Abstract
Cytoplasmic expression of the incompletely spliced RNA transcripts that encode the late, structural proteins of human immunodeficiency virus type 1 (HIV-1) is dependent on the viral Rev regulatory protein. General agreement exists that Rev acts, at least in part, by recruiting the cellular Crm1 nuclear export factor to HIV-1 transcripts bearing the Rev response element RNA target, and thereby inducing their nuclear egress. However, several groups have argued that Crm1 recruitment may not be sufficient for Rev function. Thus, several additional candidate cofactors for Rev have been proposed, and Rev has also been suggested to also inhibit the nuclear splicing of HIV-1 transcripts and/or to directly enhance their cytoplasmic translation. To examine whether Crm1 recruitment is, instead, sufficient to activate the nuclear export of viral mRNAs, we targeted a leucine-rich Crm1 binding domain, derived from a heterologous protein that normally plays no role in RNA metabolism, to HIV-1 RNAs and showed that this tethered Crm1 binding domain is sufficient to induce the nuclear export and cytoplasmic translation of late HIV-1 mRNA species. More importantly, we show that direct tethering of the Crm1 nuclear export factor to target mRNAs, by fusion to a heterologous RNA binding domain, is in and of itself sufficient to induce the nuclear export and cytoplasmic expression of the unspliced HIV-1 mRNAs that encode the viral Gag proteins.
Retroviral replication requires the cytoplasmic expression of both fully spliced and incompletely spliced forms of the initial, genome-length viral RNA transcript (reviewed in references 7 and 37). However, cells have evolved mechanisms to prevent the nuclear export of incompletely spliced cellular mRNAs, i.e., pre-mRNAs (4, 23). Retroviruses have therefore had to develop mechanisms that allow intron-containing viral transcripts to exit the nucleus in the face of this cellular proofreading mechanism. In several simple retroviruses, nuclear export of the genome-length transcript is mediated by cellular factors that are recruited to a cis-acting RNA target termed a constitutive transport element (CTE) (3, 36, 49). The cellular protein specific for the CTE found in simian type D viruses has been identified as Tap, a nuclear export factor that also plays a key role in mediating cellular mRNA export (7, 15, 19, 43).
In contrast to simple retroviruses, complex retroviruses encode a regulatory protein that is critical for the cytoplasmic expression of their incompletely spliced transcripts. In human immunodeficiency virus type 1 (HIV-1), this protein is termed Rev. Rev interacts with a cis-acting viral RNA target site, the Rev response element (RRE), and with Crm1, a host cell protein that is a member of the karyopherin or importin/exportin family of nucleocytoplasmic transport factors (12, 14, 29, 31, 35, 42, 50). Crm1 binds specifically to a short leucine-rich motif found in the Rev protein that also functions as a nuclear export signal (NES) (2, 11, 30, 48). NES binding by Crm1 requires a cellular cofactor, the GTP-bound form of the cellular G-protein Ran, and is also enhanced by a second cellular cofactor, the Ran binding protein RanBP3 (2, 12, 24).
While a general consensus exists that Crm1 is an essential cofactor for HIV-1 Rev, there remains controversy as to whether it is sufficient. Specifically, several groups have argued that Rev may also enhance the nuclear export of unspliced viral mRNAs by inhibiting the splicing of HIV-1 transcripts (4, 10, 21, 25, 38, 44). Evidence has been presented suggesting that Rev can specifically interact with a protein termed p32, which may function as a cofactor for the cellular splicing factor ASF/SF2 (25, 44). It has also been proposed that Rev can induce the recruitment of ASF/SF2 to the RRE in vitro (38) and that Rev contains a protein motif, largely coincident with the RRE binding domain, that promotes the interaction of Rev with discrete intranuclear compartments that contain high levels of splicing factors (9).
In addition to a possible role for Rev in the inhibition of viral splicing, several groups have also reported significant levels of cytoplasmic unspliced HIV-1 mRNAs in cells that lack a functional Rev protein (1, 8, 41). While controversial (16, 28, 29, 32), these data have nevertheless led to the proposal that Rev may also regulate the cytoplasmic translation of HIV-1 transcripts. Finally, it has been proposed that other potential cellular cofactors, in addition to Crm1, may also be critical for Rev NES function. These candidate cofactors include eIF-5A, which has been proposed to bind to the Rev NES directly (39).
We have taken two approaches to test the alternative hypothesis that the sole role of Rev is to induce the nuclear export of viral mRNAs by acting as an adapter between the RRE and the cellular Crm1 nuclear export factor. First, we constructed an artificial Rev protein by fusing together functional domains derived from several bacterial or mammalian proteins to form a novel nucleocytoplasm-shuttling RNA binding protein. We show that this artificial protein, which lacks any sequences known to play a role in splicing or translation, is nevertheless capable of rescuing the nuclear export and cytoplasmic expression of unspliced HIV-1 transcripts. Second, we show that direct recruitment of Crm1 is, in and of itself, sufficient to induce the expression of structural proteins from a Rev-deficient HIV-1 proviral clone. Together, these data strongly argue that Crm1 is the only cellular cofactor that is directly recruited to the RRE by the HIV-1 Rev protein.
MATERIALS AND METHODS
Construction of molecular clones.
All expression plasmids were based on pBC12/CMV (6). The following expression plasmids have been described previously: the indicator constructs pDM128/PL, pDM128/RRE, and pDM128/4xMS2 (2, 5); effector plasmids pcRev, pBC12/MS2, pBC12/MS2-Rev, and pBC12/MS2-Tap (5, 29); and the ΔCAN expression plasmid pBC12/CMV/ΔCAN and the internal control plasmid pBC12/CMV/β-gal (2).
