Abstract
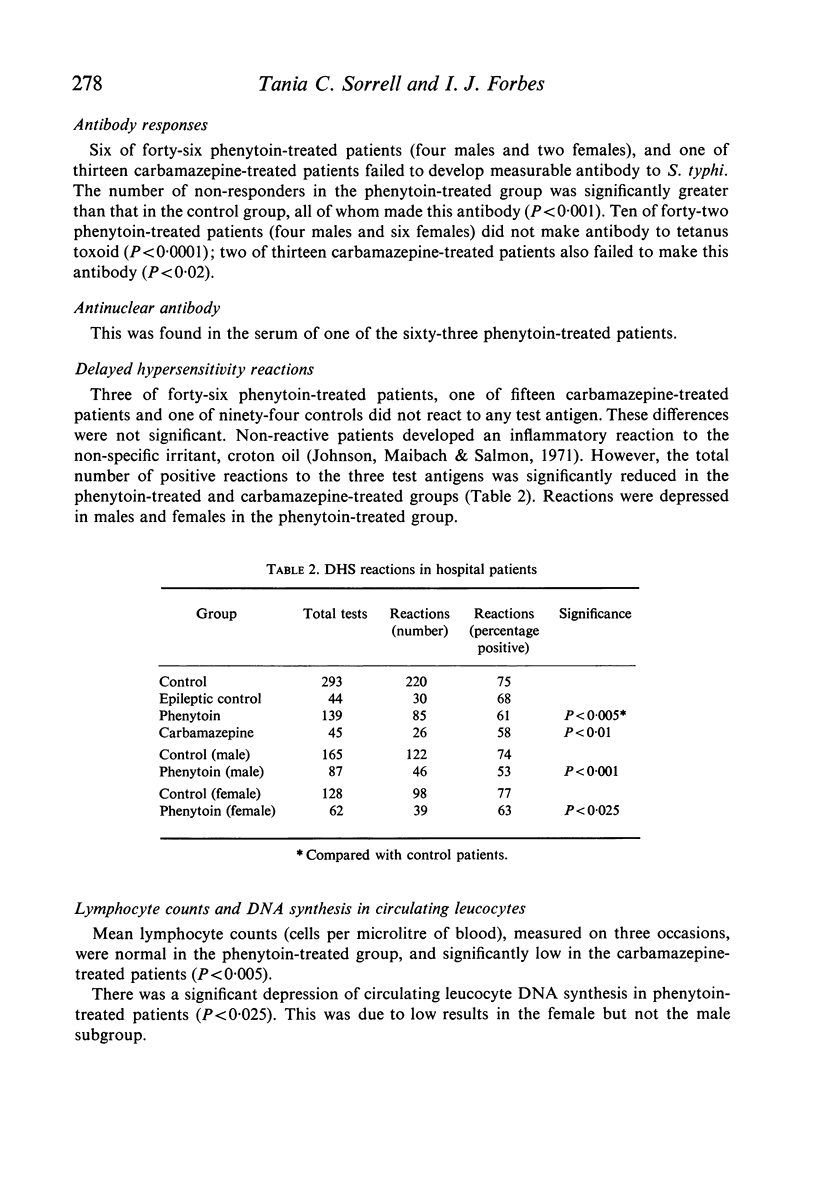
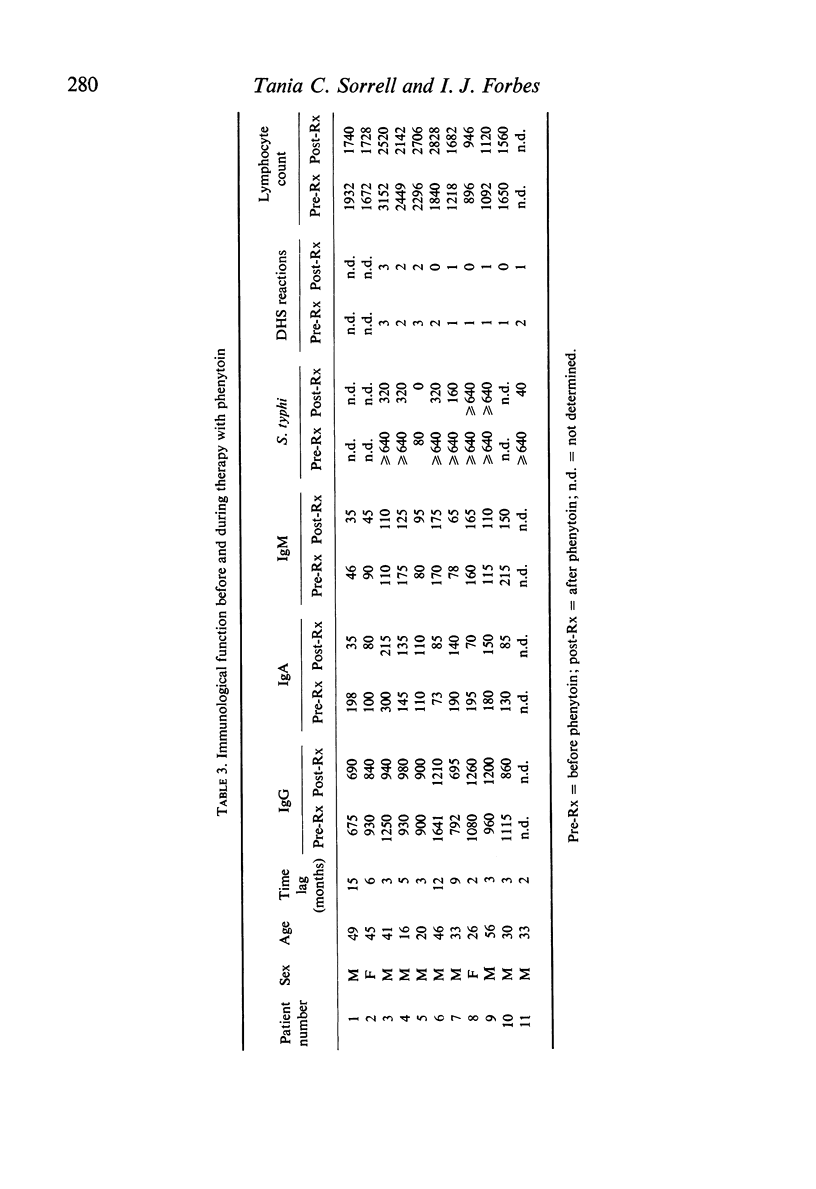
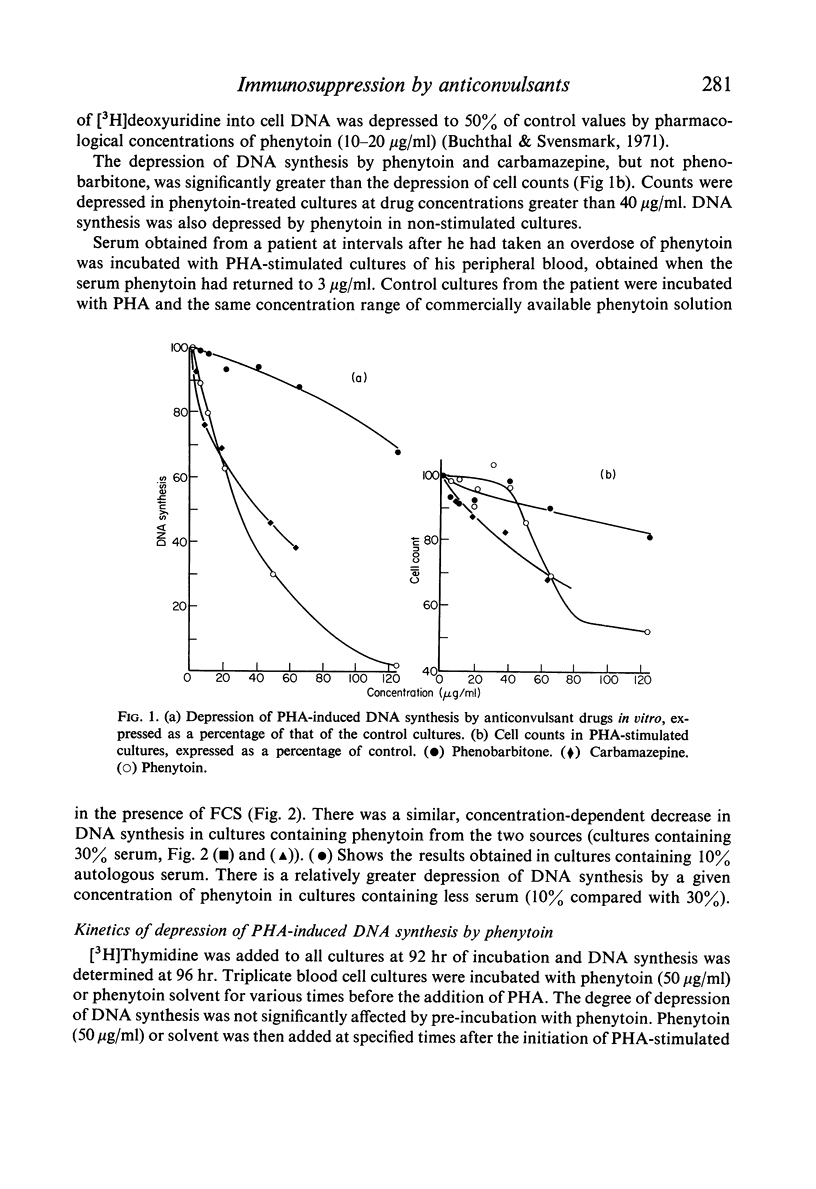
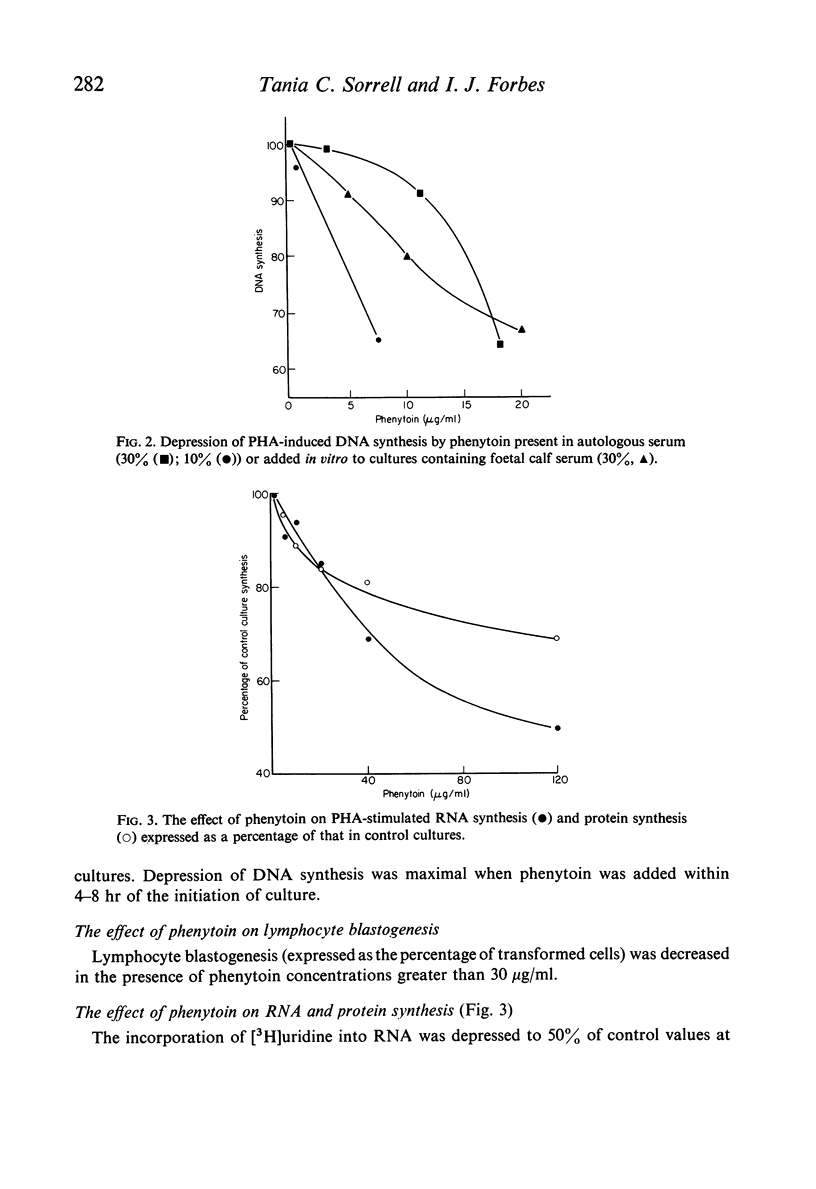
Depression of one or more parameters of cellular and/or humoral immune responses was found in 60% of general hospital patients treated with phenytoin and 47% of patients treated with carbamazepine. Phenytoin-treated patients failed to manifest delayed hypersensitivity (DHS) reactions to common antigens, and to make antibody to Salmonella typhi and tetanus toxoid. Serum levels of IgA and IgM, DNA synthesis in circulating leucocytes, and phytohaemagglutinin (PHA) induced deoxyribonucleic acid synthesis were also low. Depression of IgA, DHS reactivity and antibody responsiveness to S. typhi were shown to develop after the commencement of phenytoin therapy in a study of eleven patients. The presence of immunological defects was independent of the dosage of drug, its serum concentration, the duration of therapy and the sex of the subject. Studies in vitro provided evidence that immunosuppression was the result of a direct effect of phenytoin on the metabolism of lymphoid cells. Carbamazepine was shown to have a similar but less potent direct effect. Pharmacological concentrations of phenytoin caused a significant depression of DNA