Abstract
We have previously described postentry restriction of murine leukemia virus in mammals. Here we characterize the block in human cells. Restricted infection kinetics are multiple hit at high virus dose, and restriction is abrogated by preexposure to restricted virus. We hypothesize that restricted capsid can titrate out the restriction factor.
Phylogenetic studies of endogenous retroviral sequences in vertebrates have shown that, generally, retroviral sequences and host sequences have coevolved, suggesting that horizontal transfer between most vertebrates occurs only rarely. However, analysis of mammalian endogenous retrovirus sequences suggests that interspecies infection events between mammals are relatively frequent (7). Perhaps because of this a number of host mechanisms to restrict retroviral replication have been identified in mammalian cells, including the murine gene Fv1. We have recently described a block to N-tropic murine leukemia virus infection in a wide variety of mammalian species (11). We presumed that there were genes of Fv1-like function in other mammals and have called the human gene encoding this activity Ref1 (11). Southern blot data have shown that there are no sequences related to Fv1 in the human genome (1).
Abbreviations.
MLV, murine leukemia virus; eGFP, enhanced green fluorescent protein; LacZ, β-galactosidase; FACS, flow-assisted cell sorting; CA, capsid; N-eGFP and B-eGFP, N- and B-tropic eGFP pseudotypes, respectively; MDTF, Mus dunni tail fibroblast; IU, infectious unit; N-LNCX and B-LNCX, N- and B-tropic LNCX vector, respectively.
The Ref1-mediated block in human cells is during reverse transcription (11), whereas in murine cells restricted virus finishes reverse transcription (6). Otherwise, the restricted phenotypes are similar and virus particles containing mixed N- and B-tropic CA are restricted in human cells. This suggests that Ref1 is also a dominant inhibitor of restricted retrovirus infection (11). However, these data could also be explained by less-efficient interaction of N-tropic CA with cellular factors essential for MLV infection of human cells.
Retrovirus restriction in mice can be overcome when restricted cells are challenged with high virus doses (4). Furthermore, restriction can be abrogated by prior exposure of the target cells to restricted virus (2), suggesting that CA protein in restricted virions binds to and titrates out a limited pool of Fv1 protein. Here we show that Ref1 restriction can be abrogated in a similar manner, providing further evidence that Ref1 is a factor which blocks retrovirus infection of human cells.
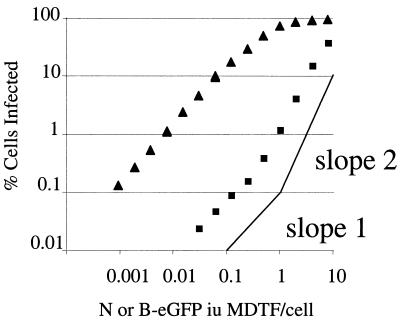
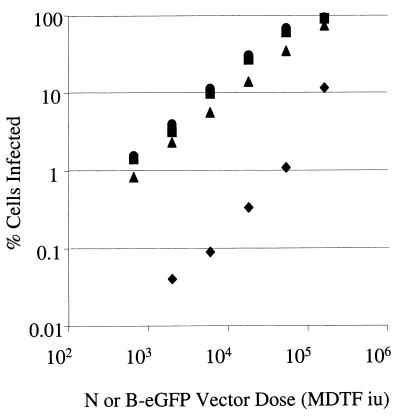
To measure retrovirus infection we packaged retroviral vectors encoding marker genes into N- and B-tropic retroviral cores pseudotyped with the G protein from vesicular stomatitis virus as previously described (11). N-eGFP and B-eGFP had similar titers on unrestricted, Fv1-null, MDTF cells (data not shown). Serial dilutions of virus were used to infect human rhabdomyosarcoma TE671 cells. Infected cells were measured by FACS. Figure 1 shows that at low virus doses B-eGFP vector infected TE671 cells approximately 100-fold more efficiently than N-tropic virus. Infection by unrestricted B-eGFP vector on TE671 cells was linear with respect to virus dose, with a gradient of 1 except at high virus doses, at which the slope decreased. This is a typical pattern of single-hit infection, where infection events increase linearly until a significant cell population is multiply infected. In contrast, the slope of restricted N-eGFP vector infection plotted versus virus dose deviated from 1 and became steeper when more than 0.5% of the cells were infected. In the range of 1 to 30% of cells being infected, the gradient was approximately 2. This suggests two-hit infection kinetics, where exposure to an initial, restricted virion facilitates infection by a second, restricted virion. At high virus doses, the difference in infection efficiency between N- and B-tropic viruses was reduced. This behavior is similar to that noted in some studies of Fv1-restricted viruses in murine cells (3, 8, 10). However, it should be noted that other studies show single-hit curves for restricted virus on murine cells (5). The reasons for these discrepancies are not clear, but as is shown here for human cells, virus dose is important. These titration experiments were also performed on HeLa cells with similar results (data not shown).