The indicator construct pDM128/1xMS2 was generated by ligation of annealed oligonucleotides encoding one MS2 RNA operator element into the BglII and Asp sites of the polylinker present in pDM128/PL. Similarly, the indicator construct pDM128/2xMS2 was generated by PCR amplification of two tandem MS2 RNA operator sequences from pIII/MS2 (40) followed by ligation into the Asp and Cla sites of the pDM128/PL polylinker. The indicator construct pDM128/8xMS2 was generated by PCR amplification of four tandem MS2 RNA elements from pDM128/4xMS2 (5), followed by ligation into the unique Asp site present in pDM128/4xMS2. Expression plasmid pBC12/MS2-APC was generated by PCR amplification of the MS2 coat protein RNA binding domain from pBC12/MS2, followed by ligation into the unique NcoI site present in the previously described pBC12/CMV/APC plasmid (34). A cDNA encoding full-length Crm1 (2, 13) was inserted between the 5" EcoRI and 3" XhoI sites present in a modified form of pBC12/MS2 to give pBC12/MS2-Crm1.
The HIV-1 proviral clone pNL4-3Rev− was generated by replacing the NheI-BamHI fragment of pNL4-3Rev− R− (33) with an NheI-BamHI fragment from the HXB3 env gene (29) containing the wild-type RRE. The resulting plasmid, pNL4-3Rev−, contains the previously described inactivating mutations in rev (33) but has an intact RRE. A DNA fragment encoding four tandem MS2 operator RNA elements flanked by XhoI sites was obtained by PCR amplification from pDM128/4xMS2 and then cloned into the unique XhoI site present in pNL4-3Rev− to generate pNL4-3Rev−/4xMS2.
Culture and transfection of 293T cells.
Human 293T cells were maintained as described previously (2) and transfected using Fugene-6 (Roche Molecular Biochemicals). All transfections used cells cultured in six-well 35-mm plates and, in most cases, included pBC12/CMV/β-gal as an internal control. Induced chloramphenicol acetyltransferase (CAT) enzyme levels were determined ≈48 h after transfection, unless otherwise indicated, and were normalized to the level of β-galactosidase (β-gal) activity present in each cell lysate (2, 5).
Immunofluorescence analysis.
The subcellular localization of the MS2-APC and MS2-Crm1 fusion proteins in transfected 293T cells was determined by indirect immunofluorescence, as previously described (19). Briefly, 293T cells were seeded onto cover slips and then transfected with pBC12/MS2-APC or pBC12/MS2-Crm1, in the latter case together with either pBC12/CMV/ΔCAN or pBC12/CMV as a negative control. Two days after transfection, cells were fixed with paraformaldehyde and then incubated with a 1:250 dilution of a rabbit polyclonal anti-MS2 antiserum, followed by a 1:1,000 dilution of a fluorescein isothiocyanate (FITC)-conjugated donkey anti-rabbit immunoglobulin G (IgG) antibody (Jackson Immunoresearch). Images were collected using a Leica DMRA fluorescence microscope and converted to grayscale using Adobe Photoshop.
RNA isolation and RNase protection assay.
Human 293T cells were transfected with 100 ng of pDM128/4xMS2 and 1,000 ng of pBC12/MS2-APC or pBC12/MS2-Crm1. pBC12/CMV was used as the negative control. Nuclear and cytoplasmic RNA fractions were isolated at ≈72 h after transfection, as previously described (2, 49), and purified using an RNeasy RNA isolation kit (Qiagen). The RNase protection assay was performed using a Hyspeed RPA kit (Ambion) following the manufacturer's protocol. The RNA probe used in this assay has been described (2, 49) and was generated by in vitro transcription. The input probe is 258 nucleotides (nt) in length and contains flanking sequences, derived from the T7 promoter, that allow the full-length input probe to be distinguished from probe fragments protected by unspliced and spliced mRNA transcripts, which have predicted lengths of 158 and 102 nt, respectively.
Virus replication assay.
Human 293T cells were transfected with 500 ng of pNL4-3Rev− or pNL4-3Rev−/4xMS2 and 1,000 ng of pBC12/CMV, pcRev, pBC12/MS2-APC, or pBC12/MS2-Crm1. At ≈72 h after transfection, the supernatant medium were collected, and p24 gag antigen production was quantified using an HIV-1 p24 enzyme-linked immunosorbent assay (ELISA) kit (NEN Life Science).
RESULTS
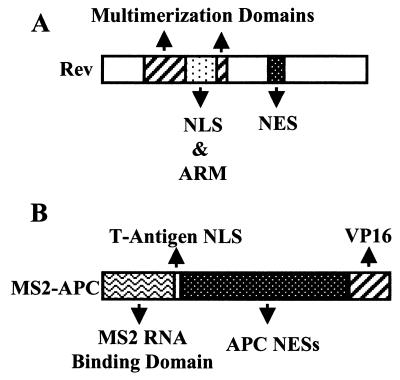
Functional domains in the 116-amino-acid (aa) HIV-1 Rev protein that play a critical role in the nuclear export of incompletely spliced HIV-1 transcripts have been extensively defined by mutational analysis (Fig. 1A) (37). These include a basic motif that functions as a nuclear localization signal (NLS) and as an arginine-rich RNA binding motif (ARM) that is highly specific for the RRE (16, 20, 26, 46). Flanking the basic domain are sequences that are required for multimerization of Rev on the RRE target, a critical but less well understood aspect of Rev function (17, 27). Finally, an essential, ≈10-aa leucine-rich NES is located towards the Rev carboxy terminus (Fig. 1A) (11, 30, 48).
FIG. 1.
Comparison of the functional organization of HIV-1 Rev with that of the artificial MS2-APC fusion protein. See text for detailed discussion.
