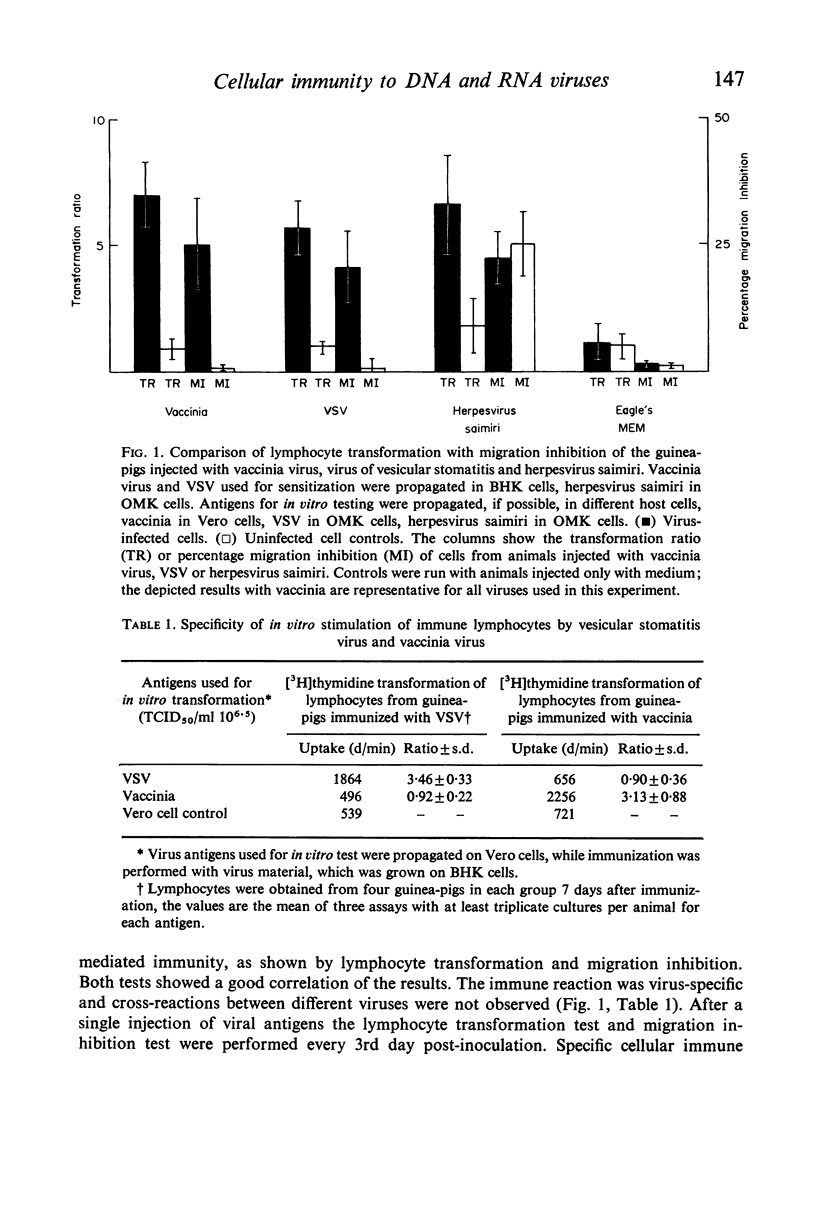
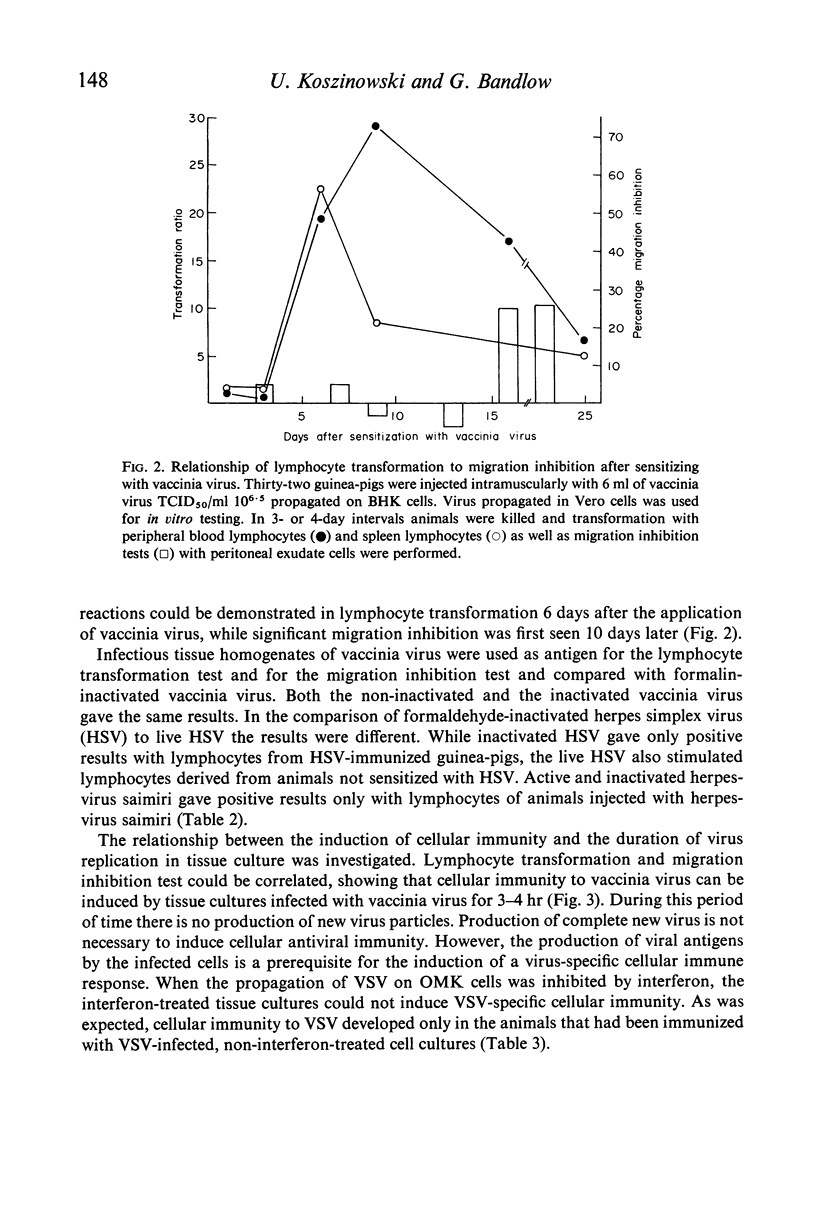
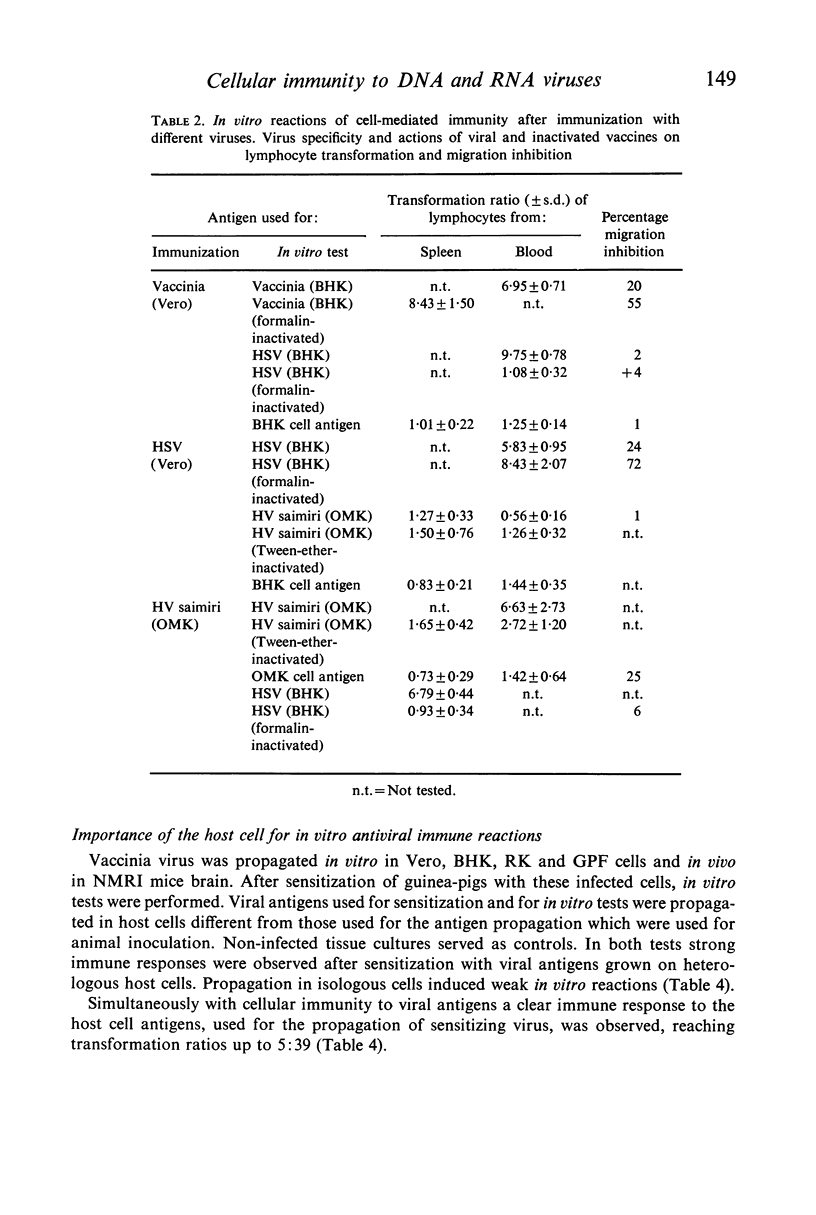
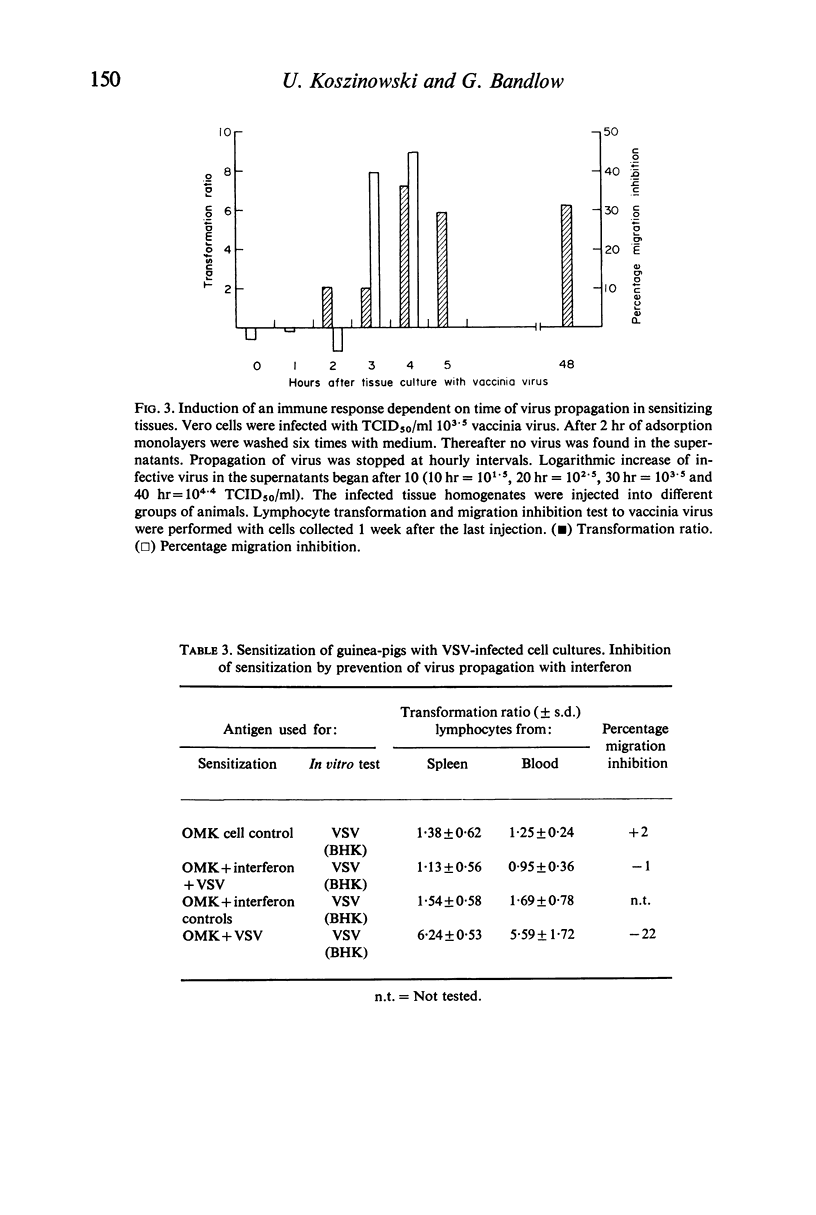
Abstract
Guinea-pigs were immunized with different cells infected with vaccinia virus, herpes simplex virus type 1, herpesvirus saimiri, and the virus of vesicular stomatitis. Development of cellular immunity against these viruses was observed with transformation of blood and spleen lymphocytes and with the migration inhibition test using peritoneal exudate cells. Cellular immunity against vaccinia virus was first seen 6 days after the inoculation of cell-bound vaccinia virus by lymphocyte transformation. The avtivation of the vaccinia virus specific cellular immune response could be induced with tissue culturrus. Since infectious virus particles are not synthesized within this time period, it is likely that virus-induced antigens in the cell surface are active in production of cellular immunity. Vaccines from heterologous host cells were more effective inducers of an immune response than syngeneic cell cultures. For in vitro testing of cellular immunity to viruses, viral antigens could be used in both infective and inactivated form. Delayed hypersensitivity to viral antigens was always accompanied by immune reactions to the host cells used for virus