FIG. 1.
Titration curve of N- and B-tropic vectors on human cells. Human TE671 cells were infected with serial dilutions of N-eGFP (▪) and B-eGFP (▴) vectors. The percentages of cells infected were determined 48 h postinfection by FACS. Data are representative of three independent experiments.
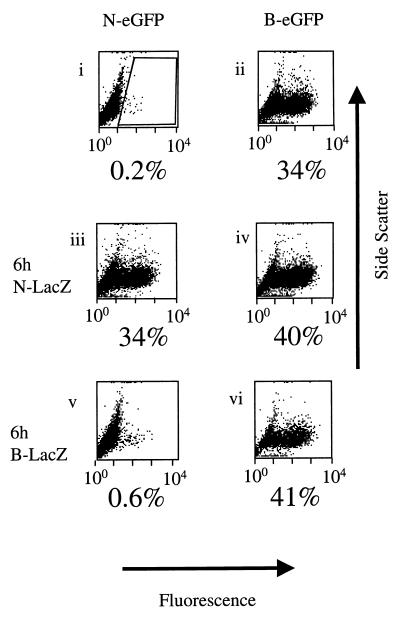
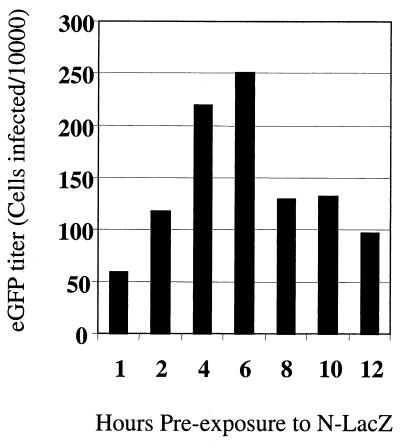
We then examined whether preexposure of TE671 cells to N- or B-tropic virus would abrogate the restriction of N-eGFP vector. Infection by restricted N-eGFP vector was measured with or without preexposure of the target cells to N- or B-tropic pseudotypes with a lacZ gene (N-lacZ or B-lacZ vectors, respectively). Figure 2, panels i and iii, show that infection of TE671 cells with restricted N-eGFP vector was largely abrogated when cells were preexposed to N-lacZ for 6 h. There was no significant increase in the infection of unrestricted B-eGFP virus after preexposure to N-lacZ (Fig. 2, panels ii and iv), and there was no significant change in the titer of N- or B-eGFP virus after preexposure to B-lacZ (Fig. 2, panels v and vi). These data show that only preexposure to restricted N-lacZ was able to affect the titer of a second input of restricted virus. The time of preexposure to lacZ vectors was also varied in order to determine the optimal condition for abrogation of Ref1 restriction in TE671 cells. TE671 cells (105) were preexposed to N-lacZ vector for 1 to 12 h and then infected with 8,000 MDTF IU of N-eGFP vector (Fig. 3). The time course shows that the maximal abrogation was achieved with 6 h of preexposure to N-lacZ vector.
FIG. 2.