If these activities are indeed the only relevant biological properties of the HIV-1 Rev protein, then we reasoned that an artificial protein consisting of the functional equivalents of each of these domains should also be able to act as a nuclear export factor for incompletely spliced HIV-1 mRNAs. To test this hypothesis, we constructed the artificial MS2-APC open reading frame shown in Fig. 1B. In this construct, the RNA binding domain is provided by an amino-terminal 130-aa segment encoding the bacteriophage MS2 coat protein (40, 47). Immediately adjacent to this is located a short, 10-aa segment encoding the simian virus 40 (SV40) T antigen (T-Ag) NLS (18, 46). The largest segment of the MS2-APC fusion protein consists of the amino-terminal 220 aa of the ≈310-kDa human adenomatous polyposis coli (APC) protein, which has been shown to contain two active, Crm1-dependent NESs (Fig. 1B) (34). Finally, at the carboxy terminus of MS2-APC, we appended a 78-aa segment that encodes a transcription activation domain derived from the VP16 protein encoded by herpes simplex virus (2, 45). This motif was added to serve as an epitope tag and to permit detection of protein-protein interactions in vivo using two-hybrid assays. While the MS2-APC protein, like Rev, therefore contains not only an RNA binding domain but also NLS and NES sequences (Fig. 1), MS2-APC does not contain a multimerization domain equivalent to the critical multimerization sequences present in Rev. However, MS2-APC is expected to dimerize via the MS2 coat protein sequence (47), and MS2-APC also contains two Crm1-dependent NESs, compared to the single NES present in HIV-1 Rev.
An important feature of the 438-aa MS2-APC fusion protein is that none of the protein segments employed in the creation of this chimera are derived from proteins known to play a role in nuclear RNA export or splicing or in the regulation of eukaryotic translation. The MS2 coat protein is of bacterial origin, while the APC sequence derives from a protein that functions as a tumor suppressor by activating the nuclear export and cytoplasmic degradation of the transcription factor β-catenin (34). The segments derived from T-Ag and VP16 protein are not only very short but are also derived from proteins that largely (T-Ag) or exclusively (VP16) function to regulate viral transcription (18, 45).
The HIV-1-based indicator constructs initially used to assess the nuclear mRNA export potential of the MS2-APC fusion are based on the previously described pDM128/PL plasmid (2, 16, 30, 32). This indicator plasmid contains splice donor and acceptor sequences, derived from HIV-1, flanking the cat indicator gene and a polylinker. As noted above, cells obstruct the nuclear export of pre-mRNAs that retain complete introns, and the unspliced mRNA encoded by pDM128/PL is therefore normally unable to access the cytoplasm effectively. As a result, cells transfected with pDM128/PL express little CAT enzyme activity. However, if sequences encoding an RNA export element, such as a CTE or the RRE, are provided in cis, and the resultant plasmid is introduced into cells expressing the cognate RNA export factor, then the unspliced cat mRNA is able to reach the cytoplasm and induce robust expression of CAT activity.
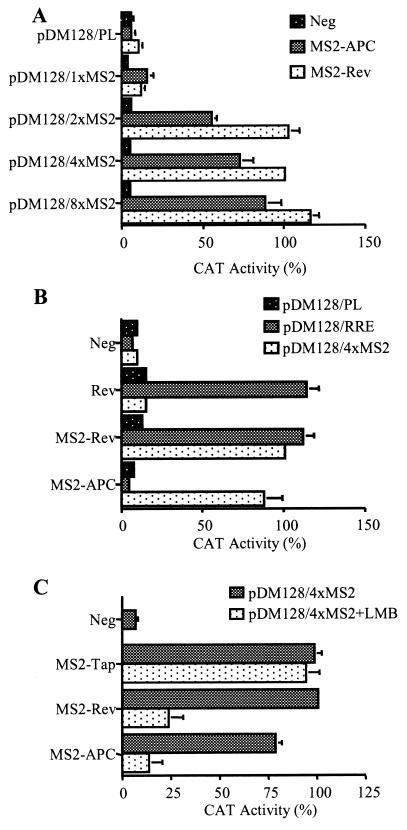
Previously, it has been demonstrated that an MS2-Rev fusion protein can activate the nuclear export of an unspliced pDM128-derived transcript containing at least two copies of the MS2 operator RNA stem-loop structure (32). This result is reproduced in Fig. 2A, which shows that the MS2-Rev fusion protein is unable to activate CAT expression in human 293T cells cotransfected with the parental pDM128/PL plasmid and has only a modest effect on pDM128/1XMS2, which contains a single copy of the MS2 operator RNA. In contrast, MS2-Rev potently activated CAT expression in cells cotransfected with pDM128 constructs encoding 2, 4, or 8 tandem copies of the MS2 operator RNA.
FIG. 2.
MS2-APC protein can function as a Crm1-dependent nuclear RNA export factor. (A) 293T cells were transfected with 25 ng of the indicated pDM128-based indicator plasmid, 1,000 ng of the pBC12/MS2-Rev or pBC12/MS2-APC effector plasmid, and 50 ng of the pBC12/CMV/β-gal internal control. The parental pBC12/CMV and pDM128/PL plasmids served as negative controls. Induced CAT and β-gal activities were assayed at ≈48 h after transfection, and CAT activity was normalized to the β-gal internal control. Averages of three independent experiments with standard deviation are indicated. (B) 293T cells were transfected with the indicated indicator and effector plasmids, as described for panel A, and induced CAT and β-gal activities were measured at ≈48 h after transfection. pBC12/CMV served as the negative (Neg) control. (C) 293T cells were transfected with the indicated indicator and effector plasmids, as described for panel A. LMB (5 ng/ml) was added at 16 h after transfection, where indicated. Induced CAT activities were measured at ≈40 h after transfection. In all three panels, data are presented relative to the level of CAT activity observed in the culture transfected with pDM128/4xMS2 and MS2-Rev, which was set at 100%.
Cotransfection of 293T cells with these pDM128-based indicator constructs and a plasmid encoding the MS2-APC fusion protein gave closely comparable results. Specifically, MS2-APC failed to induce detectable CAT activity when coexpressed with the pDM128/PL negative control, gave low but detectable activity with the pDM128/1XMS2 plasmid, and gave readily detectable CAT activity in cells cotransfected with pDM128 derivatives containing 2, 4, or 8 MS2 operator elements. Based on these data, all subsequent experiments were performed using the pDM128/4XMS2 indicator construct as the standard.
To further confirm the predicted RNA sequence specificity of MS2-APC, we next asked whether this fusion protein would be able to activate the nuclear export of pDM128-derived RNAs bearing the RRE export element. As shown in Fig. 2B, and as predicted, the MS2-APC protein was active on pDM128/4XMS2 but inactive when coexpressed with pDM128/RRE, which bears the HIV-1 RRE in place of the MS2 operator. In contrast, wild-type Rev was only active with pDM128/RRE and inactive with pDM128/4XMS2. Finally, the MS2-Rev fusion protein proved able to activate CAT expression to equivalent levels when cotransfected into 293T with either pDM128/RRE or pDM128/4XMS2.
