Abstract
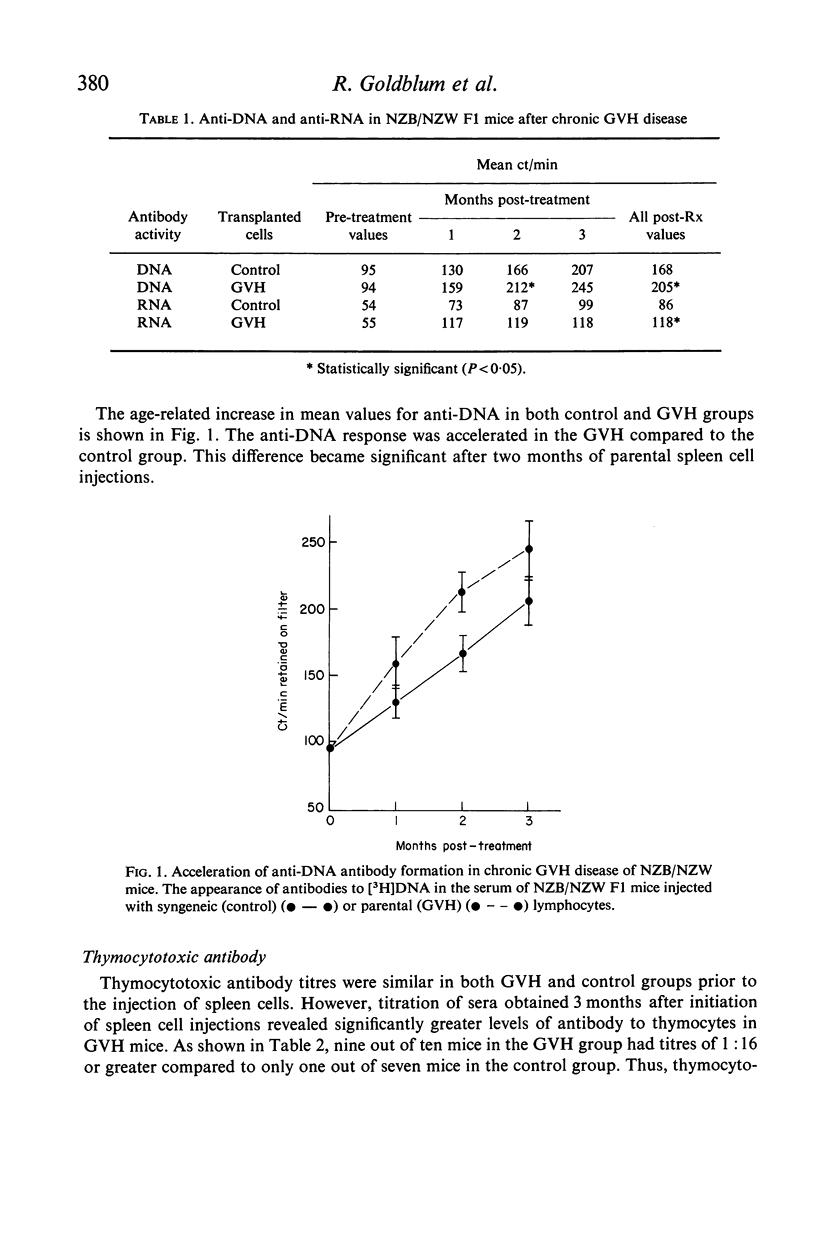
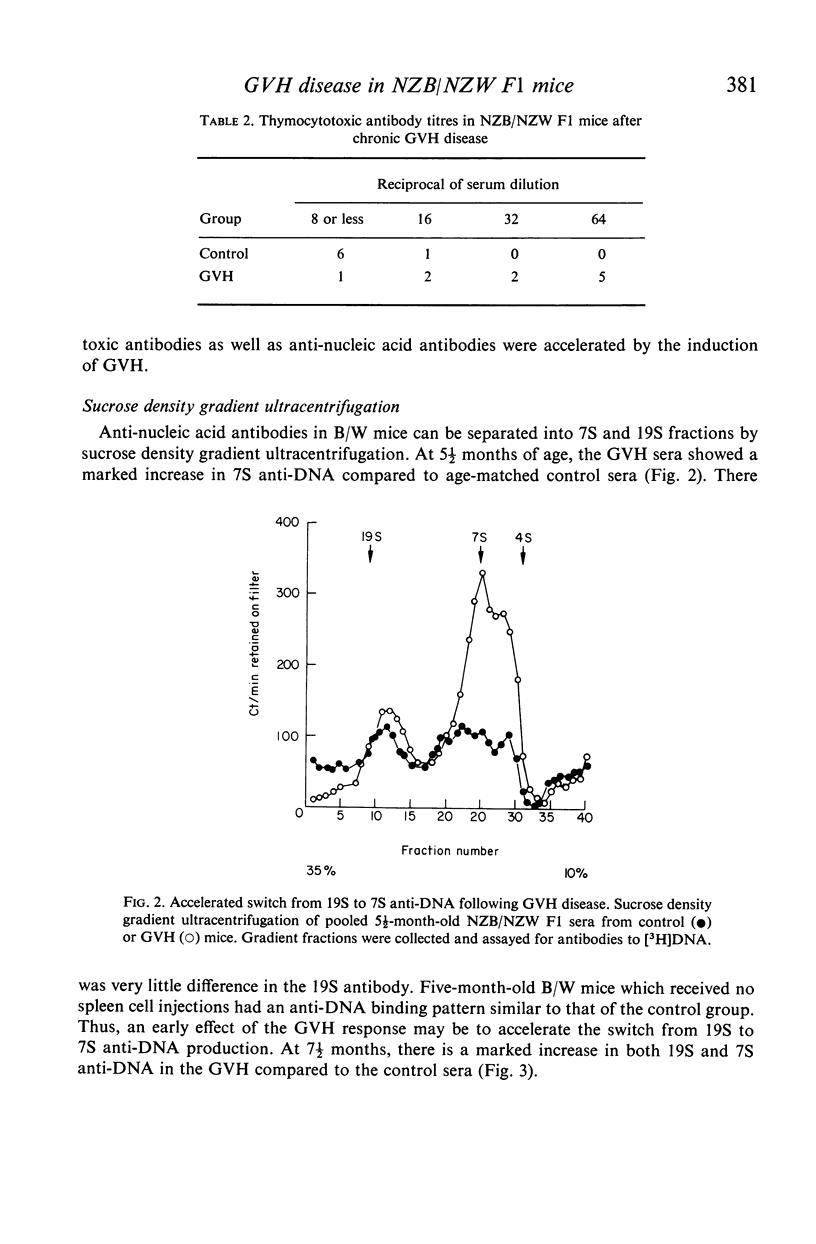
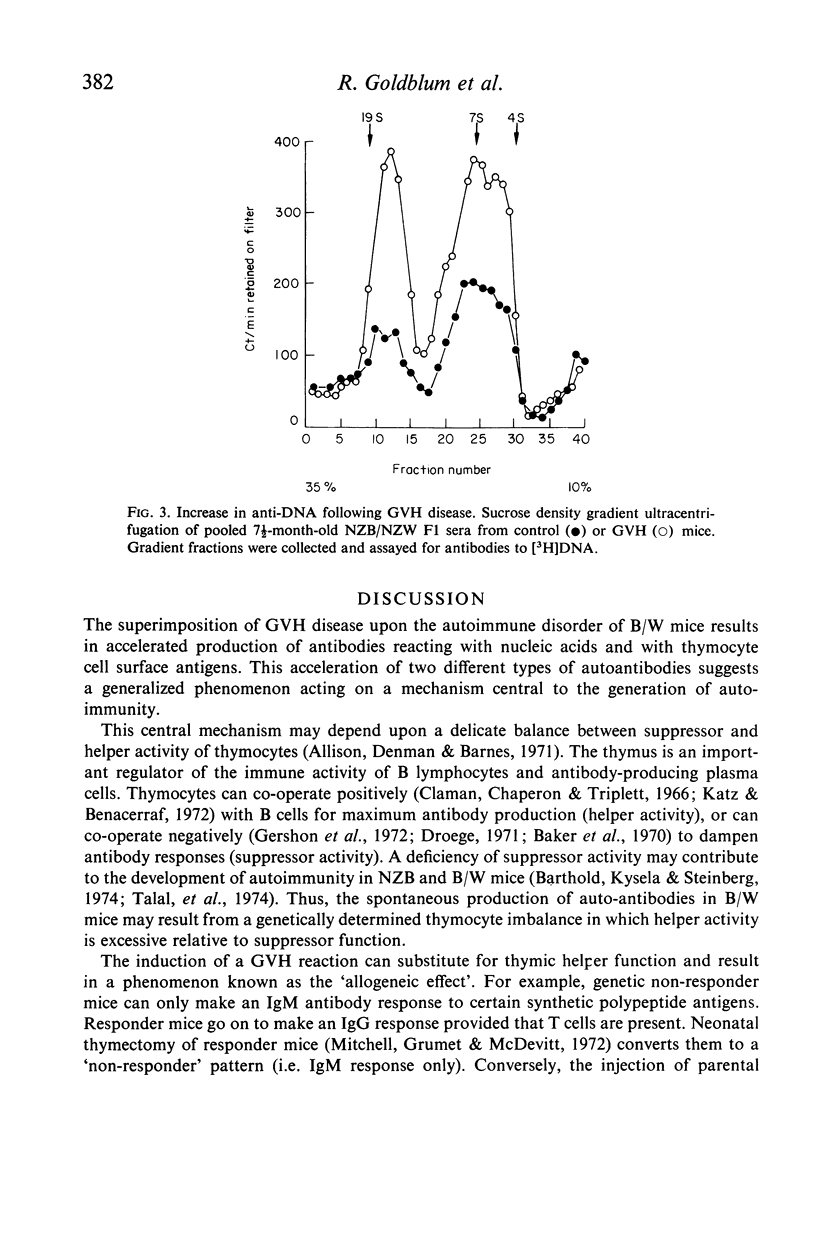
Chronic graft-versus-host (GVH) disease was induced in NZB/NZW F1 (B/W) hybrid female mice by the weekly injection of parental NZB spleen cells. Control mice received injections of syngeneic spleen cells only. The mice were assayed for antibodies to [3H]DNA and [3H]polyadenylic-polyuridylic acid by a cellulose ester filter radioimmunoassay, and for antibody to thymocytes by a cytotoxicity method. GVH disease accelerated the development of all three antibodies in B/W mice. In addition, sucrose density gradient ultracentrifugation of pooled sera suggested that an accelerated switch from 19S to 7S anti-DNA production may be an early effect of GVH. The mechanism of acceleration is discussed in terms of immunological and viral factors generated by the GVH reaction.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Denman A. M., Barnes R. D. Cooperating and controlling functions of thymus-derived lymphocytes in relation to autoimmunity. Lancet. 1971 Jul 17;2(7716):135–140. doi: 10.1016/s0140-6736(71)92306-3. [DOI] [PubMed] [Google Scholar]
- Armstrong M. Y., Black F. L., Richards F. F. Tumour induction by cell-free extracts derived from mice with graft versus host disease. Nat New Biol. 1972 Feb 2;235(57):153–154. doi: 10.1038/newbio235153a0. [DOI] [PubMed] [Google Scholar]
- Armstrong M. Y., Gleichmann E., Gleichmann H., Beldotti L., Andre-Schwartz J., Schwartz R. S. Chronic allogeneic disease. II. Development of lymphomas. J Exp Med. 1970 Sep 1;132(3):417–439. doi: 10.1084/jem.132.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attias M. R., Sylvester R. A., Talal N. Filter radioimmunoassay for antibodies to reovirus RNA in systemic lupus erythematosus. Arthritis Rheum. 1973 Nov-Dec;16(6):719–725. doi: 10.1002/art.1780160604. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Barth R. F., Stashak P. W., Amsbaugh D. F. Enhancement of the antibody response to type 3 pneumococcal polysaccharide in mice treated with antilymphocyte serum. J Immunol. 1970 May;104(5):1313–1315. [PubMed] [Google Scholar]
