Abstract
We previously reported that high-titered neutralizing antibodies directed against the human immunodeficiency virus type 1 (HIV-1) envelope can block the establishment of a simian immunodeficiency virus (SIV)/HIV chimeric virus (SHIV) infection in two monkeys following passive transfer (R. Shibata et al., Nat. Med. 5:204-210, 1999). In the present study, increasing amounts of neutralizing immunoglobulin G (IgG) were administered to 15 pig-tailed macaques in order to obtain a statistically valid protective neutralization endpoint titer in plasma. Using an in vitro assay which measures complete neutralization of the challenge SHIV, we correlated the titers of neutralizing antibodies in plasma at the time of virus inoculation (which ranged from 1:3 to 1:123) with the establishment of infection in virus-challenged animals. Ten of 15 monkeys in the present experiment were virus free as a result of neutralizing IgG administration as monitored by DNA PCR (peripheral blood mononuclear cells and lymph node cells), RNA PCR (plasma), virus isolation, and the transfer of lymph node cell suspensions (108 cells) plus 8 ml of whole blood from protected animals to naïve macaques. The titer of neutralizing antibodies in the plasma calculated to protect 99% of virus-challenged monkeys was 1:38.
There is abundant evidence that robust antiviral cellular immune responses are elicited following human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV) infections of humans and macaques, respectively (2, 3, 17, 21, 30). This is the usual pattern observed for most retrovirus infections, which typically become chronic and, in the case of the primate lentiviruses, result in debilitating and fatal clinical outcomes. An effective prophylactic vaccine for HIV-1 may have to elicit multiple immune responses, such as neutralizing antibodies and cytotoxic T lymphocytes. In experimental vaccine studies using murine retroviruses, excellent control of the virus-induced disease was achieved only when both cellular and humoral immune responses were present at the time of initial exposure to the virus; immunization of knockout mice lacking either CD8+, CD4+, or B-cell functions did not prevent chronic infection and disease development (7, 8). Nonetheless, in some human viral diseases, it is well established that virus-specific antibodies alone are capable of preventing infection or attenuating symptoms (24). This was dramatically and conclusively illustrated in a clinical trial to control poliovirus in which the administration of human immunoglobulin G (IgG) to tens of thousands of children during the spring of 1952 led to a marked reduction of paralytic disease (14). The results of this important study strongly suggested that a vaccine able to induce a robust humoral response would confer protection against this dreaded viral disease, a prediction subsequently validated in poliovirus vaccine trials (28, 29).
In the case of HIV-1, it has not been formally tested whether the induction of antibodies alone is sufficient to prevent disease. One might predict that antibody-mediated protection will not be effective for HIV infection, based on the numerous observations that only low and slowly developing levels of neutralizing antibodies can be elicited following infection or immunization (20, 22, 23). On the other hand, the potency of a targeted humoral response against primate lentivirus infections has been demonstrated in several passive-immunization studies, some of which have elicited sterilizing protection against the challenge virus (9, 11, 12, 18, 19, 25, 32). In several of these studies, the administration of monoclonal antibodies (MAbs) directed against conserved neutralizing epitopes was shown to protect hu-PBL-SCID mice against primary HIV-1 isolates (11, 12, 25) and macaque monkeys against intravenous (18) or vaginal (19) challenges with the pathogenic virus SHIV89.6PD. In the latter experiments, the resistance observed was augmented by transferring combinations of neutralizing MAbs plus polyclonal IgG, purified from the plasma of multiple HIV-1-positive individuals. A recent study, employing only the human neutralizing MAb b12, which targets the CD4-binding domain of gp120, reported the dose-dependent and complete protection in rhesus monkeys against a vaginal challenge with the R5-utilizing SHIV162P4 (26).