The MS2-APC fusion protein contains two Crm1-dependent NESs derived from the APC protein (34). MS2-APC function, like Rev function, should therefore be blocked by Crm1-specific inhibitors, such as the drug leptomycin B (LMB) (12, 14). In fact, as shown in Fig. 2C, CAT activity induced by either MS2-Rev or MS2-APC was effectively inhibited by LMB treatment. In contrast, a fusion protein consisting of the MS2 RNA binding domain linked to the Tap nuclear RNA export factor, which is known to function independently of Crm1 (19), remained fully able to activate pDM128/4XMS2-dependent CAT expression even in the presence of LMB.
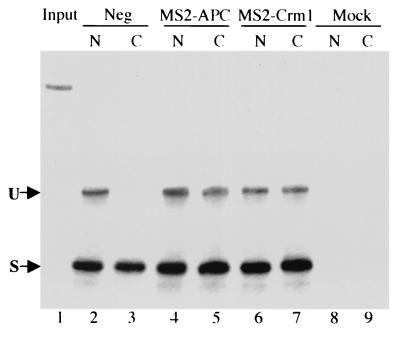
Several groups have used the pDM128 indicator plasmid to examine nuclear mRNA export, and it has been demonstrated repeatedly that activation of CAT enzyme activity correlates with the cytoplasmic appearance of the predicted unspliced cat mRNA species (16, 19, 30, 32, 49). Nevertheless, we wished to clearly demonstrate, using an RNase protection assay, that MS2-APC expression was indeed inducing the nuclear export of the unspliced mRNA encoded by pDM128/4XMS2. As shown in Fig. 3, this unspliced RNA was readily detectable in the nucleus of 293T cells transfected with pDM128/4XMS2 but was not observed in the cytoplasm in the absence of an appropriate MS2-export factor fusion (compare lanes 2 and 3). However, coexpression of the MS2-APC fusion protein induced readily detectable cytoplasmic expression of this unspliced cat mRNA species (Fig. 3, lane 5).
FIG. 3.
Analysis of nuclear and cytoplasmic mRNA levels by RNase protection assay. 293T cells were transfected with 100 ng of pDM128/4xMS2 and 1,000 ng of pBC12/MS2-APC or pBC12/MS2-Crm1. The parental pBC12/CMV plasmid served as the negative control (Neg). At ≈72 h after transfection, nuclear (N) and cytoplasmic (C) RNA fractions were isolated, and the levels of unspliced (U) and spliced (S) mRNA derived from the pDM128/4xMS2 plasmid were quantified by RNase protection analysis. The mobility of the input RNA probe is given in lane 1, which shows the signal obtained with 0.05% of the level of probe used in this assay.
Direct recruitment of Crm1 activates nuclear RNA export.
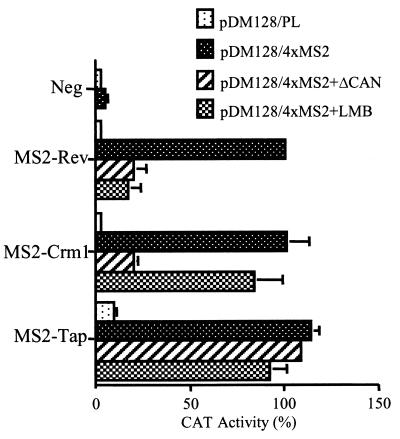
Using the pDM128/4XMS2 indicator plasmid, we have shown that an artificial protein that contains binding sites for Crm1 is sufficient to induce the nuclear export and expression of a tethered, HIV-1-derived RNA transcript (Fig. 2 and 3). However, if Crm1 recruitment to an RNA is indeed fully sufficient to induce the nuclear export of that RNA, then it is not apparent why an adapter protein such as Rev or MS2-APC would be required. Instead, it should be possible to induce the nuclear export of an mRNA by directly recruiting Crm1 to that RNA. To test this hypothesis, we expressed a fusion protein consisting of the MS2 coat protein RNA binding domain fused directly to the amino terminus of full-length Crm1 (13). In fact, the MS2-Crm1 fusion protein was very effective in inducing the cytoplasmic expression of the unspliced cat mRNA encoded by pDM128/4XMS2 when measured either at the RNA level (Fig. 3, lane 7) or at the protein level (Fig. 4).
FIG. 4.
MS2-Crm1 fusion protein can function as a nuclear mRNA export factor. 293T cells were transfected with the indicated indicator and effector plasmids, as described for Fig. 2, except that each transfection was also supplemented with 250 ng of pBC12/CMV/ΔCAN or of the pBC12/CMV control plasmid. LMB additions were performed as described for Fig. 2C. Induced CAT activities were measured at ≈40 h after transfection and are given relative to the culture transfected with pDM128/4xMS2 and pBC12/MS2-Rev in the absence of the ΔCAN expression plasmid.
To confirm that this activation indeed resulted from the normal nuclear export activity of the human Crm1 protein, we asked if this induction would be blocked by a reagent that specifically prevents Crm1 nucleocytoplasmic shuttling. The ΔCAN protein is a dominant negative form of the nucleoporin Nup214/CAN that consists solely of the short, phenylalanine-glycine (FG)-rich domain of Nup214/CAN that serves as a key binding site for Crm1 in the nuclear pore complex (13). It has previously been demonstrated that expression of ΔCAN selectively blocks not only Crm1 nucleocytoplasmic shuttling but also all nuclear export dependent on Crm1 function (2, 13). As shown in Fig. 4, ΔCAN was indeed able to effectively inhibit both MS2-Rev- and MS2-Crm1-dependent export of the unspliced cat mRNA encoded by pDM128/4XMS2, but had no effect on export mediated by the Crm1-independent nuclear export factor MS2-Tap.