synthesis in PHA-stimulated and non-stimulated blood cell cultures in vitro. High concentrations in addition caused depression of cell counts, lymphocyte blastogenesis, ribonucleic acid and protein synthesis. Phenytoin was not cytocidal at concentrations of up to 125 mug/ml. Depression of DNA synthesis by phenytoin was maximal when phenytoin was added within 4-8 hr of the addition of PHA. PHA-induced DNA synthesis was not significantly affected by pre-incubation with phenytoin. In vivo, the presence of immunological defects was not related to phenytoin-induced folic acid deficiency. High concentrations of carbamazepine, but not phenobarbitone or diazepam caused a significant depression of PHA-stimulated DNA synthesis in blood cell cultures. The data show that immunosuppression is a common side-effect of phenytoin therapy, and that lymphoma is rare. They suggest that in the presence of phenytoin-induced immunosuppression another factor, or factors are required to induce the formation of lymphoma.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony J. J. Malignant lymphoma associated with hydantoin drugs. Arch Neurol. 1970 May;22(5):450–454. doi: 10.1001/archneur.1970.00480230068008. [DOI] [PubMed] [Google Scholar]
- BAKER H., HERBERT V., FRANK O., PASHER I., HUTNER S. H., WASSERMAN L. R., SOBOTKA H. A microbiologic method for detecting folic acid deficiency in man. Clin Chem. 1959 Aug;5(4):275–280. [PubMed] [Google Scholar]
- Brown J. M. Drug-associated lymphadenopathies with special reference to the Reed-Sternberg cell. Med J Aust. 1971 Feb 13;1(7):375–378. doi: 10.5694/j.1326-5377.1971.tb87587.x. [DOI] [PubMed] [Google Scholar]
- Brown R. S., Haynes H. A., Foley H. T., Godwin H. A., Berard C. W., Carbone P. P. Hodgkin's disease. Immunologic, clinical, and histologic features of 50 untreated patients. Ann Intern Med. 1967 Aug;67(2):291–302. doi: 10.7326/0003-4819-67-2-291. [DOI] [PubMed] [Google Scholar]
- Buchthal F., Svensmark O. Serum concentrations of diphenylhydantoin (phenytoin) and phenobarbital and their relation to therapeutic and toxic effects. Psychiatr Neurol Neurochir. 1971 Mar-Apr;74(2):117–136. [PubMed] [Google Scholar]
- Buckley C. E., 3rd, Dorsey F. C. Serum immunoglobulin levels throughout the life-span of healthy man. Ann Intern Med. 1971 Nov;75(5):673–682. doi: 10.7326/0003-4819-75-5-673. [DOI] [PubMed] [Google Scholar]
- Cline M. J. Isolation and characterization of RNA from human leukocytes. J Lab Clin Med. 1966 Jul;68(1):33–46. [PubMed] [Google Scholar]
