Abstract
The lymph nodes of mice overloaded with mycobacterial products, either by the injection of whole or ultrasonicated organisms, or as a consequence of severe infection with Mycobacterium ulcerans, contain phagocytic cells which cause spontaneous transformation of the lymph node cells in a low volume, high cell density culture system. This spontaneous mitosis is unaffected by trypsinization but is inhibited by specific antigen and by PHA, and eliminated by treatment with carbonyl iron. Replacement of the macrophages removed with carbonyl iron by a critical number of peritoneal cells, restores the spontaneous transformation. Normal lymph node, thymus or peritoneal lymphocytes will also undergo mitosis if small numbers of peritoneal cells are added to them. This phenomenon therefore appears not to be antigen-dependent, but is probably due to a mediator released from macrophages. The possible role of this phenomenon in the pathogenesis of mycobacterial disease and the 'overloading' of T lymphocytes in vivo is discussed, with reference to similar macrophage-dependent mechanisms reported in other systems.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. J., Bach F. H. Lymphocyte reactivity in vitro. I. Cellular reconstitution of purified lymphocyte response. Cell Immunol. 1970 Jul;1(2):207–218. doi: 10.1016/0008-8749(70)90008-0. [DOI] [PubMed] [Google Scholar]
- Bach F. H., Alter B. J., Solliday S., Zoschke D. C., Janis M. Lymphocyte reactivity in vitro. II. Soluble reconstituting factor permitting response of purified lymphocyte. Cell Immunol. 1970 Jul;1(2):219–227. doi: 10.1016/0008-8749(70)90009-2. [DOI] [PubMed] [Google Scholar]
- Calderon J., Unanue E. R. Two biological activities regulating cell proliferation found in cultures of peritoneal exudate cells. Nature. 1975 Jan 31;253(5490):359–361. doi: 10.1038/253359a0. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell Immunol. 1970 Sep;1(3):253–265. doi: 10.1016/0008-8749(70)90047-x. [DOI] [PubMed] [Google Scholar]
- Dutton R. W., McCarthy M. M., Mishell R. I., Raidt D. J. Cell components in the immune response. IV. Relationships and possible interactions. Cell Immunol. 1970 Jul;1(2):196–206. doi: 10.1016/0008-8749(70)90007-9. [DOI] [PubMed] [Google Scholar]
- Folch H., Yoshinaga M., Waksman B. H. Regulation of lymphocyte responses in vitro. 3. Inhibition by adherent cells of the T-lymphocyte response to phytohemagglutinin. J Immunol. 1973 Mar;110(3):835–839. [PubMed] [Google Scholar]
- Frost P., Lance E. M. The cellular origin of the lymphochte trap. Immunology. 1974 Jan;26(1):175–186. [PMC free article] [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery I., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. II. The cellular source of potentiating mediator(s). J Exp Med. 1972 Jul 1;136(1):143–155. doi: 10.1084/jem.136.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., MacLean L. D. A lymphocyte-stimulating factor produced in vitro. Nature. 1965 Nov 20;208(5012):795–796. doi: 10.1038/208795a0. [DOI] [PubMed] [Google Scholar]
