticase soy broth (BBL Microbiology Systems, Cockeysville, Md.), and overnight cultures were titrated on tryptose blood agar plates (Difco Laboratories, Detroit, Mich.). For injection, the original culture was diluted in phosphate-buffered saline (PBS) to inject 5 × 103 CFU in 500 μl.
Tissue preparation.
For LCMV plaque assays, the weight of tissue samples was first determined. Thereafter, tissues were placed in 1 ml of MEM-2% FCS and frozen at −80°C before the LCMV plaque assay was performed (see below). For cryo blocks, tissue samples were placed in Tissue-Tek O.C.T. compound (Sakura Finetek Europe, Zoeterwoude, The Netherlands), frozen, and stored at −80°C. Five-micrometer cryostat sections were placed on poly-l-lysine-coated glass slides (Polysine; Menzel Gläser, Braunschweig, Germany). For in situ hybridization, slides were fixed in PBS-buffered 4% paraformaldehyde for 20 min; washed sequentially with 3× PBS, 1× PBS, and H2O; and dehydrated through a graded ethanol series. For immunohistochemistry, slides were fixed for 30 s in acetone, air dried, and stored at 4°C for up to several weeks.
LCMV plaque assay.
Virus titers of infected organs were determined in a virus plaque assay as previously described (2). Briefly, tissue samples (frozen at −80°C in MEM-2% FCS) were thawed, homogenized, and mixed with cells from the fibroblast cell line MC57G in 10-fold dilution steps in a 24-well plate (TPP, Trasadingen, Switzerland). The virus was allowed to infect MC57G cells for 4 h. Thereafter, methylcellulose (Methocel; Sigma, St. Louis, Mo.) was diluted 1:2 in 2× Dulbecco modified Eagle medium and added to inhibit budding of virus. After 48 h at 37°C and 5% CO2, plaques of virus-infected cell clusters were detected immunohistochemically using the LCMV-specific VL-4 monoclonal antibody. Peroxidase-coupled anti-rat Ig antibody (DAKO A/S, Glostrup, Denmark) was used as a second step, and a color reaction was performed using o-phenylenediamine dihydrochloride (Sigma) according to the manufacturer's instructions.
35S-labeled RNA probe preparation and in situ hybridization.
The entire coding region (1.6 kb) of the LCMV glycoprotein (GP) cDNA (generously provided by H. Pircher, Freiburg, Germany) was cloned into the expression vector pGEM-1 and used to prepare 35S-labeled RNA probe by using SP6 RNA polymerase reactions, as previously described (26). Cryostat sections and sorted cells were hybridized with a probe concentration of 2 × 105 cpm per μl of hybridization solution as previously described (2). After hybridization and washing off of nonhybridized probe, slides were dipped into NTB-2 emulsion (Eastman-Kodak, New Haven, Conn.) 1:2 diluted in 800 mM ammonium acetate and exposed in the dark at 4°C for 1 week. Slides were developed with Kodak PL 12 developer for 5 min and fixed with Kodak fixer for 8 min. Counterstaining was performed in hematoxylin for 1 min, and the blue color of the counterstaining was normalized by incubating slides for 10 min in 1 mM Na2CO3.
Antibodies.
Anti-CD11c (N418; generously provided by M. Kosco-Vilbois, Geneva, Switzerland), anti-differentiated macrophages (F4/80), anti-MAdCAM-1 (R3-1; generously provided by B. Holzmann, Munich, Germany), anti-CD4 (GK1.5), anti-CD8α (53-6.7), anti-Thy1.2 (53-2.1), and anti-CD11b/Mac-1 (M1/70) (all originally obtained from the American Type Culture Collection) were protein G purified from hybridoma supernatants. Anti-marginal zone macrophages (ERTR-9; a kind gift from G. Kraal, Amsterdam, The Netherlands) was used as the hybridoma supernatant. Anti-MOMA-1 (MCA947; Serotec, Oxford, United Kingdom) and anti-CD19 (1D3; Pharmingen) were purchased from the distributors. The N418, F4/80, and R3-1 antibodies were also biotinylated according to standard protocols. Anti-CD3 (145-2C11) was produced in a Technomouse (Integra Biosciences, Wallisellen, Switzerland) and conjugated with fluorescein isothiocyanate according to standard protocols.
Immunohistochemistry.
Slides were postfixed in acetone for 10 min and air dried. After rehydration in Tris buffered saline (pH 7.5) containing 10% horse serum, slides were incubated with the appropriate primary monoclonal antibody for 40 min. When peroxidase was used, endogenous peroxidase was quenched by incubation of slides for 20 min in methanol plus 0.3% H2O2 before the second-step incubation. Second-step incubations were performed with anti-rat Ig (DAKO) for 30 min, followed by rat alkaline phosphatase-anti-alkaline-phosphatase (DAKO) according to the manufacturer's instructions. When biotinylated primary antibodies were used, second-step incubations were performed with premixed avidin-biotin complex and avidin-peroxidase (DAKO), according to the manufacturer's instructions. Color reactions were performed with naphthol-as-biphosphate and new fuchsin (Sigma) to detect alkaline phosphatase and with 3,3"-diaminobenzidine (Fluka Chemie AG, Buchs, Switzerland) to detect peroxidase. Slides were counterstained as described above for the in situ hybridizations.
Immunohistochemistry and in situ hybridization double labelings.
For double labelings, slides were first processed for immunohistochemistry using alkaline phosphatase as described above with the following modifications. All work was performed using RNase-free reagents. RNase inhibitor (Roche Diagnostics, Rotkreuz, Switzerland) was added at 50 to 100 U/ml to antibodies and second-step reagents. After the alkaline phosphatase substrate reaction, slides were immediately fixed in freshly prepared PBS-buffered 4% paraformaldehyde as described above and were further processed for in situ hybridization.
B-cell preparation and adoptive transfer.