- Barthold D. R., Kysela S., Steinberg A. D. Decline in suppressor T cell function with age in female NZB mice. J Immunol. 1974 Jan;112(1):9–16. [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A., Triplett R. F. Thymus-marrow cell combinations. Synergism in antibody production. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1167–1171. doi: 10.3181/00379727-122-31353. [DOI] [PubMed] [Google Scholar]
- Droege W. Amplifying and suppressive effect of thymus cells. Nature. 1971 Dec 31;234(5331):549–551. doi: 10.1038/234549a0. [DOI] [PubMed] [Google Scholar]
- Feldmann M. Cell interactions in the immune response in vitro. V. Specific collaboration via complexes of antigen and thymus-derived cell immunoglobulin. J Exp Med. 1972 Oct 1;136(4):737–760. doi: 10.1084/jem.136.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkow P. J., Gilchrist C., Allison A. C. Autoimmunity in chronic graft-versus-host disease. Clin Exp Immunol. 1973 Apr;13(4):479–486. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Cohen P., Hencin R., Liebhaber S. A. Suppressor T cells. J Immunol. 1972 Mar;108(3):586–590. [PubMed] [Google Scholar]
- Greenspan J. S., Gutman G. A., Talal N., Weissman I. L., Sugai S. Thymus-antigen- and immunoglobulin- positive cells in lymph-nodes, thymus, and malignant lymphomas of NZB/NZW mice. Clin Immunol Immunopathol. 1974 Sep;3(1):32–51. doi: 10.1016/0090-1229(74)90021-x. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Black P. H., Tracy G. S., Leibowitz S., Schwartz R. S. Leukemia virus activation in chronic allogeneic disease. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1914–1917. doi: 10.1073/pnas.67.4.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Ellis D. A., Proffitt M. R., Black P. H. Effects of interferon on leukaemia virus activation in graft versus host disease. Nat New Biol. 1973 Jul 25;244(134):102–103. doi: 10.1038/newbio244102a0. [DOI] [PubMed] [Google Scholar]