propagation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BODIAN D. Experimental studies on passive immunization against poliomyelitis. II. The prophylactic effect of human gamma globulin on paralytic poliomyelitis in cynomolgus monkeys after virus feeding. Am J Hyg. 1952 Jul;56(1):78–89. [PubMed] [Google Scholar]
- Bandlow G., Fischer W., Thomssen R. Untersuchungen zum Mechanismus der immunologischen Adjuvanswirkung des Vacciniavirus. Arch Gesamte Virusforsch. 1972;38(2):192–204. [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. I. The effects of anti-thymocyte serum. J Exp Med. 1970 Nov;132(5):1035–1054. doi: 10.1084/jem.132.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouroncle B. A., Clausen K. P., Darner E. M. Replication of herpes simplex virus in cultures of phytohemagglutinin-stimulated human lymphocytes. J Natl Cancer Inst. 1970 May;44(5):1065–1078. [PubMed] [Google Scholar]
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- Feinstone S. M., Beachey E. H., Rytel M. W. Induction of delayed hypersensitivity to influenza and mumps viruses in mice. J Immunol. 1969 Oct;103(4):844–849. [PubMed] [Google Scholar]
- GEORGE M., VAUGHAN J. H. In vitro cell migration as a model for delayed hypersensitivity. Proc Soc Exp Biol Med. 1962 Nov;111:514–521. doi: 10.3181/00379727-111-27841. [DOI] [PubMed] [Google Scholar]