B cells were isolated from spleens of C57BL/6 RAG2+/− mice. Splenocytes were released by grinding spleens between frosted ends of microscopic slides. Erythrocytes were lysed by osmotic shock treatment for 15 s, and after washing, cells were resuspended in 2 ml of 37°C prewarmed Iscove's modified Dulbecco medium (IMDM) plus 5% FCS. Cells were loaded onto a nylon wool (Robbins Scientific, Sunnyvale, Calif.) column; preparation and loading of the column were performed according to instructions provided by the manufacturer. After incubation of the column for 45 min in an incubator at 37°C and 5% CO2, nonadherent cells (T cells and NK cells) were flushed out with 2 to 3 column volumes of 37°C IMDM-5% FCS, with the flow rate controlled by an attached 22-gauge needle. Adherent cells (B cells and monocytes) were released from the wool by repeated washing and squeezing out of the wool in ice-cold cytotoxicity medium (Cedarlane, Hornby, Ontario, Canada). Cells were subsequently labeled on ice for 15 min with a cocktail of 0.25 μg each of anti-CD4, anti-CD8α, anti-Thy1.2, anti-CD11b, and F4/80 antibodies per 106 cells. Labeled cells were washed and resuspended at 950 μl of cytotoxicity medium per 107 cells, and 50 μl of rabbit LowTox complement (Cedarlane) per 107 cells was added. Cells were placed for 30 min in an incubator at 37°C and 5% CO2. Afterwards cells were underlaid with Lympholyte-M (Cedarlane) and centrifuged at room temperature at 900 × g for 15 min. Cells in the interphase were harvested, and an aliquot was stained with anti-CD3-fluorescein isothiocyanate and anti-CD19-phycoerythrin. If CD19+ cells were ≤95% and/or CD3+ cells were ≥2%, as determined by fluorescence-activated cell sorter analysis, complement lysis was repeated. Otherwise, cells were resuspended at 108 cells/ml of MEM-2% FCS, and 100 μl was injected i.v. into RAG2−/− recipients.
RESULTS
Reduced LCMV production in the spleens of mice with disrupted splenic microarchitecture.
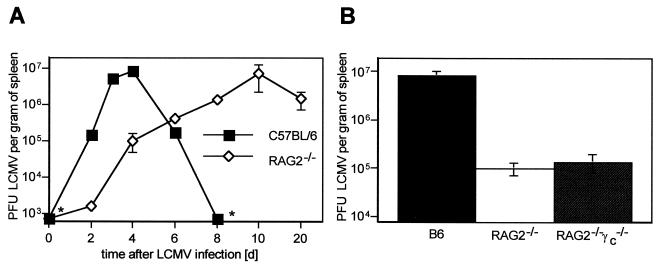
In an immunocompetent host an acute infection with LCMV is efficiently controlled by virus-specific cytotoxic T cells (CTLs). Hence, it is to be expected that RAG2−/− mice lacking functional T and B cells will exhibit rapid and continued virus replication when infected with LCMV (LCMV-WE; 500 PFU i.v.). However, when immunodeficient RAG2−/− mice were examined on day 6 after LCMV-WE infection, the amount of infectious virus recovered from the spleens slowly increased but was approximately 20-fold lower than that in the spleens of infected C57BL/6 mice at the peak of splenic virus production on day 4. On day 4, when in immunocompetent mice virus-specific CTL activity is not yet measurable, splenic virus production in C57BL/6 mice was approximately 80-fold higher than that in immunodeficient RAG2−/− mice (Fig. 1A). To assess whether enhanced activity of NK cells in RAG2−/− mice caused the delayed early LCMV production, RAG2−/− common cytokine receptor gamma-chain-negative (γc−/−) double mutant mice (9, 12), which are deficient in B, T, and NK cells, were infected with 500 PFU of LCMV-WE. Almost identical LCMV titers were detected on day 4 postinfection in RAG2−/− γc−/− mice and in RAG2−/− mice (Fig. 1B); thus, the absence of NK cells in these mice did not enhance the low LCMV production in the spleen.
FIG. 1.
Early LCMV production is reduced in the spleens of RAG2−/− mice. (A) Kinetics of LCMV-WE titers in the spleens of C57BL/6 mice and RAG2−/− mice following i.v. infection with 500 PFU of LCMV-WE. d, days. (B) Splenic LCMV titers on day 4 after LCMV-WE infection (500 PFU, i.v.) in C57BL/6, RAG2−/−, and RAG2−/− γc−/− mice (mean values for three to five mice per time point ± standard errors of the means; asterisks indicate levels below the detection limit).
Early splenic LCMV production is also not affected by increased splenic gamma interferon production as demonstrated by identical LCMV titers recovered on day 4 after LCMV infection from the spleens of otherwise untreated C57BL/6 mice and of day 6 L. monocytogenes-infected C57BL/6 mice (2. 6 × 106 ± 0.6 × 106 and 2.4 × 106 ± 0.8 × 106 PFU/spleen, respectively). The frequency of gamma interferon-producing cells in the spleen has been previously found to peak at around days 7 and 8 postinfection with L. monocytogenes (16).