- Hunter P., Kettman J. R. Mode of action of a supernatant activity from T-cell cultures that nonspecifically stimulates the humoral immune response. Proc Natl Acad Sci U S A. 1974 Feb;71(2):512–516. doi: 10.1073/pnas.71.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Paul W. E., Goidl E. A., Benacerraf B. Carrier function in anti-hapten antibody responses. 3. Stimulation of antibody synthesis and facilitation of hapten-specific secondary antibody responses by graft-versus-host reactions. J Exp Med. 1971 Feb 1;133(2):169–186. doi: 10.1084/jem.133.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. H., Dixon F. J. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968 Mar 1;127(3):507–522. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. M., Armstrong M. Y., André-Schwartz J., Muftuoglu A., Beldotti L., Schwartz R. S. Chronic allogeneic disease. I. Development of glomerulonephritis. J Exp Med. 1968 Oct 1;128(4):653–679. doi: 10.1084/jem.128.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors R. C. Autoimmune and immunoproliferative diseases of NZB/Bl mice and hybrids. Int Rev Exp Pathol. 1966;5:217–252. [PubMed] [Google Scholar]
- Mellors R. C., Huang C. Y. Immunopathology of NZB/BL mice. V. Viruslike (filtrable) agent separable from lymphoma cells and identifiable by electron microscopy. J Exp Med. 1966 Dec 1;124(6):1031–1038. doi: 10.1084/jem.124.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Grumet F. C., McDevitt H. O. Genetic control of the immune response. The effect of thymectomy on the primary and secondary antibody response of mice to poly-L(tyr, glu)-poly-D, L-ala--poly-L-lys. J Exp Med. 1972 Jan;135(1):126–135. doi: 10.1084/jem.135.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLINER H., SCHWARTZ R., DAMESHEK W. Studies in experimental autoimmune disorders. I. Clinical and laboratory features of autoimmunization (runt disease) in the mouse. Blood. 1961 Jan;17:20–44. [PubMed] [Google Scholar]