Abrogation of restriction by preexposure to N-lacZ vector. Panels are side scatter versus fluorescence FACS dot plots of human TE671 cells 48 h after infection with MLV eGFP-encoding vectors. Fluorescence units are arbitrary. The gate used to determine percentages of infected cells is shown (panel i). N- and B-tropic virus titers were equalized on MDTF cells, and the N- and B-lacZ dose was 0.3 MDTF IU/TE671 cell. The N- and B-eGFP dose was ∼8,000 MDTF IU. Cells were infected with N-eGFP vector (panels i, iii, and v) and B-eGFP vector (panels ii, iv, and vi). Cells in panels iii and iv were preexposed to N-lacZ vector for 6 h prior to eGFP vector infection, and panels v and vi were preexposed to B-lacZ vector for 6 h prior to eGFP vector infection. Cells were washed once in medium after exposure to lacZ vectors prior to exposure to eGFP vectors. These data are representative of three independent experiments.
FIG. 3.
Time course of abrogation. TE671 cells were preexposed to abrogating N-lacZ vector (0.1 MDTF IU/TE671 cell) for periods of 1 to 12 h and then infected with a fixed titer of N-eGFP vector (∼8,000 MDTF IU). Infection was measured by FACS 24 h post-N-eGFP infection. Infection of N-eGFP was maximal after 6 h of preexposure to N-lacZ. These data are representative of three independent experiments.
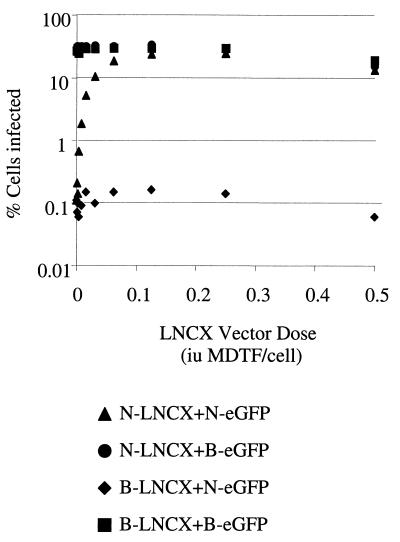
We then measured how much virus was required to cause abrogation using N- and-B tropic LNCX vector. N- and B-LNCX titers were equalized on MDTF cells by infection, followed by G418 selection and colony counting (data not shown). Serial dilutions of N- and B-LNCX vectors were used to preexpose TE671 cells, followed by infection with a fixed dose of N or B-eGFP vectors. Results are shown in Fig. 4, where the percentage of cells that are infected by N- or B-eGFP is plotted against the dose of abrogating LNCX vector used. As shown in Fig. 2, preexposure of cells had no significant effect on infection by unrestricted B-eGFP vector. However, the infection by restricted N-eGFP was increased by preexposure to N-LNCX in a dose-dependent manner. Preexposure to unrestricted B-tropic LNCX had no effect, even at high doses. Abrogation of N-eGFP restriction was achieved by preexposure to approximately 0.2 IU of N-LNCX per cell. We have previously shown that vector preparations typically contain around 300 physical particles per IU (9). This implies that a dose of around 60 particles/cell is enough to abrogate restriction.
FIG. 4.
Titration of LNCX virus for abrogation of N-eGFP vector restriction. TE671 cells were preexposed to a serial dilution of sixfold-concentrated abrogating N- or B-LNCX virus for 6 h and then infected with a fixed titer of either N- or B-eGFP vector. Cells were analyzed for eGFP expression 48 h later. N- and B-eGFP titers were equalized on MDTF cells, and around 10,000 MDTF IU was used per point. A titer of 105 IU/ml for N-LNCX and B-LNCX was measured on MDTF cells by measurement of CFU after treatment with G418 (1 mg/ml). Data are representative of several experiments performed with LNCX and lacZ abrogating vectors.
Finally, we examined the kinetics of N-eGFP infection after preexposure to lacZ vector under conditions that resulted in maximal abrogation of restriction. Cells were preexposed to 0.3 MDTF IU of N-lacZ vector per cell for 6 h and then exposed to a serial dilution of N- or B-eGFP vectors. Figure 5 shows that after preexposure to N-lacZ vector, both N- and B-eGFP vectors showed similar linear titration curves with a gradient of 1. These results indicate that abrogation of restriction by preexposure to N-tropic virions enabled N-eGFP vector to infect cells with an efficiency and a one-hit infection curve similar to those of unrestricted B-eGFP vector.