- Gyöngyössy M. I., Playfair J. H. Indirect immunofluorescence of mouse thymus-derived cells using heterologous anti-brain serum. Cell Immunol. 1973 Apr;7(1):118–123. doi: 10.1016/0008-8749(73)90187-1. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Dutton R. W. Immune response restoration with macrophage culture supernatants. Science. 1971 Jun 4;172(3987):1047–1048. doi: 10.1126/science.172.3987.1047. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Chused T. M., Herberman R. B., Holden H. T., Lavrin D. H. Evidence of suppressor cell activity in spleens of mice bearing primary tumors induced by Moloney sarcoma virus. J Exp Med. 1974 Jun 1;139(6):1473–1487. doi: 10.1084/jem.139.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene G. M., Wright D. J., Turk J. L. Cell-mediated immunity and lymphocyte transformation in syphilis. Proc R Soc Med. 1971 Apr;64(4):426–428. doi: 10.1177/003591577106400440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Lagrange P. H., Ishibashi T. The modifying effect of BCG on the immunological induction of T cells. J Exp Med. 1974 Jun 1;139(6):1540–1552. doi: 10.1084/jem.139.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrvang B., Godal T., Ridley D. S., Fröland S. S., Song Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin Exp Immunol. 1973 Aug;14(4):541–553. [PMC free article] [PubMed] [Google Scholar]
- Möller G. Induction of DNA synthesis in human lymphocytes: interaction between non-specific mitogens and antigens. Immunology. 1970 Oct;19(4):583–598. [PMC free article] [PubMed] [Google Scholar]
- Nelson D. S. Production by stimulated macrophages of factors depressing lymphocyte transformation. Nature. 1973 Nov 30;246(5431):306–307. doi: 10.1038/246306a0. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Dutton R. W. Inhibition of spleen cell DNA synthesis by autologous macrophages. J Immunol. 1966 Nov;97(5):663–669. [PubMed] [Google Scholar]
- Ptak W., Gaugas J. M., Rees R. J., Allison A. C. Immune responses in mice with murine leprosy. Clin Exp Immunol. 1970 Jan;6(1):117–124. [PMC free article] [PubMed] [Google Scholar]
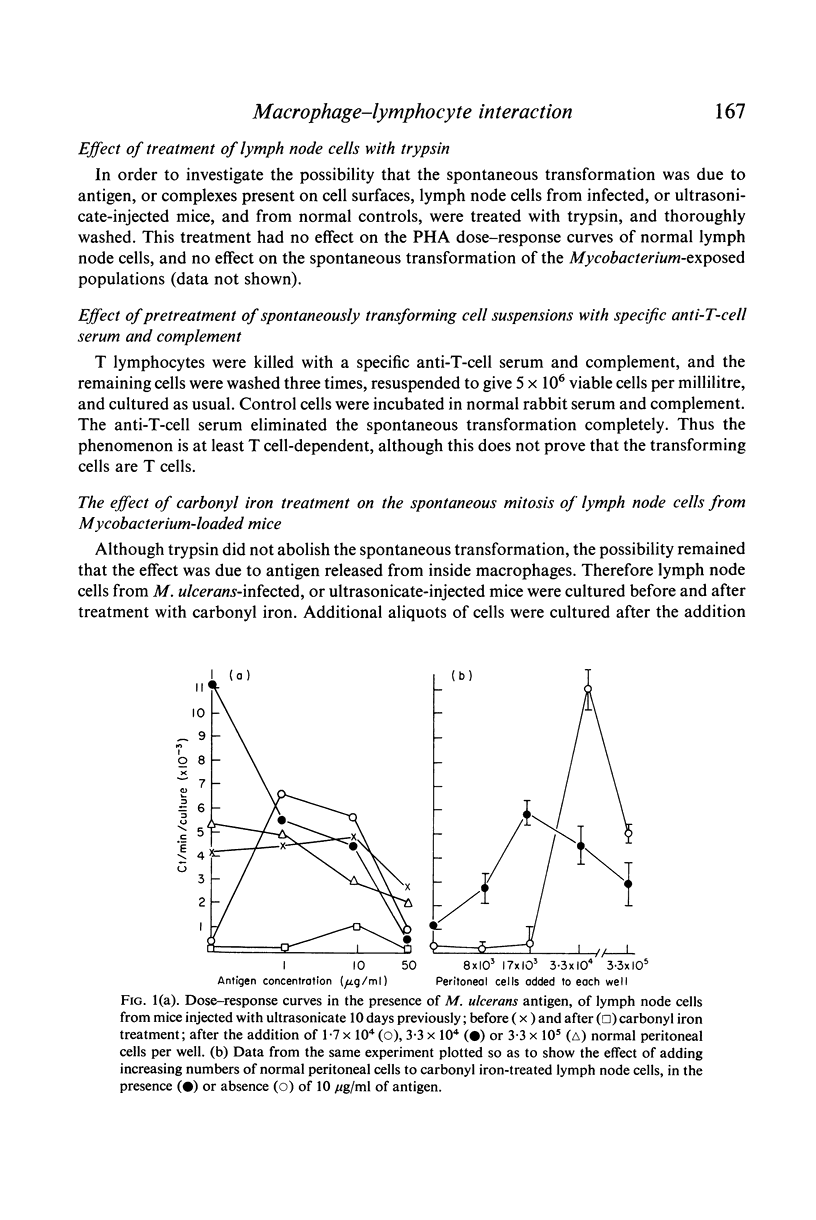
- Rook G. A. The immunological consequences of antigen overload in experimental mycobacterial infections of mice. Clin Exp Immunol. 1975 Jan;19(1):167–177. [PMC free article] [PubMed] [Google Scholar]
- Scott M. T. Biological effects of the adjuvant Corynebacterium parvum. I. Inhibition of PHA, mixed lymphocyte and GVH reactivity. Cell Immunol. 1972 Nov;5(3):459–468. doi: 10.1016/0008-8749(72)90072-x. [DOI] [PubMed] [Google Scholar]
- Shortman K., Palmer J. The requirement for macrophages in the in vitro immune response. Cell Immunol. 1971 Oct;2(5):399–410. doi: 10.1016/0008-8749(71)90051-7. [DOI] [PubMed] [Google Scholar]
- Sjöberg O. Effect of allogeneic cell interaction on the primary immune response in vitro. Cell types involved in suppression and stimulation of antibody synthesis. Clin Exp Immunol. 1972 Nov;12(3):365–375. [PMC free article] [PubMed] [Google Scholar]
- Tsakraklides V., Anastassiades O. T., Kersey J. H. Prognostic significance of regional lymph node histology in uterine cervical cancer. Cancer. 1973 Apr;31(4):860–868. doi: 10.1002/1097-0142(197304)31:4<860::aid-cncr2820310415>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Bryceson A. D. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]
- Wright D. J., Grimble A. S. Why is the infectious stage of syphilis prolonged? Br J Vener Dis. 1974 Feb;50(1):45–49. doi: 10.1136/sti.50.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]