- Ordal J. C., Grumet F. C. Genetic control of the immune response. The effect of graft-versus-host reaction on the antibody response to poly-L(Tyr, Glu)-poly-D,L-Ala--poly-L-Lys in nonresponder mice. J Exp Med. 1972 Nov 1;136(5):1195–1206. doi: 10.1084/jem.136.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. S., Beldotti L. Malignant lymphomas following allogenic disease: transition from an immunological to a neoplastic disorder. Science. 1965 Sep 24;149(3691):1511–1514. doi: 10.1126/science.149.3691.1511. [DOI] [PubMed] [Google Scholar]
- Shirai T., Mellors R. C. Natural cytotoxic autoantibody against thymocytes in NZB mice. Clin Exp Immunol. 1972 Sep;12(1):133–152. [PMC free article] [PubMed] [Google Scholar]
- Shirai T., Mellors R. C. Natural thymocytotoxic autoantibody and reactive antigen in New Zealand black and other mice. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1412–1415. doi: 10.1073/pnas.68.7.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai T., Yoshiki T., Mellors R. C. Thymus dependence of cells in peripheral lymphoid tissues and in the circulation sensitive to natural thymocytotoxic autoantibody in NZB mice. J Immunol. 1972 Jul;109(1):32–37. [PubMed] [Google Scholar]
- Steinberg A. D., Pincus T., Talal N. DNA-binding assay for detection of anti-DNA antibodies in NZB-NZW F1 mice. J Immunol. 1969 Mar;102(3):788–790. [PubMed] [Google Scholar]
- Sugai S., Pillarisetty R., Talal N. Monoclonal macroglobulinemia in NZB-NZW F1 mice. J Exp Med. 1973 Oct 1;138(4):989–1002. doi: 10.1084/jem.138.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talal N., Gallo R. C. Antibodies to a DNA:RNA hybrid in systemic lupus erythematosus measured by a cellulose ester filter radioimmunoassay. Nat New Biol. 1972 Dec 20;240(103):240–242. doi: 10.1038/newbio240240a0. [DOI] [PubMed] [Google Scholar]
- Talal N. Immunologic and viral factors in the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1970 Nov-Dec;13(6):887–894. doi: 10.1002/art.1780130620. [DOI] [PubMed] [Google Scholar]
- Talal N., Steinberg A. D., Daley G. G. Inhibition of antigodies binding polyinosinic-polycytidylic acid in human and mouse lupus sera by viral and synthetic ribonucleic acids. J Clin Invest. 1971 Jun;50(6):1248–1252. doi: 10.1172/JCI106602. [DOI] [PMC free article] [PubMed] [Google Scholar]