We previously reported the sterilizing protection of two of six pig-tailed monkeys, passively administered IgG purified from chimpanzees infected with the primary HIV-1 isolate, HIV-1DH12, and challenged intravenously with a simian-human immunodeficiency virus (SHIV) bearing the identical envelope glycoprotein (32). In that experiment, the two completely protected animals were the recipients of the largest amount of chimpanzee IgG. In the present study, we have systematically examined and quantitated the protective endpoint of anti-HIV-1 neutralizing antibodies in vivo. Passively transferring much higher amounts of neutralizing IgG than previously administered, we were able to completely protect 10 of 15 additional monkeys from infection as monitored by (i) DNA and RNA PCR analyses of peripheral blood mononuclear cells (PBMC) and plasma, respectively; (ii) virus isolation from lymph node specimens; and (iii) transfer of whole blood plus suspensions of lymph node cells from protected, virus-challenged animals to naïve macaques. An analysis of anti-SHIVDH12 neutralizing antibody levels in the plasma of the 21 monkeys in the two studies at the time of virus challenge indicated that the calculated neutralization titer capable of protecting 99% of macaques was 1:38.
MATERIALS AND METHODS
Virus.
The origin and preparation of the tissue culture-derived SHIVDH12 stock have been previously described (33). This virus stock has a titer of 1.65 × 106 50% tissue culture infective doses (TCID50)/ml measured in MT-4 cells, a human T-cell leukemia virus type 1-transformed T-lymphoid cell line (15).
Animals, virus inoculation, and sample collection.
Pig-tailed macaques (Macaca nemestrina) were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (5) and were housed in a biosafety level 2 facility; biosafety level 3 practices were followed. Phlebotomies and virus inoculations (75 TCID50 of SHIVDH12 intravenously) were performed as previously described (32). EDTA-treated blood specimens and acid citrate-dextrose A-treated samples of blood were used for the preparations of plasma and PBMC, respectively.
Antibodies.
The purification of immunoglobulin fractions from HIV-1-infected chimpanzees has been previously reported (13). The purified IgGs were administered intravenously 24 h before virus challenge.
Virus neutralization assay.
Neutralizing activities in the plasma of passively immunized monkeys were titrated in an assay that measures 100% neutralization against known amounts of virus as previously described (32, 35). Individual plasma samples were serially diluted (twofold or threefold, starting at a dilution of 1:4 or 1:6) using pre-passive-immunization plasma from each of the pig-tailed macaques as diluent. A 30-μl aliquot of each plasma dilution was incubated with 30 μl of the SHIVDH12 challenge stock (1.5 × 104 TCID50/ml) at room temperature for 1 h and was then used to infect MT-4 cells in quadruplicate. MT-4 cells (5 × 104 in 0.25 ml) were then incubated with 10 μl of the virus-plasma mixture, which contained 75 TCID50 of SHIVDH12. Infected cultures were maintained for 2 weeks, and virus replication was monitored by 32P-reverse transcriptase assays (36). Any infectious SHIVDH12 generated during the 2 weeks of incubation in MT-4 cells would be amplified to levels detectable by the assay. Neutralization antibody titers were calculated by the method of Reed and Muench (27).
Antibody concentrations in plasma.
The concentrations of chimpanzee anti-HIV-1 neutralizing IgG in monkey plasma were determined by employing a commercial enzyme-linked immunosorbent assay kit (Vironostika HIV-1 Microelisa system; Organon Teknika, Durham, N.C.). Standard curves were generated using the purified chimpanzee IgG preparation (10 mg/ml).
Quantitation of proviral DNA copies and plasma viral RNA levels.
The number of proviral DNA copies in PBMC and lymph node cells was measured by quantitative DNA PCR as previously described (33). Plasma viral RNA levels were determined by real-time PCR (ABI Prism 7700 sequence detection system; Perkin-Elmer, Foster City, Calif.) employing gag primers and probes as previously reported (10). Plasma from SHIVDH12-infected rhesus macaques and SHIVDH12-infected rhesus PBMC culture supernatants, previously quantitated by the branched DNA method (6), served as standards for the reverse transcription-PCR (RT-PCR) assay.