FIG. 5.
Titration curve of N-eGFP vector after abrogation of restriction. TE671 cells were infected with serial dilutions of N-eGFP (⧫) and B-eGFP (▪) vectors without preexposure and N-eGFP (▴) and B-eGFP (•) vectors after 6 h of preexposure to an abrogating dose (0.3 MDTF IU/TE671 cell) of N-lacZ vector. Infection was measured 48 h postinfection by FACS. Data are representative of two independent experiments.
We hypothesize that the product of a human gene, Ref1, blocks N-tropic MLV infection. The Ref1 gene product is limiting and can be titrated out by excess restricted virus particles. Direct interaction between Ref1 and restricted, but not unrestricted, CAs can explain its specificity. This hypothesis is supported by the fact that the N-eGFP titration pattern after N-lacZ preexposure resembled that of unrestricted B-eGFP (Fig. 5). We suggest that humans and other nonmurine species resistant to N-tropic infection have acquired and maintained genes that are perhaps unrelated at the sequence level to Fv1 but have very similar functions. Further study of these restrictions will lead to greater understanding of how mammals have dealt with pathogenic retroviruses in the long term and how these retroviruses have shaped the human genome during evolution.
Acknowledgments
We thank our colleagues in the Department of Immunology and Molecular Pathology at University College London for helpful discussion and J. P. Stoye of the National Institute for Medical Research for helpful discussion of unpublished data.
This work was funded by the Biotechnology and Biological Sciences Research Council, the Medical Research Council, and a research career development fellowship (number 064257) from the Wellcome Trust to G.T.
REFERENCES
- 1.Best, S., P. Le Tissier, G. Towers, and J. P. Stoye. 1996. Positional cloning of the mouse restriction gene Fv1. Nature 382:826-829. [DOI] [PubMed] [Google Scholar]
- 2.Boone, L. R., C. L. Innes, and C. K. Heitman. 1990. Abrogation of Fv-1 restriction by genome-deficient virions produced by a retrovirus packaging cell line. J. Virol. 64:3376-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Decleve, A., O. Niwa, E. Gelmann, and H. S. Kaplan. 1975. Replication kinetics of N- and B-tropic murine leukemia viruses on permissive and nonpermissive cells in vitro. Virology 65:320-332. [DOI] [PubMed] [Google Scholar]
- 4.Hartley, J. W., W. P. Rowe, and R. J. Huebner. 1970. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J. Virol. 5:221-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jolicoeur, P., and D. Baltimore. 1975. Effect of the Fv-1 locus on the titration of murine leukemia viruses. J. Virol. 16:1593-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jolicoeur, P., and E. Rassart. 1980. Effect of Fv-1 gene product on synthesis of linear and supercoiled viral DNA in cells infected with murine leukemia virus. J. Virol. 33:183-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin, J., E. Herniou, J. Cook, R. W. O'Neill, and M. Tristem. 1999. Interclass transmission and phyletic host tracking in murine leukemia virus-related retroviruses. J. Virol. 73:2442-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pincus, T., W. P. Rowe, and F. Lilly. 1971. A major genetic locus affecting resistance to infection with murine leukemia viruses. II. Apparent identity to a major locus described for resistance to friend murine leukemia virus. J. Exp. Med. 133:1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizzato, M., S. A. Marlow, E. D. Blair, and Y. Takeuchi. 1999. Initial binding of murine leukemia virus particles to cells does not require specific Env-receptor interaction. J. Virol. 73:8599-8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tennant, R. W., J. A. Otten, A. Brown, W. K. Yang, and S. J. Kennel. 1979. Characterization of Fv-1 host range strains of murine retroviruses by titration and p30 protein characteristics. Virology 99:349-357. [DOI] [PubMed] [Google Scholar]
- 11.Towers, G., M. Bock, S. Martin, Y. Takeuchi, J. P. Stoye, and O. Danos. 2000. A conserved mechanism of retrovirus restriction in mammals. Proc. Natl. Acad. Sci. USA 97:12295-12299. [DOI] [PMC free article] [PubMed] [Google Scholar]