Abstract
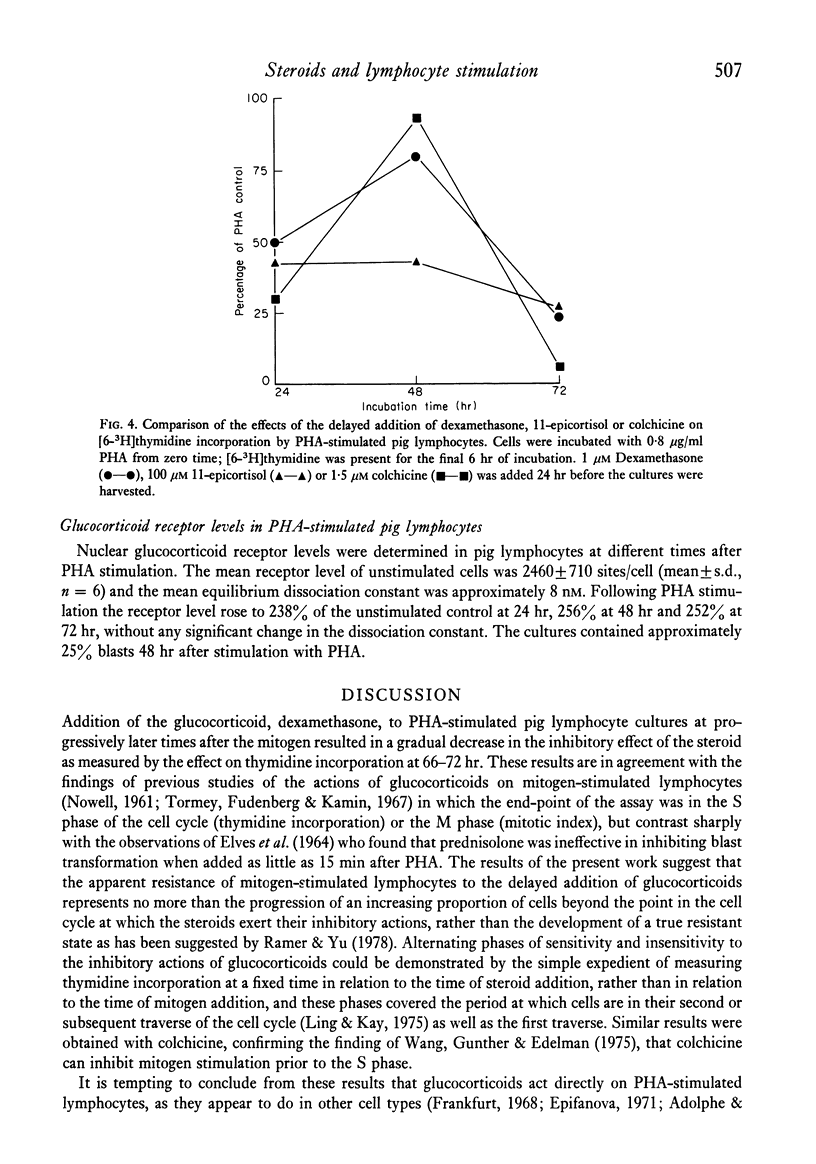
Effects of steroid hormones and colchicine on the response of pig lymphocytes to phytohaemagglutinin (PHA) were assessed by measurement of [6-3H]thymidine incorporation. At steroid concentrations of 1 microM and below, only glucocorticoids and progesterone inhibited PHA-stimulated [6-3H]thymidine incorporation but at 100 microM inhibition was also produced by oestrogens, androgens and physiologically inactive steroids. Measurement of [6-3H]thymidine incorporation 18-24 hr, 6-12 hr or 0-6 hr after the delayed addition of the synthetic glucocorticoid, dexamethasone, to PHA-stimulated lymphocytes revealed a succession of alternating phases of sensitivity and insensitivity to the effects of the steroid which suggested that it was acting, perhaps indirectly, in a cell cycle stage-specific manner to arrest the progression of activated lymphocytes from G1 to S. Similar effects were observed with colchicine, but 100 microM 11-epicortisol inhibited [6-3H]thymidine incorporation in a non-cycle-specific manner. Glucocorticoid receptor levels in pig lymphocytes were increased 2-5-fold within 24 hr of PHA stimulation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolphe M., Lechat P. Action of a steroid anti-inflammatory agent (methylprednisolone) on the cell cycle: study on synchronized cells. Biomedicine. 1974 Jan;20(1):46–53. [PubMed] [Google Scholar]
- BILLINGHAM R. E., KROHN P. L., MEDAWAR P. B. Effect of cortisone on survival of skin homografts in rabbits. Br Med J. 1951 May 26;1(4716):1157–1163. doi: 10.1136/bmj.1.4716.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N. Corticosteroids and lymphoid cells. N Engl J Med. 1972 Aug 24;287(8):388–397. doi: 10.1056/NEJM197208242870806. [DOI] [PubMed] [Google Scholar]
- Cohen I. R., Stavy L., Feldman M. Glucocorticoids and cellular immunity in vitro. Facilitation of the sensitization phase and inhibition of the effector phase of a lymphocyte anti-fibroblast reaction. J Exp Med. 1970 Dec 1;132(6):1055–1070. doi: 10.1084/jem.132.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELVES M. W., GOUGH J., ISRAUELS M. C. THE PLACE OF THE LYMPHOCYTE IN THE RETICULO-ENDOTHELIAL SYSTEM: A STUDY OF THE IN VITRO EFFECT OF PREDNISOLONE ON LYMPHOCYTES. Acta Haematol. 1964 Aug;32:100–107. doi: 10.1159/000209561. [DOI] [PubMed] [Google Scholar]
- Elliott E. V., Sinclair N. R. Effect of cortisone acetate on 19S and 7S haemolysin antibody. A time course study. Immunology. 1968 Nov;15(5):643–652. [PMC free article] [PubMed] [Google Scholar]