- GOOD R. A., VARCO R. L. A clinical and experimental study of agammaglobulinemia. J Lancet. 1955 Jun;75(6):245–271. [PubMed] [Google Scholar]
- Gardner I., Bowern N. A., Blanden R. V. Cell-mediated cytotoxicity against ectromelia virus-infected target cells. I. Specificity and kinetics. Eur J Immunol. 1974 Feb;4(2):63–67. doi: 10.1002/eji.1830040202. [DOI] [PubMed] [Google Scholar]
- Havemann K. Nucleinsäure- und Proteinstoffwechsel in Lymphocytenkulturen von Gesunden und Patienten mit Erkrankungen des lymphatischen Systems. I. Methodik und kinetische Untersuchungen bei Gesunden. Z Gesamte Exp Med. 1969;151(2):138–162. [PubMed] [Google Scholar]
- Hirsch M. S., Nahmias A. J., Murphy F. A., Kramer J. H. Cellular immunity in vaccinia infection of mice. Anti-thymocyte serum effects on primary and secondary responsiveness. J Exp Med. 1968 Jul 1;128(1):121–132. doi: 10.1084/jem.128.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lundstedt C. Interaction between antigenically different cells. Virus-induced cytotoxicity by immune lymphoid cells in vitro. Acta Pathol Microbiol Scand. 1969;75(1):139–152. [PubMed] [Google Scholar]
- Marker O., Volkert M. Studies on cell-mediated immunity to lymphocytic choriomeningitis virus in mice. J Exp Med. 1973 Jun 1;137(6):1511–1525. doi: 10.1084/jem.137.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland H. F. In vitro studies of cell-mediated immunity in an acute viral infection. J Immunol. 1974 Jul;113(1):173–180. [PubMed] [Google Scholar]
