Abstract
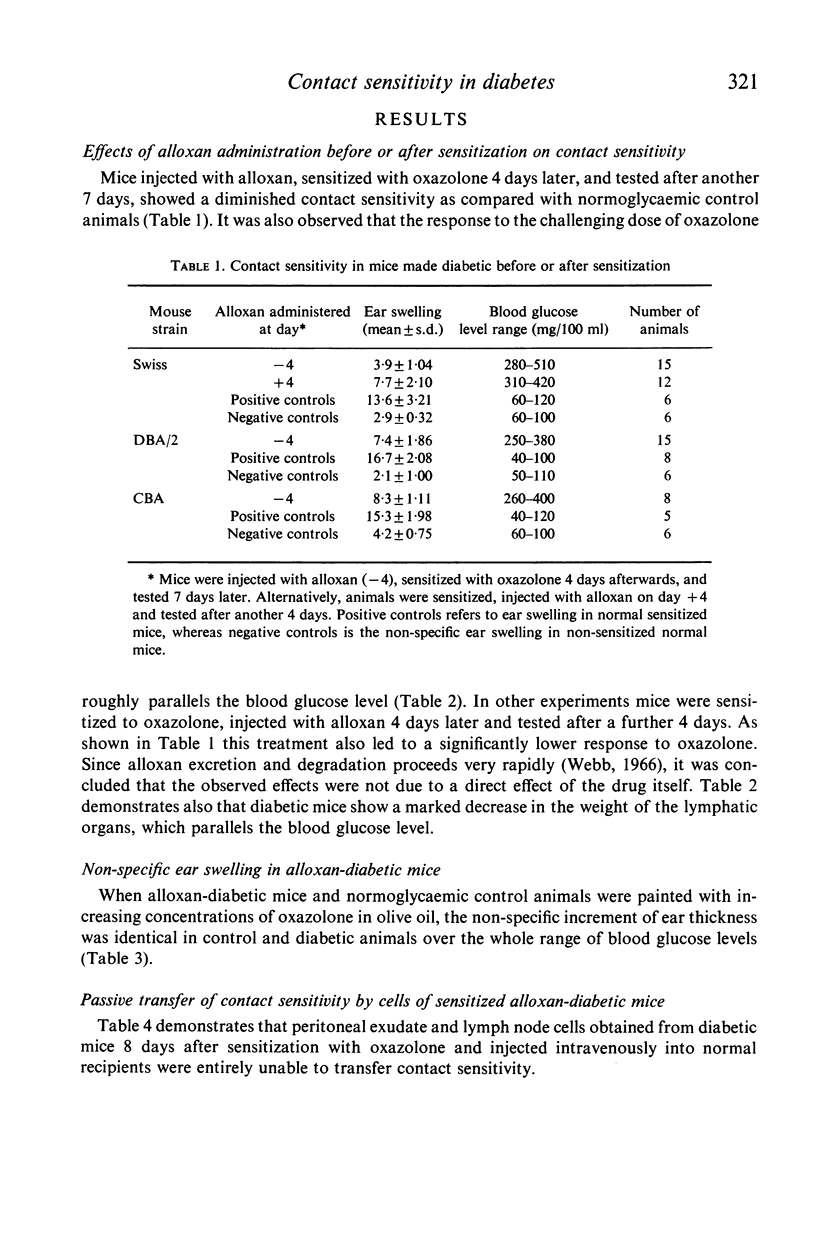
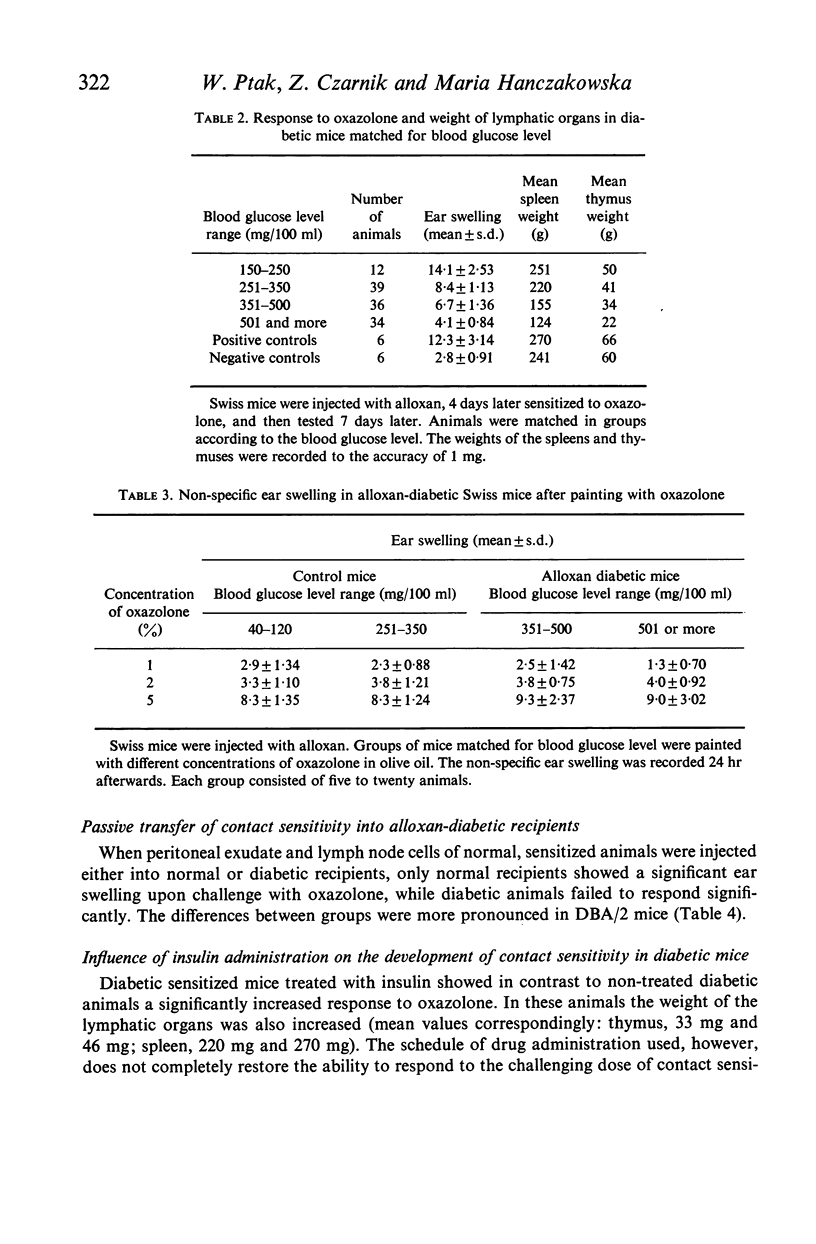
Alloxan-diabetic mice of Swiss, CBA and DBA/2 strains show a significant depression of contact sensitivity to oxazolone, as compared with normoglycaemic control animals, which is accompanied by the involution of the thymus and spleen. Insulin treatment partially restores the contact sensitivity in diabetic animals and also increases the weight of lymphatic organs. In contrast, the non-specific inflammatory response to oxazolone is not impaired in insulin-deficient mice. Further experiments have shown that neither sensitized lymphocytes of control animals given to diabetic mice, nor sensitized lymphocytes of diabetic mice injected into normoglycaemic recipients, were able to transfer passively any significant contact sensitivity. It is suggested that in alloxan-diabetic mice the function of T lymphocytes is affected.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMKIEWICZ V. W. Glycemia and immune responses. Can Med Assoc J. 1963 Apr 13;88:806–811. [PMC free article] [PubMed] [Google Scholar]
- Bagdade J. D., Root R. K., Bulger R. J. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes. 1974 Jan;23(1):9–15. doi: 10.2337/diab.23.1.9. [DOI] [PubMed] [Google Scholar]
- Boyett J. D., Hofert J. F. Stimulatory effect of insulin on glucose metabolism of thymus lymphocytes. Horm Metab Res. 1972 May;4(3):163–167. doi: 10.1055/s-0028-1094092. