Abstract
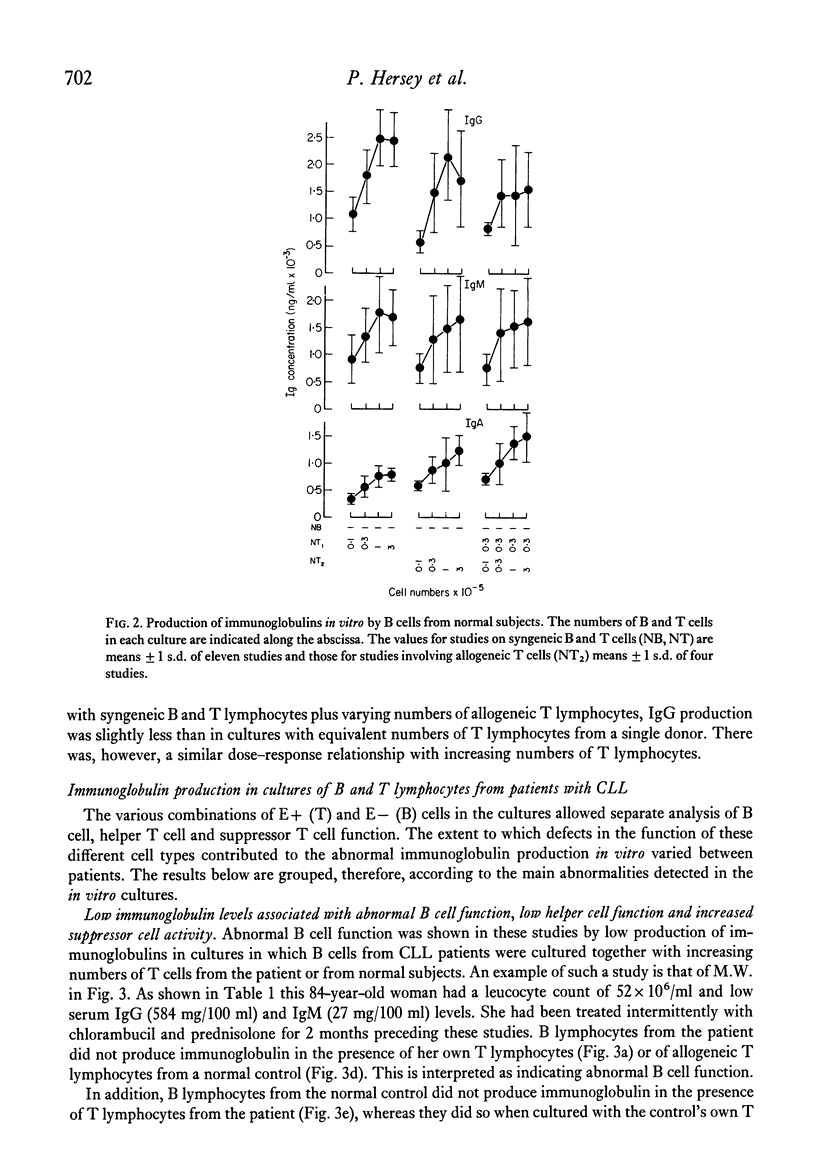
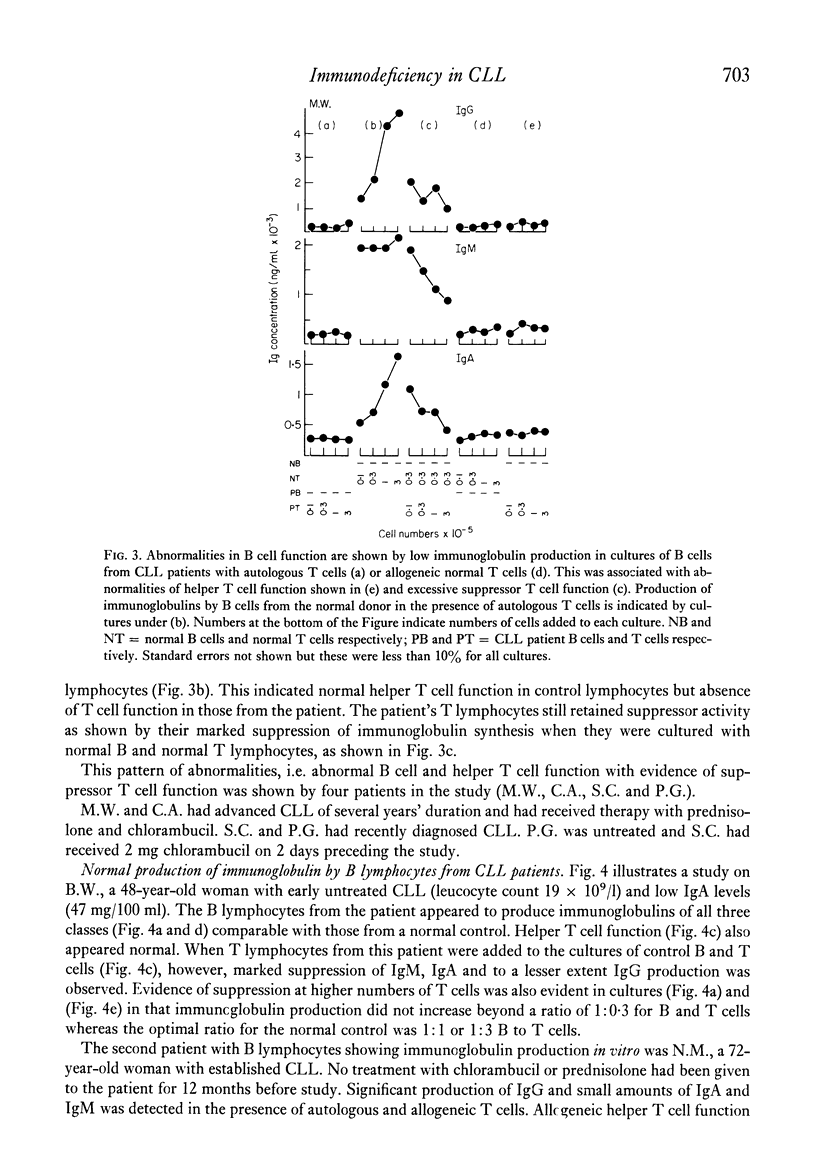
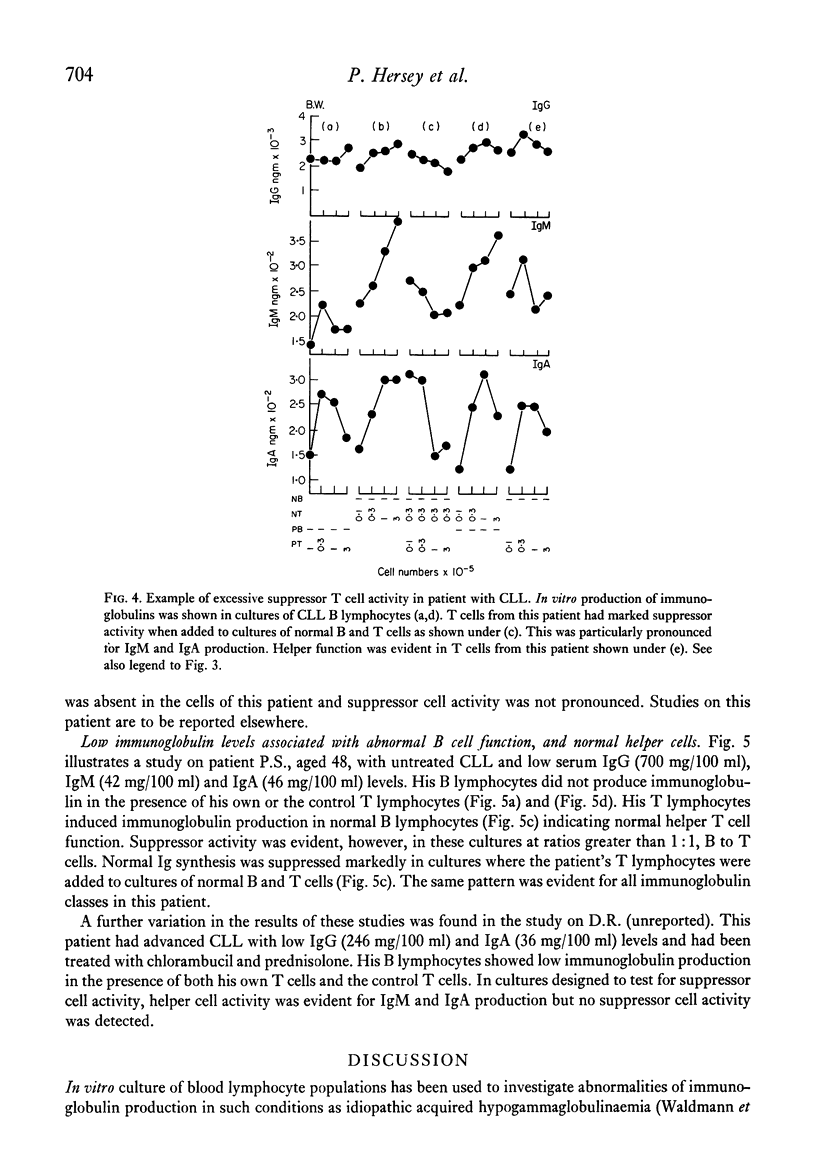
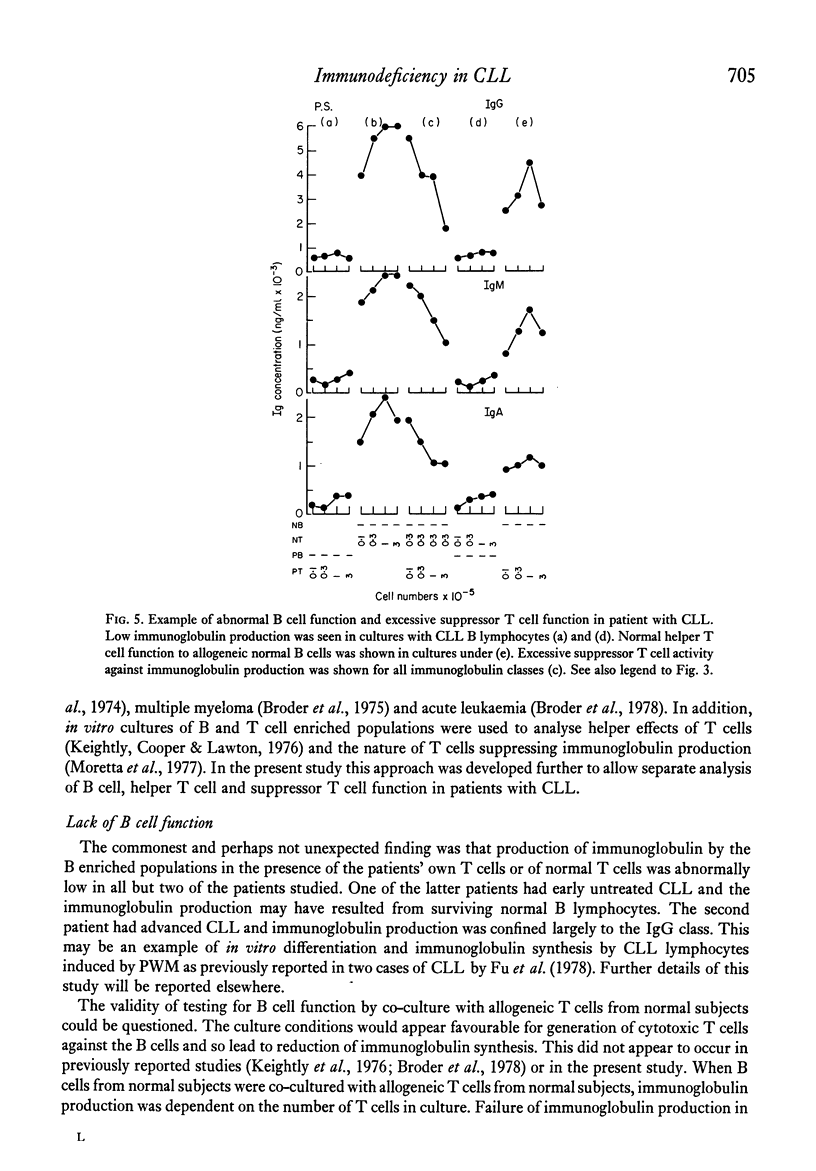
The underlying basis for hypogammaglobulinaemia in patients with chronic lymphatic leukaemia (CLL) was investigated by measurement if immunoglobulin produced in vitro in cultures of pokeweek mitogen-stimulated B and T lymphocytes. B and T cells were separated by sheep red blood cell rosette techniques and, by culture of these cells from CLL patients in various combinations with B or T cells from normal subjects, it was possible to measure independently the function of B lymphocytes and the helper or suppressor function of T lymphocytes. By these methods it was found that the B lymphocytes of six of eight patients failed to produce immunoglobulins in vitro. B lymphocytes from two patients appeared to produce immunoglobulins in vitro. T lymphocytes from five of the eight patients had low or undetectable helper T cell function and in six patients their T lymphocytes had excessive suppressor activity in comparison to T lymphocyte populations from normal subjects. Whether the primary abnormality in the CLL T cell populations was a deficiency of helper T cells or excess of suppressor T cells was uncertain