- Dill W. A., Chucot L., Chang T., Glazko A. J. Simplified benzophenone procedure for determination of diphenylhydantoin in plasma. Clin Chem. 1971 Dec;17(12):1200–1201. [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Forbes I. J. Measurement of immunological function in clinical medicine. Aust N Z J Med. 1971 May;1(2):160–170. doi: 10.1111/j.1445-5994.1971.tb02284.x. [DOI] [PubMed] [Google Scholar]
- Fröland S. S., Natvig J. B. Effect of polyspecific rabbit anti-immunoglobulin antisera on human lymphocytes in vitro. Int Arch Allergy Appl Immunol. 1970;39(2-3):121–132. doi: 10.1159/000230341. [DOI] [PubMed] [Google Scholar]
- Hulliger L., Blazkovec A. A. A simple and efficient method of separating peripheral-blood leucocytes for in-vitro studies. Lancet. 1967 Jun 17;1(7503):1304–1305. doi: 10.1016/s0140-6736(67)91596-6. [DOI] [PubMed] [Google Scholar]
- Hyman G. A., Sommers S. C. The development of Hodgkin's disease and lymphoma during anticonvulsant therapy. Blood. 1966 Sep;28(3):416–427. [PubMed] [Google Scholar]
- Johnson M. W., Maibach H. I., Salmon S. E. Skin reactivity in patients with cancer. Impaired delayed hypersensitivity or faulty inflammatory response? N Engl J Med. 1971 Jun 3;284(22):1255–1257. doi: 10.1056/NEJM197106032842210. [DOI] [PubMed] [Google Scholar]
- Junge U., Hoekstra J., Wolfe L., Deinhardt F. Microtechnique for quantitative evaluation of in vitro lymphocyte transformation. Clin Exp Immunol. 1970 Sep;7(3):431–437. [PMC free article] [PubMed] [Google Scholar]