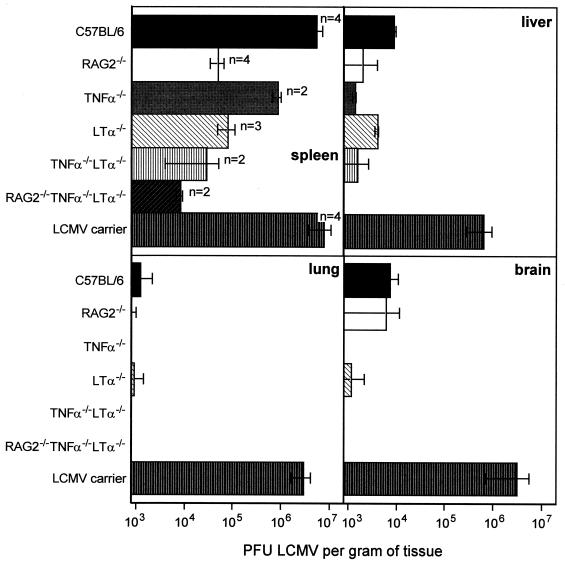
In the absence of functional B and T cells, the splenic architecture is greatly impaired in RAG2−/− and RAG2−/− γc−/− mice. In particular, the splenic marginal zone, which had been previously shown to be instrumental for the clearance of blood-borne LCMV (34), is poorly developed in RAG2−/− mice and in a number of other mutant mice. In order to assess how disruption of the splenic microarchitecture might affect early LCMV replication in the spleen, RAG2−/−, TNF-α−/−, LTα−/−, TNF-α−/− LTα−/−, and RAG2−/− TNF-α−/− LTα−/− mice were assessed for LCMV titers in various organs on day 4 postinfection. Neonatally infected C57BL/6 mice (LCMV carrier mice) were used as a positive control. In contrast to the case for LCMV carrier mice, no infectious virus was detected in the blood of all mice tested on day 4 postinfection with 500 PFU i.v. (data not shown). As shown in Fig. 2, on day 4 after LCMV infection the spleen represents the main LCMV-producing organ in all mouse strains analyzed. When mouse strains representing variable degrees of disrupted splenic microarchitecture were compared, a clear correlation between the reduction in the amount of virus recovered from the spleen and the extent of splenic microarchitecture disruption was found (Fig. 2): whereas in TNF-α−/− mice the splenic virus production was reduced by a factor of 10 compared with C57BL/6 mice, markedly lower virus titers were determined in the spleens of day 4 infected LTα−/− mice, TNF-α−/− LTα−/− mice, and particularly RAG2−/− TNF-α−/− LTα−/− mice. The disrupted splenic microarchitecture, however, did not lead to preferential spreading of LCMV to other susceptible organs, since the amount of virus recovered from liver, lung, or brain of day 4 infected mutant mice was always lower than that recovered from the respective organs of C57BL/6 mice (Fig. 2).
FIG. 2.
LCMV titers in the spleen, liver, lung, and brain in mutant and wild-type mice. C57BL/6, RAG2−/−, TNF-α−/−, LTα−/−, TNF-α−/− LTα−/−, and RAG2−/− TNF-α−/− LTα−/− were infected i.v. with a low dose (500 PFU) of LCMV-WE. Four days later LCMV in the indicated organs was titrated. For comparison, LCMV titers in organs from neonatally LCMV-infected C57BL/6 mice (LCMV carrier mice) were also assessed. Bars represent mean values from n animals. Error bars show the range (for n = 2) or standard error of the mean (for n > 2).
In mice with disorganized splenic architecture, fewer spleen cells are infected upon low-dose LCMV infection.
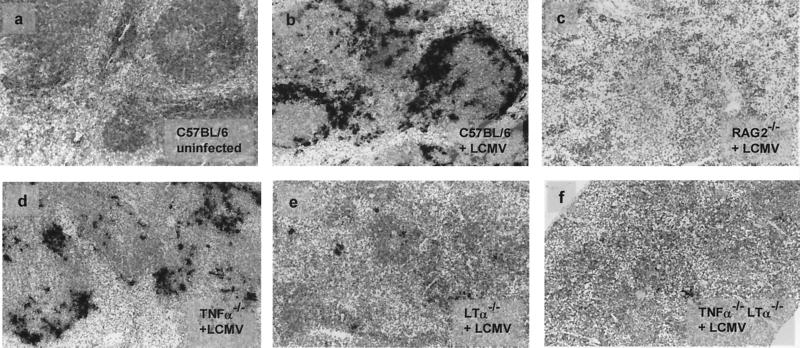
The reduced amount of virus recovered from the spleens of mutant mice with disrupted splenic microarchitecture can be ascribed to a smaller amount of virus produced per infected cell and/or reduced numbers of infected cells. To discriminate between these possibilities, in situ hybridizations were performed on splenic tissue sections. Splenic tissue sections from C57BL/6, RAG2−/−, TNF-α−/−, LTα−/−, and TNF-α−/− LTα−/− mice hybridized in situ with LCMV GP mRNA-specific probes showed greatly reduced numbers of LCMV-infected cells on day 4 (Fig. 3). Morphometric analyses revealed an approximately 25-fold-smaller area covered by the in situ hybridization signal on LTα−/− spleen sections and a 100-fold-smaller area in RAG2−/− and TNF-α−/− LTα−/− mice compared with LCMV-infected C57BL/6 spleens. This reduction in the number of LCMV-infected cells correlated with the smaller amount of virus recovered from the spleens of mouse strains with disrupted splenic microarchitecture (Table 1). In situ hybridizations with LCMV nucleoprotein-specific probes revealed expression levels and patterns identical to those for LCMV GP expression (data not shown). This is compatible with earlier reports indicating that the observed time-dependent down-modulation of LCMV GP expression (31) is due to posttranscriptional regulation (14).
FIG. 3.
Decreased LCMV GP-specific in situ hybridization signals in spleens of mutant mice. C57BL/6, RAG2−/−, TNF-α−/−, LTα−/−, and TNF-α−/− LTα−/− mice were infected with 500 PFU of LCMV-WE i.v., and spleens were harvested 4 days later. Cryosections of spleens were used for hybridization with an RNA probe specific for LCMV GP mRNA. Representative frozen tissue sections of a noninfected C57BL/6 mouse (a) and day 4 infected C57BL/6 (b), RAG2−/− (c), TNF-α−/− (d), LTα−/− (e), and TNF-α−/− LTα−/− (f) mice, hybridized with an LCMV GP-specific RNA probe (mRNA-specific cells, black silver grains), are shown (magnification, ×125).
TABLE 1.
Morphometric analysis of spleen sections hybridized with an LCMV GP-specific mRNA probe and amounts of virus isolated from the corresponding spleensa
| Mouse strainb | % Black area of total tissue | LCMV CPFU/g of spleen |
|---|---|---|
| C57BL/6, uninfected | 0 | 0 |
| C57BL/6 | 22.2 | 5.4 × 106 |
| RAG2−/− | 0.2 | 4.7 × 104 |
| TNF-α−/− | 9.0 | 9.8 × 105 |
| LTα−/− | 0.9 | 7.4 × 104 |
| TNF-α−/− LTα−/− | 0.2 | 3.7 × 104 |
Longitudinally cut spleen tissue samples from individual mice were frozen for subsequent in situ hybridization or used for LCMV plaque assays. The fraction of tissue area covered by black silver grains upon hybridization with an LCMV GP-specific RNA probe was determined by computer-assisted morphometric analysis.