- Frankfurt O. S. Effect of hydrocortisone, adrenalin and actinomycin D on transition of cells to the DNA synthesis phase. Exp Cell Res. 1968 Sep;52(1):222–232. doi: 10.1016/0014-4827(68)90561-2. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Mendelsohn J., Multer M. M., Bernheim J. L. Inhibition of human lymphocyte stimulation by steroid hormones: cytokinetic mechanisms. Clin Exp Immunol. 1977 Jan;27(1):127–134. [PMC free article] [PubMed] [Google Scholar]
- NOWELL P. C. Inhibition of human leukocyte mitosis by prednisolone in vitro. Cancer Res. 1961 Dec;21:1518–1521. [PubMed] [Google Scholar]
- Neifeld J. P., Lippman M. E., Tormey D. C. Steroid hormone receptors in normal human lymphocytes. Induction of glucocorticoid receptor activity by phytohemagglutinin stimulation. J Biol Chem. 1977 May 10;252(9):2972–2977. [PubMed] [Google Scholar]
- Ramer S. J., Yu D. T. Effect of corticosteroids on committed lymphocytes. Clin Exp Immunol. 1978 Jun;32(3):545–553. [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., Franks D. Effect of prednisolone on cell-mediated cytotoxicity in vitro. Demonstration of a short sensitive induction phase. Int Arch Allergy Appl Immunol. 1975;48(5):610–620. doi: 10.1159/000231349. [DOI] [PubMed] [Google Scholar]
- Schechter B., Feldman M. Hydrocortisone affects tumor growth by eliminating precursors of suppressor cells. J Immunol. 1977 Nov;119(5):1563–1568. [PubMed] [Google Scholar]
- Segal S., Cohen I. R., Feldman M. Thymus-derived lymphocytes: humoral and cellular reactions distinguished by hydrocortisone. Science. 1972 Mar 10;175(4026):1126–1128. doi: 10.1126/science.175.4026.1126. [DOI] [PubMed] [Google Scholar]
- Sloman J. C., Bell P. A. The dependence of specific nuclear binding of glucocorticoids by rat thymus cells on cellular ATP levels. Biochim Biophys Acta. 1976 Apr 23;428(2):403–413. doi: 10.1016/0304-4165(76)90048-9. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Crabtree G. R., Kennedy S. J., Munck A. U. Glucocorticoid receptors and glucocorticoid sensitivity of mitogen stimulated and unstimulated human lymphocytes. Nature. 1977 Jun 9;267(5611):523–526. doi: 10.1038/267523a0. [DOI] [PubMed] [Google Scholar]
- Tormey D. C., Fudenberg H. H., Kamin R. M. Effect of prednisolone on synthesis of DNA and RNA by human lymphocytes in vitro. Nature. 1967 Jan 21;213(5073):281–282. doi: 10.1038/213281a0. [DOI] [PubMed] [Google Scholar]
- Wang J. L., Gunther G. R., Edelman G. M. Inhibition by colchicine of the mitogenic stimulation of lymphocytes prior to the S phase. J Cell Biol. 1975 Jul;66(1):128–144. doi: 10.1083/jcb.66.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N. A., Appleton D. R., Morley A. R. Effect of dexamethasone on cell population kinetics in the adrenal cortex of the prepubertal male rat. J Endocrinol. 1974 Sep;62(3):527–536. doi: 10.1677/joe.0.0620527. [DOI] [PubMed] [Google Scholar]