from these studies. These results suggest that immunoglobulin production by B lymphocytes from most patients with CLL was abnormal but also that T cells from CLL patients may be abnormal in respect to their role in immunoglobulin production at an early stage of the disease. These findings may assist in understanding the pathogenesis of this disease and lead to new approaches in treatment.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- Broder S., Muul L., Waldmann T. A. Suppressor cells in neoplastic disease. J Natl Cancer Inst. 1978 Jul;61(1):5–11. doi: 10.1093/jnci/61.1.5. [DOI] [PubMed] [Google Scholar]
- Broder S., Poplack D., Whang-Peng J., Durm M., Goldman C., Muul L., Waldmann T. A. Characterization of a suppressor-cell leukemia. Evidence for the requirement of an interaction of two T cells in the development of human suppressor effector cells. N Engl J Med. 1978 Jan 12;298(2):66–72. doi: 10.1056/NEJM197801122980202. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- CONE L., UHR J. W. IMMUNOLOGICAL DEFICIENCY DISORDERS ASSOCIATED WITH CHRONIC LYMPHOCYTIC LEUKEMIA AND MULTIPLE MYELOMA. J Clin Invest. 1964 Dec;43:2241–2248. doi: 10.1172/JCI105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaton C. D., Maxwell K. W., Smith R. S., Creveling R. L. Use of laser nephelometry in the measurement of serum proteins. Clin Chem. 1976 Sep;22(9):1465–1471. [PubMed] [Google Scholar]
