Abstract
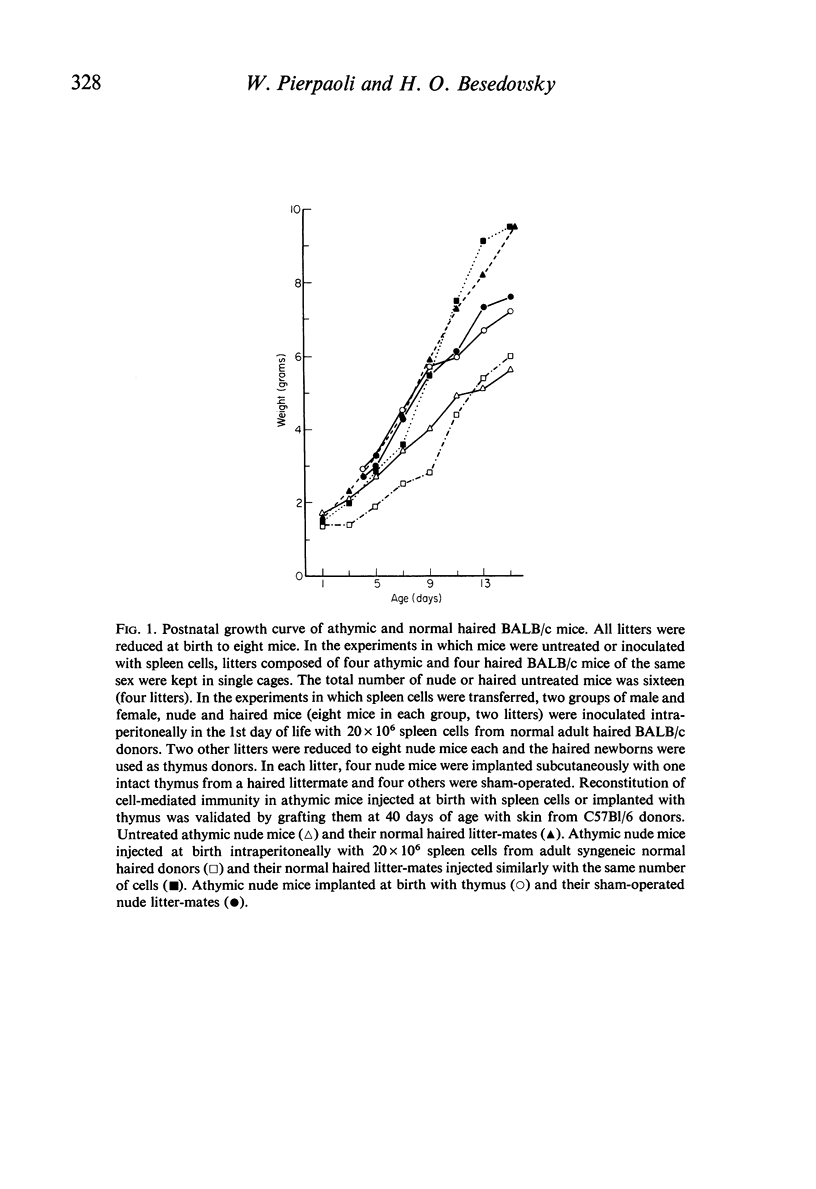
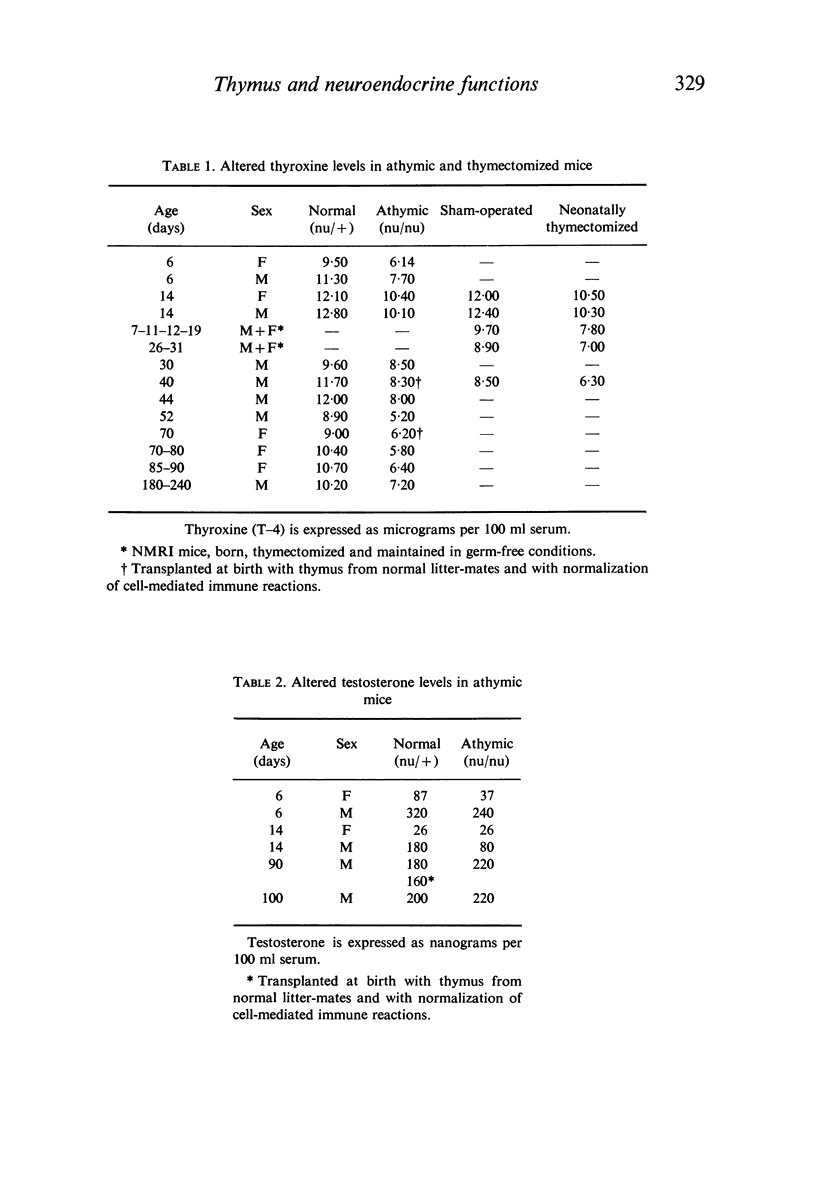
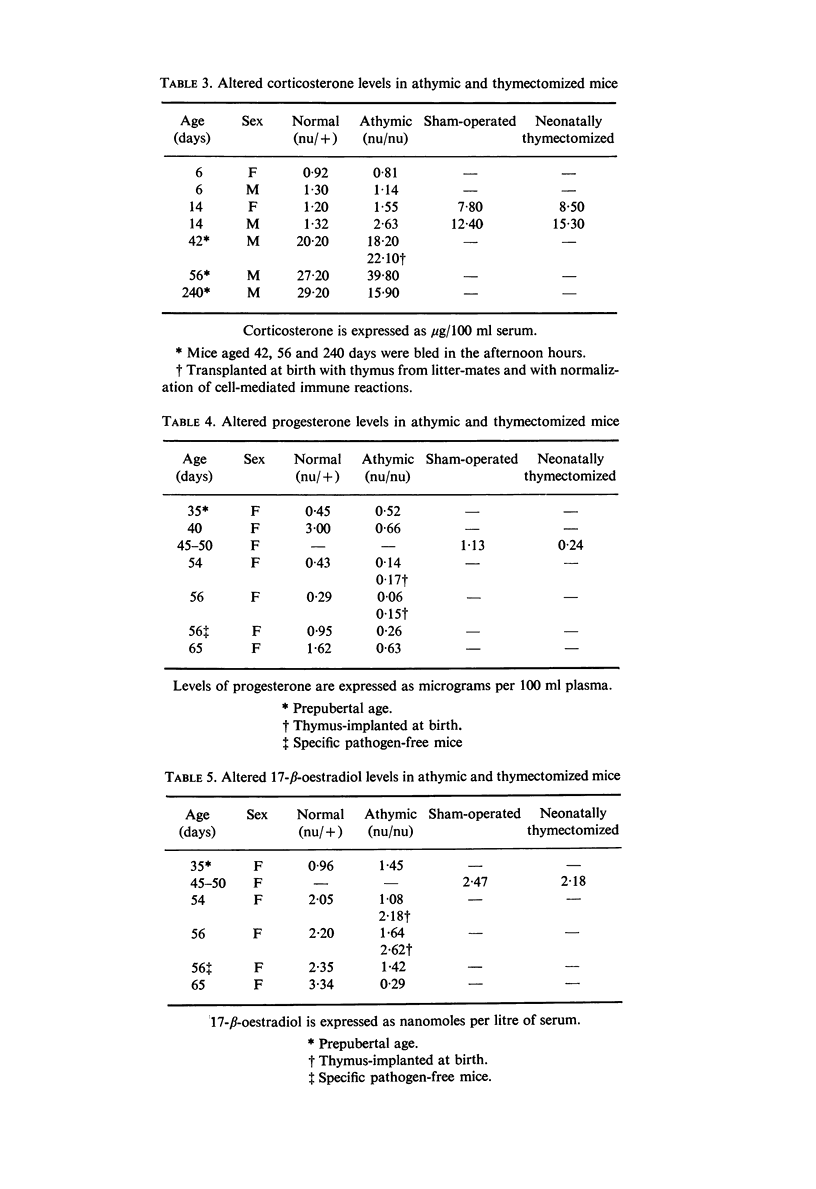
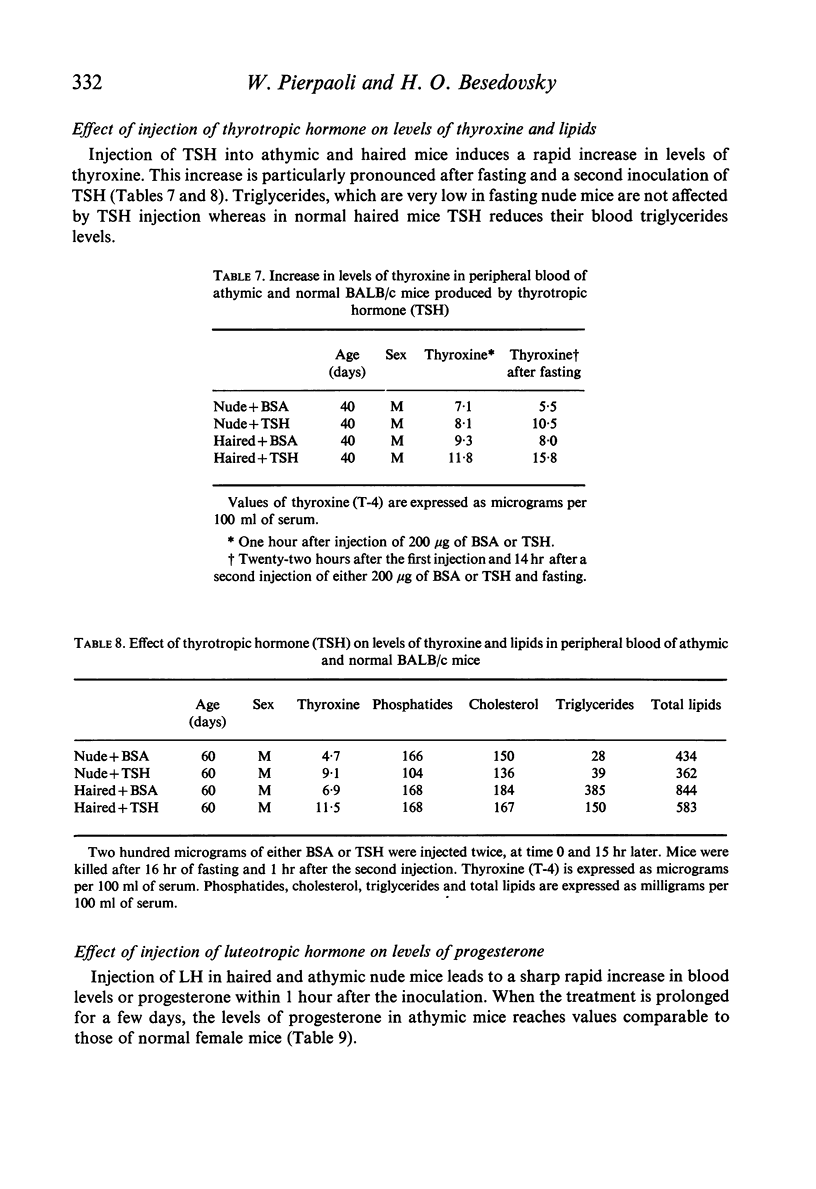
Specific derangements of thyroid and gonadal functions were observed in athymic nude and neonatally thymectomized mice. Such endocrine alterations are already established during the perinatal period and maintained through adult life. Passive transfer of lymphoid cells from normal donors does not prevent such alterations. In nude mice thymus implantation at birth fully reconstitutes oestrogenic function, but thyroid and progestational functions remain defective. Peripheral endocrine glands (thyroid, adrenals and ovaries) respond normally to ACTH, TSH and LH. Thus the thymus may well have a basic role in the organization of the adult hypothalamus-pituitary axis for thyroid and sexual functions.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G. E. Solid-phase radioimmunoassay of estradiol-17 beta. J Clin Endocrinol Metab. 1969 Jun;29(6):866–870. doi: 10.1210/jcem-29-6-866. [DOI] [PubMed] [Google Scholar]
- Alexander D. P., Britton H. G., Forsling M. L., Nixon D. A., Ratcliffe J. G. The concentrations of adrenocorticotrophin, vasopressin and oxytocin in the foetal and maternal plasma of the sheep in the latter half of gestation. J Endocrinol. 1971 Jan;49(1):179–180. doi: 10.1677/joe.0.0490179. [DOI] [PubMed] [Google Scholar]