Samples were taken 4 days after LCMV infection unless otherwise noted.
Spleen cells infected early with LCMV are preferentially located in the marginal zone.
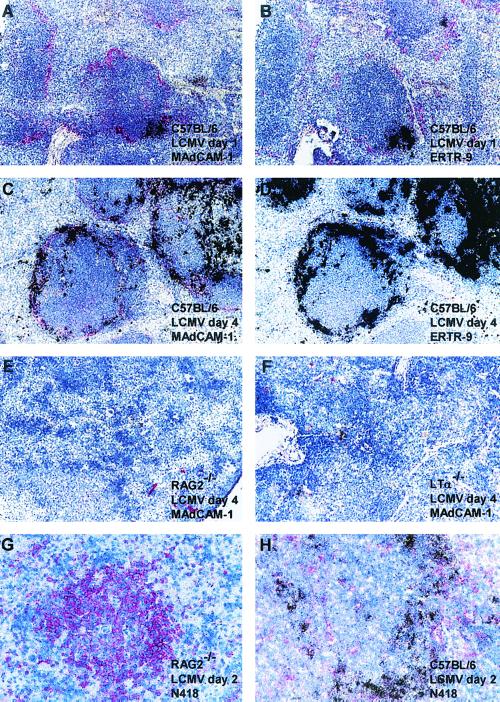
To obtain more information on the structural requirements and the cell subsets required for a potent early LCMV propagation, serial sections of the spleens from LCMV-infected mice were subjected to immunohistochemical analysis using monoclonal antibodies against CD11c (N418), CD11b (M1/70), differentiated macrophages (F4/80), marginal zone macrophages (ERTR-9), metallophilic macrophages (MOMA-1), and the mucosal vascular addressin MAdCAM-1 and in situ hybridization for LCMV GP mRNA. At 24 h after i.v. infection with 500 PFU of LCMV-WE, virus-infected cells were easily detected in the splenic marginal zones of C57BL/6 mice (Fig. 4). The localization of LCMV-infected splenocytes as assessed by in situ hybridization was associated with a small fraction of MAdCAM-1-positive reticular endothelial cells and ERTR-9-positive macrophages in the splenic marginal zone (Fig. 4A and B). Unlike that of MAdCAM-1, the staining intensity of ERTR-9 decreased rapidly after 24 h post-LCMV infection and was virtually undetectable by day 4 (Fig. 4C and D). This made it difficult to assess the initial ERTR-9 phenotype of all LCMV-infected cells in the splenic marginal zone (Fig. 4C and D). Apart from scattered cells of undetermined phenotype, MAdCAM-1 is virtually undetectable in the spleens of RAG2−/− and LTα−/− mice 4 days after LCMV infection (Fig. 4E and F, respectively). The reduced early LCMV production in the spleens of RAG2−/− mice cannot be attributed to the absence of CD11c-positive dendritic cells, since numerous CD11c-positive dendritic cells were seen in the spleens of C57BL/6 and RAG2−/− mice; these cells, however, remained uninfected in RAG2−/− mice and generally also in C57BL/6 mice during the first 48 h of infection (Fig. 4G and H). Early after infection, the presence of LCMV-WE-infected cells in the splenic marginal zones of C57BL/6 mice correlated mostly with the localization of metallophilic macrophages (MOMA-1+). However, in mutant mice with disrupted splenic microarchitecture, no correlation was observed between the localizations of the few LCMV-infected cells and the numerous MOMA-1+ cells scattered throughout the entire spleen. No evidence for a correlation between the extent and localization of LCMV-WE-infected cells and the staining patterns for CD11b (M1/70) or differentiated macrophages (F4/80) was observed in the early stage of splenic LCMV infection at 24 h or 4 days postinfection (data not shown).
FIG. 4.
Presence of MAdCAM-1+ reticular endothelial cells in the splenic marginal zone and of ERTR-9+ marginal zone macrophages but lack (or much less) of N418-positive dendritic cells is associated with extensive early virus production in the spleen following LCMV-WE infection (500 PFU, i.v.). Representative examples of spleen sections from C57BL/6 mice at 24 h postinfection (5 × 103 PFU of LCMV per g of spleen) (A and B) and 4 days postinfection (8.2 × 106 PFU of LCMV per g of spleen) (C and D), from a RAG2−/− mouse (7 × 104 PFU of LCMV per g of spleen) (E) and an LTα−/− mouse (8 × 104 PFU of LCMV per g of spleen) (F) at 4 days postinfection, and from a RAG2−/− mouse (1.6 × 103 PFU of LCMV per g of spleen) (G) and a C57BL/6 mouse (1.5 × 105 PFU of LCMV per g of spleen) (H) at 48 h postinfection, with immunostaining for MAdCAM-1 (A, C, E, and F), ERTR-9 (B and D), or CD11c (N418) (G and H) (positive reaction in red), were hybridized in situ with 35S-labeled RNA probes specific for LCMV GP (mRNA-positive cells, black silver grains). Magnifications, ×125 (A to D) and ×250 (E to H).
Reconstitution of the splenic architecture in RAG2−/− mice by adoptive transfer of B cells leads to increased LCMV production.
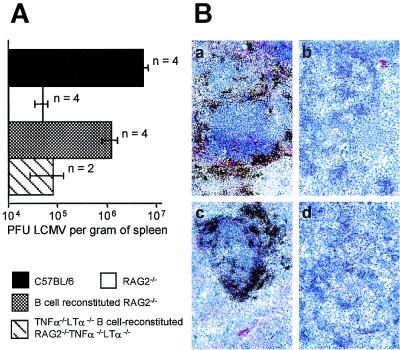
Previous work with lymphocyte-reconstituted immunodeficient SCID mice (17) and RAG1−/− mice (15) indicated a crucial role of B-cell-derived LTα in establishing the splenic microarchitecture. Notably, adoptively transferred B lymphocytes were able to induce MAdCAM-1 expression on marginal zone reticular endothelial cells (10), and reconstitution of RAG1−/−, SCID, and μMT mice with fetal liver cells from T-cell receptor α−/− mice resulted in the formation of follicular dendritic cell-containing splenic germinal centers (21). Hence, we assessed the consequences for splenic LCMV production of an adoptive transfer of B cells into RAG2−/− mice. As seen in Fig. 5, the reappearance of MAdCAM-1-positive cells in the marginal zones of the spleens from B-cell-reconstituted RAG2−/− mice coincided with an increased frequency of LCMV-infected cells and increased splenic LCMV production that almost equaled values found in day 4 infected C57BL/6 mice. Reconstitution of RAG2−/− mice with B cells in the absence of TNF-α and LTα failed to restore the splenic virus production. Again, the low splenic LCMV production by TNF-α−/− LTα−/− B-cell-reconstituted RAG2−/− TNF-α−/− LTα−/− mice correlated with the complete absence of well-organized marginal zones containing MAdCAM-1-expressing cells (Fig. 5).