We also examined whether addition of LMB would inhibit nuclear mRNA export mediated by the MS2-Crm1 fusion protein. As expected, LMB potently inhibited nuclear mRNA export mediated by the MS2-Rev protein but not by MS2-Tap (Fig. 4). However, LMB failed to inhibit nuclear mRNA export mediated by the MS2-Crm1 fusion protein. These results are consistent with previous data showing that LMB effectively blocks the interaction between Crm1 and leucine-rich NESs but does not inhibit the nucleocytoplasmic shuttling of the Crm1 protein (12, 14).
Both MS2-APC and MS2-Crm1 are nucleocytoplasmic shuttle proteins.
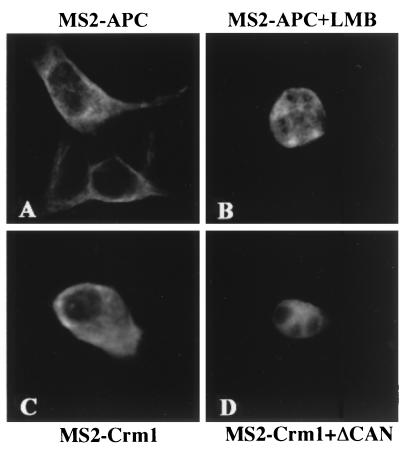
The observation that MS2-APC and MS2-Crm1 are able to induce the cytoplasmic expression of unspliced mRNAs bearing MS2 binding sites (Fig. 2 to 4) strongly implies that both proteins are nucleocytoplasmic shuttle proteins. To examine this issue, we first determined the steady-state localization of both fusion proteins in transfected 293T cells using an anti-MS2 antiserum. As shown in Fig. 5, both MS2-APC (panel A) and MS2-Crm1 (panel C) were largely cytoplasmic at steady state. If both proteins are, in fact, constantly shuttling into and out of the nucleus, then specific inhibition of their nuclear export should result in their relocalization from the cytoplasm to the nucleus. In fact, inhibition of Crm1-dependent NES function using LMB resulted in the rapid relocalization of MS2-APC into the cell nucleus (Fig. 5B). Similarly, inhibition of Crm1 function using ΔCAN also resulted in relocalization of MS2-Crm1 to the cell nucleus (Fig. 5D). Therefore, these data strongly support the hypothesis that both MS2-APC and MS2-Crm1 are nucleocytoplasmic shuttle proteins. Of interest, this nuclear retention did not result in any nucleolar concentration of either MS2-APC or MS2-Crm1, which appeared instead to be largely confined to the nucleoplasm. In contrast, the HIV-1 Rev protein has been shown to concentrate in the nucleoli of expressing cells, a property that has been mapped to the unusual arginine-rich NLS observed in HIV-1 Rev (16, 26, 32, 46).
FIG. 5.
Subcellular localization of the MS2-APC and MS2-Crm1 fusion proteins. 293T cells were seeded on cover slips and then transfected with 500 ng of pBC12/MS2-APC (A and B) or with 1,000 ng of pBC12/MS2-Crm1 together with 250 ng of the pBC12/CMV control plasmid (C) or 250 ng of pBC12/CMV/ΔCAN (D). At ≈16 h after transfection, LMB (5 ng/ml) was added to the culture visualized in panel B. At ≈40 h after transfection, cells were fixed and stained using a rabbit anti-MS2 antiserum, followed by FITC-conjugated donkey anti-rabbit IgG. Images were collected using a Leica DMRA fluorescence microscope.
Both MS2-APC and MS2-Crm1 can rescue HIV-1 structural protein expression.
The data presented thus far demonstrate that both MS2-APC and MS2-Crm1 can induce the sequence-specific nuclear export of a model unspliced mRNA. However, the key question, in terms of their ability to effectively mimic Rev function, is whether they are able to rescue the cytoplasmic expression of the incompletely spliced mRNAs encoded by a Rev-deficient HIV-1 provirus. The pNL4-3Rev− plasmid encodes a full-length HIV-1 provirus that contains a mutationally inactivated rev gene (33). As a result, pNL4-3Rev− is not able to express the viral Gag proteins, which are encoded by the unspliced, genome-length HIV-1 transcript, unless Rev is provided in trans.
To test whether MS2-APC or MS2-Crm1 would be able to substitute for Rev in inducing p24 gag expression, we inserted four tandem copies of the MS2 RNA operator into a unique XhoI site present in the dispensable HIV-1 nef gene and then assayed p24 gag production upon cotransfection of 293T cells with a wild-type Rev, MS2-APC, or MS2-Crm1 expression plasmid, together with either the pNL4-3Rev− or the pNL4-3Rev−/4XMS2 provirus. As shown in Table 1, only the Rev protein was able to rescue p24 gag expression from the parental Rev-deficient pNL4-3Rev− proviral clone. However, MS2-APC and MS2-Crm1 were both able to rescue p24 gag expression upon cotransfection with the pNL4-3 Rev−/4xMS2 proviral clone, with MS2-Crm1 being essentially as active as Rev in this assay. We therefore conclude that both MS2-APC and MS2-Crm1 are indeed able to substitute for Rev in mediating the nuclear export and cytoplasmic expression of the unspliced HIV-1 mRNA encoding the viral Gag structural protein.
TABLE 1.
Rescue of HIV-1 Gag expression from a Rev provirus by MS2 fusion proteinsa
| Proviral clone | Avg p24 released (ng/ml) ± SD
|
|||
|---|---|---|---|---|
| Negative control | Rev | MS2-Crm1 | MS2-APC | |
| pNL4-3Rev− | 0.48 ± 0.03 | 126.5 ± 12.1 | 0.51 ± 0.05 | 0.41 ± 0.05 |
| pNL4-3Rev−/4×MS2 | 0.48 ± 0.06 | 129.8 ± 33.9 | 78.2 ± 11.9 | 13.6 ± 2.3 |
293T cells were transfected with 500 ng of the pNL4-3Rev− or pNL4-3Rev−/4×MS2 proviral clone together with 1,000 ng of a plasmid expressing Rev or the indicated MS2 fusion protein. The parental pBC12/CMV plasmid served as the negative control. The level of p24 antigen released into the medium of the transfected cultures was assayed at ∼72 h posttransfection by ELISA. Averages of three independent experiments with standard deviation are indicated.