- Anderson D. C. A simple and clinically useful method for plasma testosterone-like substances by competitive protein binding. Clin Chim Acta. 1970 Sep;29(3):513–522. doi: 10.1016/0009-8981(70)90023-9. [DOI] [PubMed] [Google Scholar]
- Bassett J. M., Thorburn G. D., Wallace A. L. The plasma growth hormone concentration of the foetal lamb. J Endocrinol. 1970 Oct;48(2):251–263. doi: 10.1677/joe.0.0480251. [DOI] [PubMed] [Google Scholar]
- Besedovsky H. O., Sorkin E. Thymus involvement in female sexual maturation. Nature. 1974 May 24;249(455):356–358. doi: 10.1038/249356a0. [DOI] [PubMed] [Google Scholar]
- Biachi E., Pierpaoli W., Sorkin E. Cytological changes in the mouse anterior pituitary after neonatal thymectomy: a light ane electron microscopical study. J Endocrinol. 1971 Sep;51(1):1–6. doi: 10.1677/joe.0.0510001. [DOI] [PubMed] [Google Scholar]
- Brown-Grant K., Sherwood M. R. The "early androgen syndrome" in the guinea-pig. J Endocrinol. 1971 Feb;49(2):277–291. doi: 10.1677/joe.0.0490277. [DOI] [PubMed] [Google Scholar]