FIG. 5.
Reconstitution of the splenic follicular microarchitecture with wild-type B cells restores impaired, early LCMV production in RAG2−/− mice. (A) Various mice or chimeras were infected with 500 PFU of LCMV-WE i.v., and 4 days later LCMV titers were determined. Bars represent mean values for n animals. Error bars show the range (for n = 2) or standard error of the mean (for n = 4). (B) Representative examples of immunohistochemical analysis with MAdCAM-1+ antibodies and LCMV GP-specific in situ hybridization of C57BL/6 (a), RAG2−/− (b), B-cell-reconstituted RAG2−/− (c), and TNF-α−/− LTα−/− B-cell-reconstituted RAG2−/− TNF-α−/− LTα−/− (d) mice at 4 days after LCMV-WE infection. Magnification, ×125.
Early splenic LCMV production after low-dose i.v. infection depends on the presence of an intact splenic architecture but not necessarily on the presence of serum IgM antibodies.
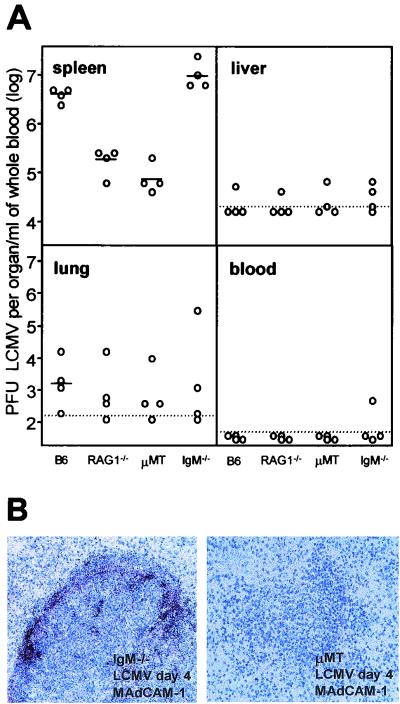
Natural antibodies have recently been found to be instrumental in trapping high doses (106 to 107 infectious units) of circulating virus (vesicular stomatitis virus or LCMV) and bacteria (L. monocytogenes) in the marginal zone of the spleen (28). Hence, one might speculate that a reduced splenic virus production in RAG2−/− mice may be due to inefficient virus trapping in the absence of LCMV-binding natural IgM antibodies in RAG2−/− mice. To determine the relative contributions of the splenic microarchitecture and of IgM natural antibodies to early LCMV production, IgM−/− mice (24) and μMT mice (20) were infected with 500 PFU of LCMV-WE. Due to the complete absence of B cells, μMT mice show a vastly impaired splenic microarchitecture. In contrast, IgM−/− mice, which lack natural serum IgM antibodies, still have IgD and are able to mount specific B-cell responses and isotype class switch to all other Ig classes following immunization (24). IgM−/− mice show a microarchitecture in the spleen that is within normal ranges. Four days after i.v. infection with 500 PFU of LCMV-WE, virus production in the spleens of RAG1−/− mice and that in the spleens of B-cell-deficient μMT mice were comparably low. In IgM−/− mice, however, the amount of virus recovered from the spleens equaled, or even exceeded, values obtained from the spleens of day 4 infected C57BL/6 mice. This indicates that upon low-dose infection with LCMV, trapping of the virus in the spleen occurs independently from the presence of natural serum IgM antibodies. Alternatively, it cannot be excluded completely that natural antibodies of other classes may mediate splenic trapping of LCMV in IgM−/− mice, although in the presence of IgM natural antibodies, targeting of circulating bacteria and viruses by non-IgM isotype natural antibodies seems to be minimal (28).
On day 4, virus titers determined in the liver, lung, and blood of infected mice were comparable in the four different mouse strains analyzed (Fig. 6A). Reduced LCMV titers in the spleens of μMT mice compared with the spleens of IgM−/− mice correlated well with the absence of a MAdCAM-1+ marginal zone (Fig. 6B). The impaired LCMV replication in the spleens of low-dose-infected μMT mice could not be significantly increased by the injection of normal C57BL/6 serum prior to infection with LCMV, and the amount of LCMV recovered from the brain, lung, liver, and blood on day 4 was not significantly influenced by the transferred serum (data not shown). The differential splenic virus production in IgM−/− mice and μMT mice was already apparent on day 1 after LCMV infection, when LCMV titers in the spleens of IgM−/− mice were similar to, or even higher than, those found in the spleens of day 1 infected C57BL/6 mice (1.2 × 104 ± 0.9 × 104 PFU and 3.3 × 103 ± 1.4 × 103 PFU per spleen, respectively), whereas at 24 h after LCMV infection virus was below detectable levels in the spleens of μMT mice. These results indicate that the absence of natural antibodies of the IgM class in serum does not affect trapping of low doses of LCMV and early virus replication in the spleen if an intact microarchitecture is available.
FIG. 6.
Early splenic LCMV production after low-dose infection i.v. with 5 × 102 PFU of LCMV-WE depends on an intact splenic architecture but not on IgM in serum. (A) Four mice of each indicated strain were infected with 500 PFU of LCMV i.v., and the LCMV-WE titers in the indicated organs from individual mice are shown. LCMV detection limits for each organ are indicated as dotted lines. (B) Spleen sections from an IgM−/− C57BL/6 mouse (with no serum IgM but all other Ig classes) (left) and a μMT mouse (no serum Ig) at 4 days postinfection, immunostained for MAdCAM-1 (positive reaction in red) and hybridized in situ with 35S-labeled RNA probes specific for LCMV GP. Magnification, ×250.