Virus isolation from lymph nodes of passively immunized macaques.
Inguinal and axillary lymph node samples were collected between weeks 2 and 10 postchallenge. Suspensions of more than 5 × 105 lymph node cells were cocultivated with MT-4 cells in RPMI 1640 medium, supplemented with 10% heat-inactivated fetal bovine serum (HyClone). Virus production was monitored by reverse transcriptase assay after 4 weeks of culture (36).
Data analysis.
Calculation of the protective neutralizing antibody titer in plasma, resulting in the protection of 50 or 99% of the virus-challenged animals, was performed using the method of Reed and Muench (27).
RESULTS
Characterization and transfer of anti-HIV-1 IgG to pig-tailed macaques.
In our earlier study, we reported that the passive transfer of high-titered neutralizing IgGs from chimpanzees chronically infected with HIV-1DH12 resulted in sterilizing protection of two of six pig-tailed macaques following an intravenous challenge with a SHIV bearing the identical envelope glycoprotein (32). Because these two animals received the highest amounts of neutralizing IgG, we were unable to determine the endpoint of protective neutralizing antibody levels in plasma in vivo. This endpoint titer might be of value as a predictive indicator of an effective humoral immune response directed against primate lentiviruses.
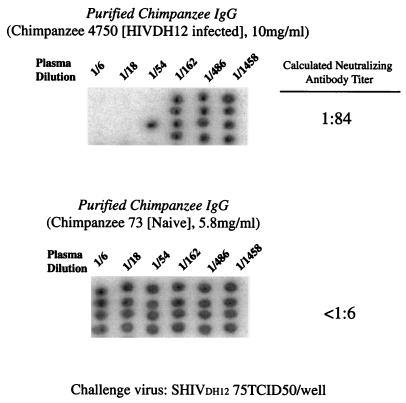
For these present experiments, IgG was collected and prepared in 2000 from the plasma of asymptomatic chimpanzee 4750, which had been inoculated in 1993 with three independent primary HIV-1 isolates, including HIV-1DH12 (34). These viral strains had been selected because of their tropism for chimpanzee PBMC (31). IgG, collected from naïve chimpanzee 73 (32), served as a negative control. Employing an assay that measures complete neutralization of virus, not percent reduction of progeny virion release, we initially determined the neutralizing activity of the chimpanzee 4750 IgG preparation (Fig. 1). By convention, the biological activity of non-plaque-forming viruses like primate lentiviruses is determined by endpoint dilution experiments and is reported as the dilution of virus resulting in the infection of 50% of replicate cultures. The virus infectivity titers obtained are reported in units of TCID50. Similarly, virus-neutralizing activity is determined by terminally diluting serum from an individual or animal previously exposed to a virus or immunogen and incubating the diluted samples with a standard amount of virus (commonly 100 TCID50). The antivirus neutralization titer is determined by calculating the highest dilution of serum that prevents infection of 50% of replicate inoculations. The calculated neutralization titer (the dilution of IgG in which two of four quadruplicate infections were blocked) against 75 TCID50 of SHIVDH12 was 1:84. No neutralization was observed when the IgG from the naïve chimpanzee was tested (titer = <1:6).
FIG. 1.
Endpoint titrations of anti-SHIVDH12 neutralizing activities present in purified chimpanzee IgGs. The virus-neutralizing activities in IgGs purified from a chimpanzee chronically infected with HIV-1DH12 (top) or a virus-naïve animal (bottom) were evaluated in quadruplicate cultures of MT-4 cells following a 1-h incubation of virus (75 TCID50) with threefold serial dilutions of plasma. The presence or absence of progeny virus production was measured by 32P-reverse transcriptase assays performed on aliquots of the culture supernatant from day 14 of infection. The black spots shown in the autoradiograms indicate the presence of virion-associated reverse transcriptase activity (i.e., no virus neutralization).