- Demetriou J. A., Austin F. G. A rapid competitive protein-binding assay for plasma progesterone. Clin Chim Acta. 1971 Jun;33(1):21–32. doi: 10.1016/0009-8981(71)90244-0. [DOI] [PubMed] [Google Scholar]
- HARRIS G. W. SEX HORMONES, BRAIN DEVELOPMENT AND BRAIN FUNCTION. Endocrinology. 1964 Oct;75:627–648. doi: 10.1210/endo-75-4-627. [DOI] [PubMed] [Google Scholar]
- Jost A., Vigier B., Prépin J., Perchellet J. P. Studies on sex differentiation in mammals. Recent Prog Horm Res. 1973;29:1–41. doi: 10.1016/b978-0-12-571129-6.50004-x. [DOI] [PubMed] [Google Scholar]
- KLIMAN B., PETERSON R. E. Double isotope derivative assay of aldosterone in biological extracts. J Biol Chem. 1960 Jun;235:1639–1648. [PubMed] [Google Scholar]
- MCINTIRE K. R., SELL S., MILLER J. F. PATHOGENESIS OF THE POST-NEONATAL THYMECTOMY WASTING SYNDROME. Nature. 1964 Oct 10;204:151–155. doi: 10.1038/204151a0. [DOI] [PubMed] [Google Scholar]
- MUELLER J. ALDOSTERONE STIMULATION IN VITRO. I. EVALUATION OF ASSAY PROCEDURE AND DETERMINATION OF ALDOSTERONE-STIMULATING ACTIVITY IN A HUMAN URINE EXTRACT. Acta Endocrinol (Copenh) 1965 Feb;48:283–296. [PubMed] [Google Scholar]
- Mikhail G., Wu C. H., Ferin M., Vande Wiele R. L. Radioimmunoassay of plasma estrone and estradiol. Steroids. 1970 Mar;15(3):333–352. doi: 10.1016/s0039-128x(70)80053-8. [DOI] [PubMed] [Google Scholar]