DISCUSSION
The splenic marginal zone is a dynamic structure formed by sessile reticular endothelial cells that can express the mucosal addressin MAdCAM-1 and metallophilic and marginal zone macrophages (22). Blood-borne B and T cells are continuously migrating through this network of antigen-presenting cells, strategically located for the removal, processing, and presentation of circulating infectious agents. Splenic marginal zone macrophages have been previously shown to be an essential cellular component in the clearance and retention of LCMV in the spleen (34). The present study demonstrates that an intact structure of the marginal zone is also instrumental for early rapid virus production in the spleen, since mutant mice with disrupted marginal zones showed dramatically reduced early LCMV replication compared to wild-type mice. Intriguingly, transfer of TNF- and LT-competent B cells into RAG2−/− mice led to the appearance of a defined marginal zone and greatly enhanced early virus production in the spleen, whereas reconstitution of RAG2−/− mice with TNF- and LT-deficient B cells failed to restore the formation of a defined marginal zone and to increase early LCMV production (Fig. 5) despite the presence of numerous B cells throughout the entire spleen in these mice (data not shown).
α-Dystroglycan has been defined as one of perhaps several LCMV, if not arenavirus, receptors (7). The immunosuppressive LCMV strains exhibit a higher avidity for α-dystroglycan than less immunosuppressive strains (38). Besides these virus strain dependencies, the initial dose of infection, the degree of immunocompetence of the host, and the route of infection are also important in defining the virus-host balance away from or towards immunopathology, immunosuppression, and persistence. A major difficulty has been, and still is, that important parameters are always linked, not only on the virus side but also on the host immunity side. For example there are no slowly replicating, persistent LCMV strains which induce immunosuppression in the host, and there is no LCMV that does not initially replicate predominantly in the marginal zone, but only in dendritic cells or in red pulp macrophages (30, 36, 38). In addition, the formation of a marginal zone, antibody formation, and functional TNF-LT systems are all interrelated and cannot be analyzed separately in short-term reconstitution experiments. In general, this situation renders a discussion of the relative importance of the various parameters still difficult and inconclusive. What seems evident is that the marginal zone is an important site of early LCMV replication and correlates with early initiation of a T-cell immune response (28). It remains to be determined whether the observed preferential virus production in the marginal zone of the spleen can be attributed solely to the strategic location of the early virus-producing cells at this predominant site of antigen filtration or whether it may also be due to an enhanced expression of the cellular receptor(s) for LCMV-WE, including α-dystroglycan, on these cells.
Early infection of the cellular constituents of the marginal zone allows an optimal priming of the host's immune system with the antigen present at sufficient concentrations at the primary inductive site of an immune response (41), thus ensuring the efficient elimination of the virus and the establishment of memory. The observations that LTβ−/− mice infected with LCMV-ARM mounted severely impaired virus-specific CTL responses (3) and that LTα−/− mice infected with a low dose (500 PFU) of LCMV were not able to induce an efficient CTL response capable of clearing the virus (data not shown) support this notion.
The comparable LCMV propagation observed after infection of RAG2−/− and NK cell-deficient RAG2−/− γc−/− double mutant mice indicates that NK cells are unable to control LCMV production in the absence of T and B cells and hence cannot be considered responsible for the low virus titers observed in infected RAG2−/− mice. These results are compatible with earlier results demonstrating that augmented rejection of adoptively transferred, LCMV-infected target cells by nonimmune mice is largely independent of the presence of NK cells (4). A similar requirement for an intact splenic microarchitecture for enhanced immunogenicity and infectivity has been previously noted in infection with LCMV plus vesicular stomatitis virus (30) or scrapie infection in mice (21).
The comparative analysis of in situ hybridizations for the detection of LCMV-specific transcripts and the virus titers isolated from infected organs demonstrated that the reduced virus replication in mouse strains with impaired splenic microarchitecture is due to a lower frequency of LCMV-infected cells rather than to a reduced virus production by infected cells. The lower frequency of infected cells may be due to reduced expression of cellular receptors for LCMV on the potential virus-producing cells, reduced numbers of target cells that support early LCMV production, and impaired access of LCMV to susceptible target cells in the absence of an intact splenic microarchitecture, characterized by the absence of MAdCAM-1+ marginal sinus-lining cells.
The precise phenotype of the cell subsets supporting the initial replication of LCMV in an intact splenic microarchitecture could not be conclusively determined. The observed presence of clusters of virus-infected cells in the spleens of LCMV-WE-infected C57BL/6 mice (Fig. 4), however, may indicate that upon initial replication in cells of the marginal zone, the virus may spread from one cell type to another into the splenic follicle.
The early recruitment of viruses and bacteria to secondary lymphoid organs is greatly enhanced by the presence of natural IgM antibodies (28), at least if high doses of infectious agents are studied, as had to be used in the study by Ochsenbein et al. (28) for technical reasons. Under viremic and bacterimic conditions, natural antibodies binding the infectious agents may prevent infection of nonlymphoid organs. The results of the comparative analysis of IgM−/− mice and the Ig- and B-cell-deficient μMT mice, which lack natural IgM antibodies but differ in the presence or absence of other Ig classes and in the quality of the splenic microarchitecture, demonstrate that for the retention of small amounts of circulating LCMV in the spleen, serum antibodies and/or an intact splenic microarchitecture with a well-defined marginal zone and distinct B- and T-cell areas is required. In the absence of appropriate experimental tools, it cannot be conclusively determined whether this trapping of virus in IgM−/− mice early after infection with a low dose of LCMV-WE is completely independent of natural antibodies other than IgM. The only limiting effect that reconstitution of μMT mice with normal serum has on early splenic virus production, however, indicates that in the absence of an intact splenic architecture, natural antibodies of all isotypes are unable to efficiently target circulating LCMV to the phagocytic cells of the spleen.