- Fernandez L. A., MacSween J. M., Langley G. R. T cell function in untreated B cell chronic lymphocytic leukemia. Cancer. 1977 Mar;39(3):1168–1174. doi: 10.1002/1097-0142(197703)39:3<1168::aid-cncr2820390323>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Fu S. M., Chiorazzi N., Kunkel H. G., Halper J. P., Harris S. R. Induction of in vitro differentiation and immunoglobulin synthesis of human leukemic B lymphocytes. J Exp Med. 1978 Dec 1;148(6):1570–1578. doi: 10.1084/jem.148.6.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNZ F. W., ANGUS H. B. LEUKEMIA AND CANCER IN THE SAME PATIENT. Cancer. 1965 Feb;18:145–152. doi: 10.1002/1097-0142(196502)18:2<145::aid-cncr2820180204>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Patterns of gene expression and the cellular origins of human leukaemias. Biochim Biophys Acta. 1978 Oct 27;516(2):193–230. doi: 10.1016/0304-419x(78)90008-2. [DOI] [PubMed] [Google Scholar]
- Hersey P., Edwards A., Edwards J. Characterization of mononuclear effector cells in human blood. Clin Exp Immunol. 1976 Jan;23(1):104–113. [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Keightley R. G., Cooper M. D., Lawton A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol. 1976 Nov;117(5 Pt 1):1538–1544. [PubMed] [Google Scholar]