DISCUSSION
While the evidence demonstrating that Rev functions as a Crm1-dependent nuclear mRNA export factor appears conclusive, it has remained unclear whether this is the only biological activity of Rev or whether the activation of HIV-1 structural protein expression is additionally dependent on other activities. The main alternative proposed activity for Rev is a role in inhibiting HIV-1 mRNA splicing. Consistent with this hypothesis, data have been presented suggesting that Rev can inhibit the splicing of RRE-containing RNAs in vitro (21), that Rev interacts with p32, a potential cofactor of the splicing factor ASF/SF2 (25, 44), and that Rev can induce recruitment of ASF/SF2 to the RRE in vitro (38). In addition, Rev has been proposed to contain a functional domain, coincident with the Rev ARM/NLS, that promotes recruitment of Rev to subnuclear domains enriched for known splicing factors (9). On the other hand, Rev does not increase the level of nuclear unspliced HIV-1 mRNAs in most cell systems (4, 30), and point mutants of the Rev NES, which might be predicted to be inhibited only for Crm1 recruitment, have no enhancing effect on either the nuclear or cytoplasmic expression of incompletely spliced HIV-1 RNAs (26, 30).
A second possible activity of Rev is at the level of translation. While several groups have argued that little or no unspliced HIV-1 mRNA reaches the cytoplasm in cells infected or transfected with a Rev− HIV-1 provirus (4, 29, 30, 32), others have suggested that unspliced HIV-1 mRNA does enter the cytoplasm in the absence of Rev yet is not translated (1, 8, 41). These groups have therefore suggested that Rev also activates the translation of RRE-containing HIV-1 transcripts. A proposed functional domain that allows the association of Rev with currently undefined cytoplasmic components and that coincides with sequences required for Rev multimerization could play a role in this hypothetical Rev activity (22). Finally, in addition to these two novel activities for Rev, it has also been suggested that other proteins, in addition to Crm1, can interact directly with Rev to promote nuclear mRNA export. Of these, the most prominent is eIF-5A, which has been proposed to bind directly to the Rev NES (39).
Domains in Rev that mediate splicing inhibition or translational activation either have not been defined or have been suggested to coincide with domains important for Crm1-dependent nuclear RNA export (9, 22). As a result, it has not been possible to clearly address the functional relevance of these hypothetical Rev activities. Our initial approach to this problem was to build an artificial RNA binding protein that undergoes Crm1-dependent nucleocytoplasmic shuttling, using defined functional domains from proteins that play no role in either splicing or translation. The resultant artificial protein, termed MS2-APC, is similar to Rev in that it contains an RNA binding domain (from the bacteriophage MS2 coat protein), a short NLS (from SV40 T-Ag), and Crm1-dependent NESs (from the tumor suppressor protein APC). While MS2-APC does not contain multimerization sequences comparable to those seen in HIV-1 Rev, which are known to be essential for Rev function (17, 27). MS2-APC should dimerize via the MS2 coat protein sequence (47), and MS2-APC also contains two NESs, i.e., one more than Rev (34). Finally, we cloned from two to eight tandem MS2 RNA operator sequences into indicator constructs designed to detect MS2-APC-dependent nuclear RNA export in order to facilitate recruitment of multiple MS2-APC molecules.
Despite the predicted poor multimerization activity of MS2-APC, this protein proved to be almost as active as a similar MS2-Rev fusion protein, or indeed wild-type Rev, in mediating the sequence-specific nuclear export and cytoplasmic expression of the unspliced cat mRNA encoded by indicator constructs based on pDM128 (Fig. 2 and 3). Perhaps surprisingly, MS2-Rev and MS2-APC appeared similar in that both required a minimum of two MS2 RNA binding sites for significant export activity (Fig. 2A). As predicted (34), MS2-APC-induced nuclear RNA export proved to be Crm1 dependent (Fig. 2C), as was MS2-APC nucleocytoplasmic shuttling (Fig. 5). Finally, MS2-APC also proved able to rescue the expression of Gag protein by a Rev− HIV-1 provirus, although with only modest efficiency compared to wild-type HIV-1 Rev (Table 1). Nevertheless, these data clearly indicate that MS2-APC contains all of the functional domains that are essential for the Crm1-dependent nuclear export of tethered RNA species.
While the results obtained with MS2-APC were clearly consistent with the hypothesis that Crm1 recruitment is the key biological activity of Rev, we wished to address this question more directly. For this purpose, we therefore expressed a fusion protein consisting of the MS2 RNA binding domain fused to full-length human Crm1 and asked whether the direct recruitment of Crm1, without an intervening adapter protein, would also suffice to rescue the sequence-specific nuclear export of tethered unspliced mRNAs. In fact, MS2-Crm1 proved highly effective at inducing the cytoplasmic expression of unspliced cat mRNAs derived from the pDM128 reporter plasmid (Fig. 3 and 4) and was almost as effective as wild-type Rev in rescuing structural gene expression from a Rev− HIV-1 provirus (Table 1). As predicted (2, 13), MS2-Crm1 activity and nucleocytoplasmic shuttling were specifically blocked by the dominant negative ΔCAN mutant of the nucleoporin Nup214/CAN (Fig. 4 and 5). We therefore conclude that Crm1 recruitment is not only necessary but also sufficient to induce the efficient nuclear export and cytoplasmic translation of incompletely spliced HIV-1 mRNA species. While these data are fully consistent with the hypothesis that Crm1 recruitment to the RRE is the only physiologically relevant role of Rev in the HIV-1 life cycle, it remains formally possible that Rev has other, as yet undiscovered functions, distinct from its role in activating late HIV-1 protein expression, that were not addressed in these experiments.
Acknowledgments
We thank George Pavlakis, Tristram Parslow, Kristi Neufeld, Gerard Grosveld, Minoru Yoshida, and Marvin Wickens for reagents used in this study.
This research was funded by the Howard Hughes Medical Institute.