- KLIPSTEIN F. A. SUBNORMAL SERUM FOLATE AND MACROCYTOSIS ASSOCIATED WITH ANTICONVULSANT DRUG THERAPY. Blood. 1964 Jan;23:68–86. [PubMed] [Google Scholar]
- Lamvik J. O. Separation of lymphocytes from human blood. Acta Haematol. 1966 May;35(5):294–303. doi: 10.1159/000209135. [DOI] [PubMed] [Google Scholar]
- MacKinney A. A., Booker H. E. Diphenylhydantoin effects on human lymphocytes in vitro and in vivo. An hypothesis to explain some drug reactions. Arch Intern Med. 1972 Jun;129(6):988–992. [PubMed] [Google Scholar]
- Sorrell T. C., Forbes I. J., Burness F. R., Rischbieth R. H. Depression of immunological function in patients treated with phenytoin sodium (sodium diphenylhydantoin). Lancet. 1971 Dec 4;2(7736):1233–1235. doi: 10.1016/s0140-6736(71)90547-2. [DOI] [PubMed] [Google Scholar]
- Stewart C. C., Ingram M. A method for counting phytohemagglutinin-stimulated lymphocytes. Blood. 1967 Apr;29(4 Suppl):628–639. [PubMed] [Google Scholar]
- Sutherland R. M., Inch W. R., McCredie J. A. Phytohemagglutinin (PHA)-induced transformation of lymphocytes from patients with cancer. Cancer. 1971 Mar;27(3):574–578. doi: 10.1002/1097-0142(197103)27:3<574::aid-cncr2820270310>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]