- Norén L., Espmark A., Fagraeus A., Holm S. E., Lindahl J., Lycke E., Marquardt J. Vaccinia antigens for skin testing. Preparation of antigens and quantitative studies of skin reactions in "standard populations". Acta Med Scand Suppl. 1966;464:162–169. doi: 10.1111/j.0954-6820.1966.tb05084.x. [DOI] [PubMed] [Google Scholar]
- Pilchard E. I., Gold E. E., Gray H. K. A study of delayed hypersensitivity of guinea pigs to vaccinia virus. Comparison of inhibition of cell migration to a local passive transfer reaction for the quantification of delayed hypersensitivity. Int Arch Allergy Appl Immunol. 1970;37(4):337–343. doi: 10.1159/000230795. [DOI] [PubMed] [Google Scholar]
- Rogers H. W., Scott L. V., Patnode R. A. Sensitization of guinea pigs to herpes simplex virus. J Immunol. 1972 Oct;109(4):801–807. [PubMed] [Google Scholar]
- Rosenberg G. L., Farber P. A., Notkins A. L. In vitro stimulation of sensitized lymphocytes by herpes simplex virus and vaccinia virus. Proc Natl Acad Sci U S A. 1972 Mar;69(3):756–760. doi: 10.1073/pnas.69.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G. L., Wohlenberg C., Nahmias A. J., Notkins A. L. Differentiation of type 1 and type 2 herpes simplex virus by in vitro stimulation of immune lymphocytes. J Immunol. 1972 Aug;109(2):413–414. [PubMed] [Google Scholar]