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Buell D. N., Roth J. Water-soluble insulin receptors from human lymphocytes. Science. 1972 Oct 13;178(4057):168–169. doi: 10.1126/science.178.4057.168. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Gorden P., Roth J., Archer J. A., Buell D. N. Characteristics of the human lymphocyte insulin receptor. J Biol Chem. 1973 Mar 25;248(6):2202–2207. [PubMed] [Google Scholar]
- Goldfine I. D., Gardner J. D., Neville D. M., Jr Insulin action in isolated rat thymocytes. I. Binding of 125 I-insulin and stimulation of -aminoisobutyric acid transport. J Biol Chem. 1972 Nov 10;247(21):6919–6926. [PubMed] [Google Scholar]
- HYVARINEN A., NIKKILA E. A. Specific determination of blood glucose with o-toluidine. Clin Chim Acta. 1962 Jan;7:140–143. doi: 10.1016/0009-8981(62)90133-x. [DOI] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Wilson E. E., Good R. A., Coffey R. G. Direct action of insulin on plasma membrane ATPase activity in human lymphocytes. Nat New Biol. 1972 Feb 9;235(58):174–177. doi: 10.1038/newbio235174a0. [DOI] [PubMed] [Google Scholar]
- Lundin P. M., Angervall L. Effect of insulin on rat lymphoid tissue. Pathol Eur. 1970;5(3):273–278. [PubMed] [Google Scholar]
- THOMPSON G. E. Alloxan and hypersensitivity. Nature. 1961 May 27;190:822–822. doi: 10.1038/190822a0. [DOI] [PubMed] [Google Scholar]
- Thompson G. E. Enhancing effect of insulin on the tuberculin reaction in the albino rat. Nature. 1967 Aug 12;215(5102):748–749. doi: 10.1038/215748a0. [DOI] [PubMed] [Google Scholar]