Hence, by the preferential infection of the highly organized splenic microarchitecture, LCMV seems to successfully exploit one of the key elements in the chain of the adaptive immune system required for the induction of a potent immune response. Although the price for the successful elimination of virus-infected cells in the spleen by the virus-specific CTLs, i.e., the loss of antigen-presenting cells resulting in a transient immune suppression, may be considered high, it does efficiently help to deprive LCMV of some of its most efficient virus-producing target cells; however, it also deprives the host of key antigen-trapping cells and thereby causes rather general immunosuppression (30). The present data demonstrate the importance of a well-organized splenic microarchitecture in the induction of a potent antivirus response and in the subsequent control of virus infections and may explain the complications often observed in splenectomized patients in mounting an immune response not only against encapsulated bacteria but also during some virus infections (35). On the other hand, the striking reduction in viremia following splenectomy in human immunodeficiency virus patients during the asymptomatic phase despite the increased CD4 T-cell counts in the peripheral blood may indicate that the exploitation of the intact splenic microarchitecture for efficient virus production may also hold true for other viruses (40).
Acknowledgments
We thank Thomas Brunner for helpful discussions; Ernst Wagner, Hans-Pietro Eugster, and N. Corazza for providing mouse strains; H. Pircher for providing LCMV GP cDNA; Marie Kosco-Vilbois, Bernhard Holzmann, and Georg Kraal for providing antibodies; and Ann M. Pullen for critically reading the manuscript.
This work was supported by Swiss National Science Foundation grant 31-53961.98 to C.M. and grant SNF-RZI 31-50900.97 to R.M.Z. and by a grant from the Novartis Jubiläumsstiftung to C.M. J.P.D. is supported by the Institut Pasteur and INSERM.
REFERENCES
- 1.Althage, A., B. Odermatt, D. Moskophidis, T. Kundig, U. Hoffman-Rohrer, H. Hengartner, and R. M. Zinkernagel. 1992. Immunosuppression by lymphocytic choriomeningitis virus infection: competent effector T and B cells but impaired antigen presentation. Eur. J. Immunol. 22:1803-1812. [DOI] [PubMed] [Google Scholar]
- 2.Battegay, M., S. Cooper, A. Althage, J. Banziger, H. Hengartner, and R. M. Zinkernagel. 1991. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods 33:191-198. (Erratum, 35:115, 1991; erratum, 38:263, 1992.) [DOI] [PubMed]
- 3.Berger, D. P., D. Naniche, M. T. Crowley, P. A. Koni, R. A. Flavell, and M. B. Oldstone. 1999. Lymphotoxin-beta-deficient mice show defective antiviral immunity. Virology 260:136-147. [DOI] [PubMed] [Google Scholar]
- 4.Biron, C. A., S. Habu, K. Okumura, and R. M. Welsh. 1984. Lysis of uninfected and virus-infected cells in vivo: a rejection mechanism in addition to that mediated by natural killer cells. J. Virol. 50:698-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow, P., C. F. Evans, and M. B. Oldstone. 1995. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J. Virol. 69:1059-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butz, E. A., and M. J. Bevan. 1998. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8:167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao, W., M. D. Henry, P. Borrow, H. Yamada, J. H. Elder, E. V. Ravkov, S. T. Nichol, R. W. Compans, K. P. Campbell, and M. B. Oldstone. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:2079-2081. [DOI] [PubMed] [Google Scholar]
- 8.Cihak, J., and F. Lehmann-Grube. 1978. Immunological tolerance to lymphocytic choriomeningitis virus in neonatally infected virus carrier mice: evidence supporting a clonal inactivation mechanism. Immunology 34:265-275. [PMC free article] [PubMed] [Google Scholar]
- 9.Colucci, F., M. Turner, E. Schweighoffer, D. Guy-Grand, V. Di Bartolo, M. Salcedo, V. L. Tybulewicz, and J. P. Di Santo. 1999. Redundant role of the Syk protein tyrosine kinase in mouse NK cell differentiation. J. Immunol. 163:1769-1774. [PubMed] [Google Scholar]
- 10.Crowley, M. T., C. R. Reilly, and D. Lo. 1999. Influence of lymphocytes on the presence and organization of dendritic cell subsets in the spleen. J. Immunol. 163:4894-4900. [PubMed] [Google Scholar]
- 11.De Togni, P., J. Goellner, N. H. Ruddle, P. R. Streeter, A. Fick, S. Mariathasan, S. C. Smith, R. Carlson, L. P. Shornick, J. Strauss-Schoenberger, J. H. Russell, R. Karr, and D. D. Chaplin. 1994. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264:703-707. [DOI] [PubMed] [Google Scholar]
- 12.DiSanto, J. P., W. Muller, D. Guy Grand, A. Fischer, and K. Rajewsky. 1995. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. USA 92:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eugster, H.-P., M. Müller, U. Karrer, B. D. Car, B. Schnyder, V. M. Eng, G. Woerly, M. Le Hir, F. di Padova, M. Aguet, R. Zinkernagel, H. Bluethmann, and B. Ryffel. 1996. Multiple immune abnormalities in tumor necrosis factor and lymphotoxin-alpha double-deficient mice. Int. Immunol. 8:23-36. [DOI] [PubMed] [Google Scholar]
- 14.Francis, S. J., and P. J. Southern. 1988. Molecular analysis of viral RNAs in mice persistently infected with lymphocytic choriomeningitis virus. J. Virol. 62:1251-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu, Y. X., G. Huang, Y. Wang, and D. D. Chaplin. 1998. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin alpha-dependent fashion. J. Exp Med. 187:1009-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gessner, A., D. Moskophidis, and F. Lehmann-Grube. 1989. Enumeration of single IFN-gamma-producing cells in mice during viral and bacterial infection. J. Immunol. 142:1293-1298. [PubMed] [Google Scholar]