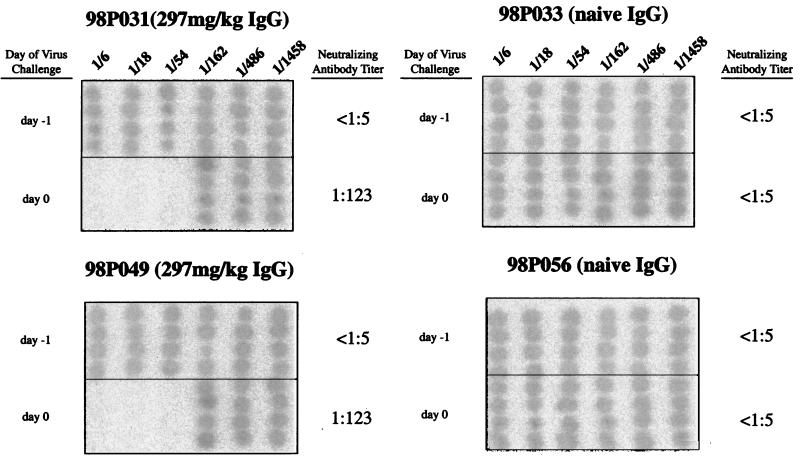
In passive-immunization studies, the dose of IgG administered is usually calculated from the results of ex vivo neutralization assays, the estimated plasma volume in the recipient, and an unknown compensation factor to offset the extravascular redistribution or degradation of the transferred IgG. In this study, our goals were to passively transfer sufficient amounts of anti-HIV-1 IgG to completely protect a monkey from a subsequent virus challenge and then determine the neutralizing titer in plasma that would confer sterilizing immunity on 50% of exposed animals (the endpoint protective titer) by serially diluting the amount of IgG administered. Based on its neutralization titer, the anti-HIV-1 IgG (10 mg/ml) could theoretically be diluted 84-fold and still prevent infection of 50% of animals inoculated with 75 TCID50 of SHIVDH12. Assuming that a 3-kg monkey has a plasma volume of approximately 150 ml (5% of body weight), the transfer of 17.8 mg of the chimpanzee IgG (10 mg/ml × 1/84 × 150 ml) would achieve this titer. However, because it is highly unlikely that this minimal amount of IgG would remain physically within the vascular compartment, 50-fold more of the neutralizing IgG (297 mg/kg of body weight) as well as a large excess of the control IgG was intravenously administered to pairs of macaques in the initial experiment (Fig. 2). Twenty-four hours following IgG transfer, the anti-SHIVDH12 neutralization titer in the plasma of monkeys 98P031 and 98P049, determined in an ex vivo assay, was calculated to be 1:123. No neutralizing activity (<1:3) was measurable in the recipients of the control IgG (animals 98P033 and 98P056). Because these anti-SHIVDH12 neutralization titers in plasma far exceeded the levels necessary to block a viral inoculum of 75 TCID50, twofold serial dilutions of IgG were transferred to groups of two or three animals. The neutralization titers in the plasma of these animals at the time of virus challenge are indicated in Table 1. It is also worth noting that the concentrations of IgG actually present in the plasma closely approximate the calculated IgG values for this compartment based on body weight (Table 1).
FIG. 2.
Neutralization titers in plasma following the passive transfer of large amounts of purified chimpanzee IgG to naïve pig-tailed macaques. Shown are endpoint neutralization titers in pairs of monkeys receiving the maximum amounts of IgG from an HIV-1-infected (left panels) or naïve (right panels) chimpanzee. The neutralization assays were performed with samples collected immediately prior to IgG administration (day −1) and SHIVDH12 challenge (day 0). Assays were carried out as described in the legend to Fig. 1.
TABLE 1.