- Murphy B. E., Pattee C. J., Gold A. Clinical evaluation of a new method for the determination of serum thyroxine. J Clin Endocrinol Metab. 1966 Mar;26(3):247–256. doi: 10.1210/jcem-26-3-247. [DOI] [PubMed] [Google Scholar]
- Pierce J. G., Liao T., Howard S. M., Shome B., Cornell J. S. Studies on the structure of thyrotropin: its relationship to luteinizing hormone. Recent Prog Horm Res. 1971;27:165–212. doi: 10.1016/b978-0-12-571127-2.50029-8. [DOI] [PubMed] [Google Scholar]
- Pierpaoli W., Fabris N., Sorkin E. Developmental hormones and immunological maturation. Ciba Found Study Group. 1970;36:126–153. [PubMed] [Google Scholar]
- Pierpaoli W., Sorkin E. Alterations of adrenal cortex and thyroid in mice with congenital absence of the thymus. Nat New Biol. 1972 Aug 30;238(87):282–285. doi: 10.1038/newbio238282a0. [DOI] [PubMed] [Google Scholar]
- Pierpaoli W., Sorkin E. Hormones, thymus and lymphocyte functions. Experientia. 1972 Nov 15;28(11):1385–1389. doi: 10.1007/BF01965362. [DOI] [PubMed] [Google Scholar]
- Uettwiller A. Testosteron-Bestimmung in Plasma und Urin mittels "Competitive Protein Binding Method". Z Klin Chem Klin Biochem. 1970 May;8(3):225–230. [PubMed] [Google Scholar]