- 17.Gonzalez, M., F. Mackay, J. L. Browning, M. H. Kosco-Vilbois, and R. J. Noelle. 1998. The sequential role of lymphotoxin and B cells in the development of splenic follicles. J. Exp Med. 187:997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kägi, D., B. Ledermann, K. Bürki, P. Seiler, B. Odermatt, K. J. Olsen, E. R. Podack, R. M. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369:31-37. [DOI] [PubMed] [Google Scholar]
- 19.Karrer, U., A. Althage, B. Odermatt, C. W. Roberts, S. J. Korsmeyer, S. Miyawaki, H. Hengartner, and R. M. Zinkernagel. 1997. On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11(−)/−) mutant mice. J. Exp. Med. 185:2157-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 21.Klein, M. A., R. Frigg, A. J. Raeber, E. Flechsig, I. Hegyi, R. M. Zinkernagel, C. Weissmann, and A. Aguzzi. 1998. PrP expression in B lymphocytes is not required for prion neuroinvasion. Nat. Med. 4:1429-1433. [DOI] [PubMed] [Google Scholar]
- 22.Kraal, G. 1992. Cells in the marginal zone of the spleen. Int. Rev. Cytol. 132:31-74. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann-Grube, F. 1971. Lymphocytic choriomeningitis virus. Virol. Monogr. 10:1-173. [Google Scholar]
- 24.Lutz, C., B. Ledermann, M. H. Kosco-Vilbois, A. F. Ochsenbein, R. M. Zinkernagel, G. Kohler, and F. Brombacher. 1998. IgD can largely substitute for loss of IgM function in B cells. Nature 393:797-801. [DOI] [PubMed] [Google Scholar]
- 25.Mims, C. A., and S. Wainwright. 1968. The immunodepressive action of lymphocytic choriomeningitis virus in mice. J. Immunol. 101:717-724. [PubMed] [Google Scholar]
- 26.Mueller, C., H. K. Gershenfeld, C. G. Lobe, C. Y. Okada, R. C. Bleackley, and I. L. Weissman. 1988. A high proportion of T lymphocytes that infiltrate H-2-incompatible heart allografts in vivo express genes encoding cytotoxic cell-specific serine proteases, but do not express the MEL-14 defined lymph node homing receptor. J. Exp Med. 167:1124-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 28.Ochsenbein, A. F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R. M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 286:2156-2159. [DOI] [PubMed] [Google Scholar]
- 29.Ochsenbein, A. F., D. D. Pinschewer, B. Odermatt, M. C. Carroll, H. Hengartner, and R. M. Zinkernagel. 1999. Protective T cell-independent antiviral antibody responses are dependent on complement. J. Exp. Med. 190:1165-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odermatt, B., M. Eppler, T. P. Leist, H. Hengartner, and R. M. Zinkernagel. 1991. Virus-triggered acquired immunodeficiency by cytotoxic T-cell-dependent destruction of antigen-presenting cells and lymph follicle structure. Proc. Natl. Acad. Sci. USA 88:8252-8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oldstone, M. B., and M. J. Buchmeier. 1982. Restricted expression of viral glycoprotein in cells of persistently infected mice. Nature 300:360-362. [DOI] [PubMed] [Google Scholar]
- 32.Pasparakis, M., L. Alexopoulou, M. Grell, K. Pfizenmaier, H. Bluethmann, and G. Kollias. 1997. Peyer's patch organogenesis is intact yet formation of B lymphocyte follicles is defective in peripheral lymphoid organs of mice deficient for tumor necrosis factor and its 55-kDa receptor. Proc. Natl. Acad. Sci. USA 94:6319-6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Planz, O., P. Seiler, H. Hengartner, and R. M. Zinkernagel. 1996. Specific cytotoxic T cells eliminate cells producing neutralizing antibodies. Nature 382:726-729. [DOI] [PubMed] [Google Scholar]
- 34.Seiler, P., P. Aichele, B. Odermatt, H. Hengartner, R. M. Zinkernagel, and R. A. Schwendener. 1997. Crucial role of marginal zone macrophages and marginal zone metallophils in the clearance of lymphocytic choriomeningitis virus infection. Eur. J. Immunol. 27:2626-2633. [DOI] [PubMed] [Google Scholar]
- 35.Sekikawa, T., and C. H. Shatney. 1983. Septic sequelae after splenectomy for trauma in adults. Am. J. Surg. 145:667-673. [DOI] [PubMed] [Google Scholar]
- 36.Sevilla, N., S. Kunz, A. Holz, H. Lewicki, D. Homann, H. Yamada, K. P. Campbell, J. C. de La Torre, and M. B. Oldstone. 2000. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J. Exp. Med. 192:1249-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shinkai, Y., G. Rathbun, K. P. Lam, E. M. Oltz, V. Stewart, M. Mendelsohn, J. Charron, M. Datta, F. Young, A. M. Stall, et al. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855-867. [DOI] [PubMed] [Google Scholar]
- 38.Smelt, S. C., P. Borrow, S. Kunz, W. Cao, A. Tishon, H. Lewicki, K. P. Campbell, and M. B. Oldstone. 2001. Differences in affinity of binding of lymphocytic choriomeningitis virus strains to the cellular receptor alpha-dystroglycan correlate with viral tropism and disease kinetics. J. Virol. 75:448-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tishon, A., P. J. Southern, and M. B. Oldstone. 1988. Virus-lymphocyte interactions. II. Expression of viral sequences during the course of persistent lymphocytic choriomeningitis virus infection and their localization to the L3T4 lymphocyte subset. J. Immunol. 140:1280-1284. [PubMed] [Google Scholar]
- 40.Tsoukas, C. M., N. F. Bernard, M. Abrahamowicz, H. Strawczynski, G. Growe, R. T. Card, and P. Gold. 1998. Effect of splenectomy on slowing human immunodeficiency virus disease progression. Arch. Surg. 133:25-31. [DOI] [PubMed] [Google Scholar]
- 41.Zinkernagel, R. M., S. Ehl, P. Aichele, S. Oehen, T. Kundig, and H. Hengartner. 1997. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol. Rev. 156:199-209. [DOI] [PubMed] [Google Scholar]
- 42.Zinkernagel, R. M., O. Planz, S. Ehl, M. Battegay, B. Odermatt, P. Klenerman, and H. Hengartner. 1999. General and specific immunosuppression caused by antiviral T-cell responses. Immunol. Rev. 168:305-315. [DOI] [PubMed] [Google Scholar]