Macaques and purified IgG infused
| Animals | Chimp IgG administered (mg/kg) | Calculated plasma IgG concn (μg/ml)a | Observed plasma IgG concn at 24 h (mean ± SD; μg/ml) | Neutralizing titer in plasma |
|---|---|---|---|---|
| 98P031 | 297 | 5,900 | 5,932 ± 770 | 1:123 |
| 98P049 | 297 | 5,900 | 7,687 ± 1,177 | 1:123 |
| 98P024 | 149 | 2,980 | 2,643 ± 241 | 1:40 |
| 98P043 | 149 | 2,980 | 2,199 ± 797 | 1:40 |
| 98P023 | 74 | 1,480 | 1,278 ± 157 | 1:15 |
| 98P046 | 74 | 1,480 | 1,507 ± 148 | 1:18 |
| 98P045 | 74 | 1,480 | 1,905 ± 366 | 1:18 |
| 98P021 | 37 | 740 | 724 ± 180 | 1:13 |
| 98P061 | 37 | 740 | 591 ± 137 | 1:12 |
| 98P035 | 18.6 | 380 | 399 ± 104 | 1:7 |
| 98P042 | 18.6 | 380 | 333 ± 82 | 1:6 |
| 96P082 | 18.6 | 380 | 409 ± 82 | 1:7 |
| 96P076 | 9.3 | 180 | 124 ± 7 | 1:3 |
| 96P081 | 8.9 | 180 | 149 ± 42 | 1:4 |
| 96P088 | 10.2 | 180 | 164 ± 23 | 1:4 |
| 98P033 | 34.5 (naïve IgG) | Not done | NDb | ND |
| 98P056 | 34.5 (naïve IgG) | Not done | ND | ND |
Plasma volume is calculated as 5% of the body weight.
ND, none detected.
SHIVDH12 challenge of passively immunized monkeys.
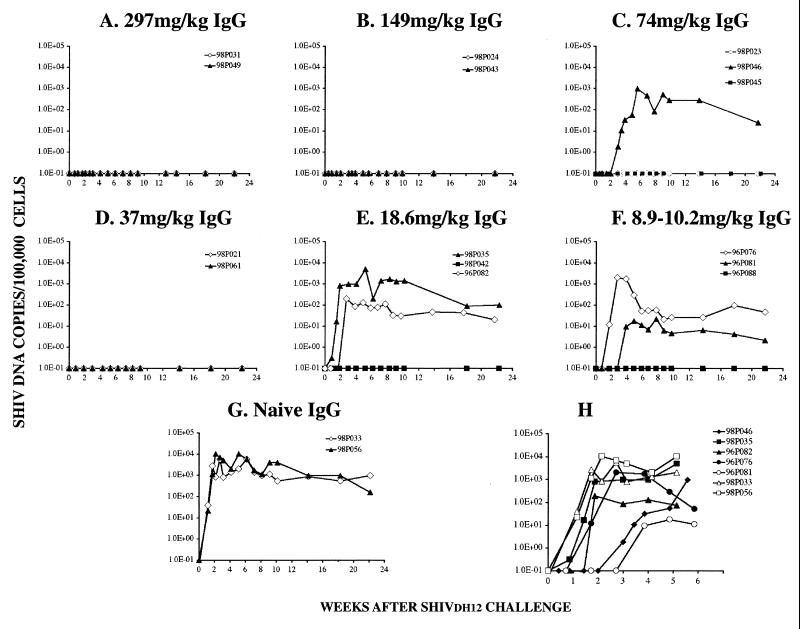
The 15 recipients of anti-HIV-1 IgG and the 2 animals administered control antibodies were inoculated intravenously with 75 TCID50 of virus 24 h after passive transfer. Evidence of robust virus infections in the two control macaques became apparent by day 8 of infection, as monitored by DNA and RT-PCR (Fig. 3G and 4G). Eight of nine animals with neutralization titers in pl asma of 1:12 or greater (or an IgG dose of 37 mg/kg or greater) at the time of virus challenge resisted the SHIV infection. It is worth noting that the infection of the single animal (98P046) in this group was delayed several weeks compared to that for the control macaques (Fig. 3C and 4C). In contrast, only two of six animals with anti-SHIVDH12 neutralizing titers in the range of 1:3 to 1:7 (or an IgG dose of 8.9 to 18.6 mg/kg) were protected. However, the SHIV infection in two of the four virus-positive monkeys was delayed and peak plasma virus loads were markedly reduced (Fig. 3H), suggesting that administration of even low amounts of anti-HIV-1 IgG conferred partial protection in some animals.