REFERENCES
- 1.Arrigo, S. J., and I. S. Y. Chen. 1991. Rev is necessary for translation but not cytoplasmic accumulation of HIV-1 vif, vpr, and env/vpu2 RNAs. Genes Dev. 5:808-819. [DOI] [PubMed] [Google Scholar]
- 2.Bogerd, H. P., A. Echarri, T. M. Ross, and B. R. Cullen. 1998. Inhibition of human immunodeficiency virus Rev and human T-cell leukemia virus Rex function, but not Mason-Pfizer monkey virus constitutive transport element activity, by a mutant human nucleoporin targeted to Crm1. J. Virol. 72:8627-8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray, M., S. Prasad, J. W. Dubay, E. Hunter, K.-T. Jeang, D. Rekosh, and M.-L. Hammarskjold. 1994. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc. Natl. Acad. Sci. USA 91:1256-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, D. D., and P. A. Sharp. 1989. Regulation by HIV Rev depends upon recognition of splice sites. Cell 59:789-795. [DOI] [PubMed] [Google Scholar]
- 5.Coburn, G. A., H. L. Wiegand, Y. Kang, D. N. Ho, M. M. Georgiadis, and B. R. Cullen. 2001. Using viral species specificity to define a critical protein/RNA interaction surface. Genes Dev. 15:1194-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullen, B. R. 1986. trans-Activation of human immunodeficiency virus occurs via a bimodal mechanism. Cell 46:973-982. [DOI] [PubMed] [Google Scholar]
- 7.Cullen, B. R. 2000. Nuclear RNA export pathways. Mol. Cell. Biol. 20:4181-4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Agostino, D. M., B. K. Felber, J. E. Harrison, and G. N. Pavlakis. 1992. The Rev protein of human immunodeficiency virus type 1 promotes polysomal association and translation of gag/pol and vpu/env mRNAs. Mol. Cell. Biol. 12:1375-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Agostino, D. M., T. Ferro, L. Zotti, F. Meggio, L. A. Pinna, L. Chieco-Bianchi, and V. Ciminale. 2000. Identification of a domain in human immunodeficiency virus type 1 Rev that is required for functional activity and modulates association with subnuclear compartments containing splicing factor SC35. J. Virol. 74:11899-11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feinberg, M. S., R. F. Jarrett, A. Aldovini, R. C. Gallo, and F. Wong-Staal. 1986. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell 46:807-817. [DOI] [PubMed] [Google Scholar]
- 11.Fischer, U., J. Huber, W. C. Boelens, I. W. Mattaj, and R. Luhrmann. 1995. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell 82:475-483. [DOI] [PubMed] [Google Scholar]
- 12.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. Crm1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 13.Fornerod, M., J. van Deursen, S. van Baal, A. Reynolds, D. Davis, K. G. Murti, J. Fransen, and G. Grosveld. 1997. The human homologue of yeast Crm1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J. 16:807-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuda, M., S. Asano, T. Nakamura, M. Adachi, M. Yoshida, M. Yanagida, and E. Nishida. 1997. Crm1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390:308-311. [DOI] [PubMed] [Google Scholar]
- 15.Gruter, P., C. Tabernero, C. von Kobbe, C. Schmitt, C. Saavedra, A. Bachi, M. Wilm, B. K. Felber, and E. Izaurralde. 1998. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol. Cell 1:649-659. [DOI] [PubMed] [Google Scholar]
- 16.Hope, T. J., X. Huang, D. McDonald, and T. G. Parslow. 1990. Steroid-receptor fusion of the human immunodeficiency virus type 1 Rev transactivator: mapping cryptic functions of the arginine-rich motif. Proc. Natl. Acad. Sci. USA 87:7787-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain, C., and J. G. Belasco. 2001. Structural model for the cooperative assembly of HIV-1 Rev multimers on the RRE as deduced from analysis of assembly-defective mutants. Mol. Cell 7:603-614. [DOI] [PubMed] [Google Scholar]
- 18.Kalderon, D., B. L. Roberts, W. D. Richardson, and A. E. Smith. 1984. A short amino acid sequence able to specify nuclear location. Cell 39:499-509. [DOI] [PubMed] [Google Scholar]
- 19.Kang, Y., and B. R. Cullen. 1999. The human Tap protein is a nuclear mRNA export factor that contains novel RNA-binding and nucleocytoplasmic transport sequences. Genes Dev. 13:1126-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kjems, J., B. J. Calnan, A. D. Frankel, and P. A. Sharp. 1992. Specific binding of a basic peptide from HIV-1 Rev. EMBO J. 11:1119-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kjems, J., A. D. Frankel, and P. A. Sharp. 1991. Specific regulation of mRNA splicing in vitro by a peptide from HIV-1 Rev. Cell 67:169-178. [DOI] [PubMed] [Google Scholar]
- 22.Kubota, S., and R. J. Pomerantz. 1998. A cis-acting peptide signal in human immunodeficiency virus type 1 Rev which inhibits nuclear entry of small proteins. Oncogene 16:1851-1861. [DOI] [PubMed] [Google Scholar]
- 23.Legrain, P., and M. Rosbash. 1989. Some cis- and trans-acting mutants for splicing target pre-mRNA to the cytoplasm. Cell 57:573-583. [DOI] [PubMed] [Google Scholar]
- 24.Lindsay, M. E., J. M. Holaska, K. Welch, B. M. Paschal, and I. G. Macara. 2001. Ran-binding protein 3 is a cofactor for Crm1-mediated nuclear protein export. J. Cell Biol. 153:1391-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo, Y., H. Yu, and B. M. Peterlin. 1994. Cellular protein modulates effects of human immunodeficiency virus type 1 Rev. J. Virol. 68:3850-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malim, M. H., S. Bohnlein, J. Hauber, and B. R. Cullen. 1989. Functional dissection of the HIV-1 Rev trans-activator--derivation of a trans-dominant repressor of Rev function. Cell 58:205-214. [DOI] [PubMed] [Google Scholar]