- Kumagai K., Abo T., Sekizawa T., Sasaki M. Studies of surface immunoglobulins on human B lymphocytes. I. Dissociation of cell-bound immunoglobulins with acid pH or at 37 degrees C. J Immunol. 1975 Oct;115(4):982–987. [PubMed] [Google Scholar]
- MILLER D. G., KARNOFSKY D. A. Immunologic factors and resistance to infection in chronic lymphatic leukemia. Am J Med. 1961 Nov;31:748–757. doi: 10.1016/0002-9343(61)90159-0. [DOI] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. M., Hagon E. E., Vincent P. C., Gunz F. W. Quantitative studies on the response of normal and leukaemic lymphocytes to phytohaemagglutinin. Aust J Exp Biol Med Sci. 1974 Feb;52(1):87–97. doi: 10.1038/icb.1974.7. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Wells J. V., Fudenberg H. H. Metabolism of radio-iodinated IgG in patients with abnormal IgG levels. I. Hypergamma-globulinaemia. Clin Exp Immunol. 1971 Dec;9(6):761–774. [PMC free article] [PubMed] [Google Scholar]
- Wybran J., Chantler S., Fudenberg H. H. Isolation of normal T cells in chronic lymphatic leukaemia. Lancet. 1973 Jan 20;1(7795):126–129. doi: 10.1016/s0140-6736(73)90196-7. [DOI] [PubMed] [Google Scholar]