- Seligmann M., Fudenberg H. H., Good R. A. A proposed classification of primary immunologic deficiencies. Am J Med. 1968 Dec;45(6):817–825. doi: 10.1016/0002-9343(68)90180-0. [DOI] [PubMed] [Google Scholar]
- Speel L. F., Osborn J. E., Walker D. L. An immuno-cytopathogenic interaction between sensitized leukocytes and epithelial cells carrying a persistent noncytocidal myxovirus infection. J Immunol. 1968 Sep;101(3):409–417. [PubMed] [Google Scholar]
- Tompkins W. A., Adams C., Rawls W. E. An in vitro measure of cellular immunity to fibroma virus. J Immunol. 1970 Feb;104(2):502–510. [PubMed] [Google Scholar]
- Ueda Y., Tagaya I., Amano H., Ito M. Studies on the early antigens induced by vaccinia virus. Virology. 1972 Sep;49(3):794–800. doi: 10.1016/0042-6822(72)90535-1. [DOI] [PubMed] [Google Scholar]
- Ueda Y., Tagaya I. Induction of skin resistance to vaccinia virus in rabbits by vaccinia-soluble early antigens. J Exp Med. 1973 Nov 1;138(5):1033–1043. doi: 10.1084/jem.138.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. W., Ortiz de Landazuri M., Herberman R. B. Immune response to Gross virus-induced lymphoma: comparison of two in vitro assays of cell-mediated immunity. J Natl Cancer Inst. 1973 Apr;50(4):947–954. doi: 10.1093/jnci/50.4.947. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]