- 27.Malim, M. H., and B. R. Cullen. 1991. HIV-1 structural gene expression requires the binding of multiple Rev monomers to the viral RRE: Implications for HIV-1 latency. Cell 65:241-248. [DOI] [PubMed] [Google Scholar]
- 28.Malim, M. H., and B. R. Cullen. 1993. Rev and the fate of pre-mRNA in the nucleus: implications for the regulation of RNA processing in eukaryotes. Mol. Cell. Biol. 13:6180-6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malim, M. H., J. Hauber, S.-Y Le, J. V. Maizel, and B. R. Cullen. 1989. The HIV-1 Rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature 338:254-257. [DOI] [PubMed] [Google Scholar]
- 30.Malim, M. H., D. F. McCarn, L. S. Tiley, and B. R. Cullen. 1991. Mutational definition of the human immunodeficiency virus type 1 Rev activation domain. J. Virol. 65:4248-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malim, M. H., L. S. Tiley, D. F. McCarn, J. R. Rusche, J. Hauber, and B. R. Cullen. 1990. HIV-1 structural gene expression requires binding of the Rev trans-activator to its RNA target sequence. Cell 60:675-683. [DOI] [PubMed] [Google Scholar]
- 32.McDonald, D., T. J. Hope, and T. G. Parslow. 1992. Posttranscriptional regulation by the human immunodeficiency virus type 1 Rev and human T-cell leukemia virus type I Rex proteins through a heterologous RNA binding site. J. Virol. 66:7232-7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nappi, F., R. Schneider, A. Zolotukhin, S. Smulevitch, D. Michalowski, J. Bear, B. K. Felber, and G. N. Pavlakis. 2001. Identification of a novel posttranscriptional regulatory element by using a rev- and RRE-mutated human immunodeficiency virus type 1 DNA proviral clone as a molecular trap. J. Virol. 75:4558-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neufeld, K. L., D. A. Nix, H. Bogerd, Y. Kang, M. C. Beckerle, B. R. Cullen, and R. L. White. 2000. Adenomatous polyposis coli protein contains two nuclear export signals and shuttles between the nucleus and cytoplasm. Proc. Natl. Acad. Sci. USA 97:12085-12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neville, M., F. Stutz, L. Lee, L. I. Davis, and M. Rosbash. 1997. The importin-beta family member Crm1p bridges the interaction between Rev and the nuclear pore complex during nuclear export. Curr. Biol. 7:767-775. [DOI] [PubMed] [Google Scholar]
- 36.Ogert, R. A., L. H. Lee, and K. L. Beemon. 1996. Avian retroviral RNA element promotes unspliced RNA accumulation in the cytoplasm. J. Virol. 70:3834-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollard, V. W., and M. H. Malim. 1998. The HIV-1 Rev protein. Annu. Rev. Microbiol. 52:491-532. [DOI] [PubMed] [Google Scholar]
- 38.Powell, D. M., M. C. Amaral, J. Y. Wu, T. Maniatis, and W. C. Greene. 1997. HIV Rev-dependent binding of SF2/ASF to the Rev response element: possible role in Rev-mediated inhibition of HIV RNA splicing. Proc. Natl. Acad. Sci. USA 94:973-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruhl, M., M. Himmelspach, G. M. Bahr, F. Hammerschmid, H. Jaksche, B. Wolff, H. Aschauer, G. K. Farrington, H. Probst, D. Bevec, and J. Hauber. 1993. Eukaryotic initiation factor 5A is a cellular target of the human immunodeficiency virus type 1 Rev activation domain mediating trans-activation. J. Cell Biol. 123:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.SenGupta, D. J., B. Zhang, B. Kraemer, P. Pochart, and S. Fields. 1996. A three-hybrid system to detect RNA-protein interactions in vivo. Proc. Natl. Acad. Sci. USA 93:8496-8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sodroski, J., W. C. Goh, C. Rosen, A. Dayton, E. Terwilliger, and W. Haseltine. 1986. A second post-transcriptional trans-activator gene required for HTLV-III replication. Nature 321:412-417. [DOI] [PubMed] [Google Scholar]
- 42.Stade, K., C. S. Ford, C. Guthrie, and K. Weis. 1997. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 90:1041-1050. [DOI] [PubMed] [Google Scholar]
- 43.Tan, W., A. S. Zolotukhin, J. Bear, D. J. Patenaude, and B. K. Felber. 2000. The mRNA export in Caenorhabditis elegans is mediated by Ce-NXF-1, an ortholog of human TAP/NXF and Saccharomyces cerevisiae Mex67p. RNA 6:1762-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tange, T. O., T. H. Jesen, and J. Kjems. 1996. In vitro interaction between immunodeficiency virus type 1 Rev protein and splicing factor ASF/SF2-associated protein, p32. J. Biol. Chem. 271:10066-10072. [DOI] [PubMed] [Google Scholar]
- 45.Triezenberg, S. J., R. C. Kingsbury, and S. L. McKnight. 1988. Functional dissection of VP16, the trans-activator of herpes simples virus immediate early gene expression. Genes Dev. 2:718-729. [DOI] [PubMed] [Google Scholar]
- 46.Truant, R., and B. R. Cullen. 1999. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin β-dependent nuclear localization signals. Mol. Cell. Biol. 19:1210-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Worm, S. H. E., N. J. Stonehouse, K. Valegard, J. B. Murray, C. Walton, K. Fridborg, P. G. Stockley, and L. Liljas. 1998. Crystal structures of MS2 coat protein mutants in complex with wild-type RNA operator fragments. Nucleic Acids Res. 26:1345-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wen, W., J. L. Meinkoth, R. Y. Tsien, and S. S. Taylor. 1995. Identification of a signal for rapid export of proteins from the nucleus. Cell 82:463-473. [DOI] [PubMed] [Google Scholar]
- 49.Yang, J., and B. R. Cullen. 1999. Structural and functional analysis of the avian leukemia virus constitutive transport element. RNA 5:1645-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zapp, M. L., and M. R. Green. 1989. Sequence-specific RNA binding by the HIV-1 Rev protein. Nature 342:714-716. [DOI] [PubMed] [Google Scholar]