FIG. 3.
PBMC-associated viral DNA loads in passively immunized pig-tailed macaques challenged with SHIVDH12. (A to G) DNA was prepared from PBMC, collected from recipients of anti-HIV-1DH12 IgG, and analyzed by quantitative DNA PCR over a 4-month period for the presence of SHIVDH12. (H) Levels of PBMC-associated viral DNA in the five immunized and two naïve monkeys which became infected are shown in an expanded time scale.
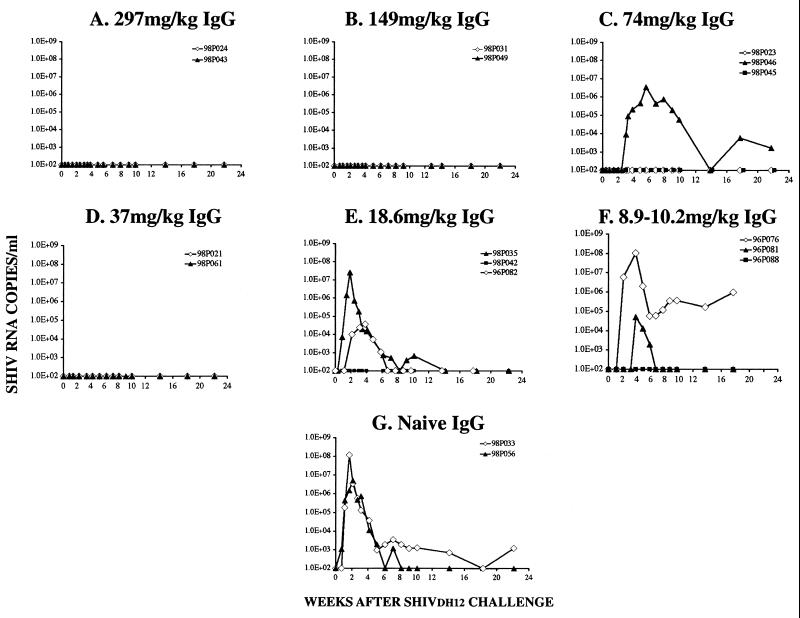
FIG. 4.
Plasma viral RNA levels in passively immunized pig-tailed macaques challenged with SHIVDH12. The number of SHIVDH12 RNA copies in IgG recipients was determined over a 4-month period following an intravenous SHIVDH12 challenge by quantitative RT-PCR.
Several additional experiments were conducted to validate the apparent sterilizing immunity observed in 10 of the pig-tailed macaques following transfer of the antiviral IgG. DNA PCR analyses and virus isolations from lymph node samples collected between weeks 2 and 10 postchallenge yielded no evidence of virus infection (Table 2). In contrast, SHIVDH12 was detected in the lymph node specimens from the five unprotected and two control animals. Finally, in an attempt to verify that biologically active virus was not present in protected monkeys, in vivo infectivity assays were performed in which lymph node suspensions and whole blood from putatively protected animals were inoculated into naïve pig-tailed macaques. In this experiment, approximately 108 lymph node cells plus 8 ml of blood from nine of the protected animals were pooled in groups of three and infused intravenously into three uninfected monkeys. As shown in Table 3, none of the three recipient animals became infected, as monitored on multiple occasions by DNA and RT-PCR assays of PBMC and plasma samples, respectively, during a 26- to 28-week observation period.
TABLE 2.
Neutralizing antibody titers and infection status in passively immunized monkeys
| Animals | Neutralizing titer in plasma | Infection
|
|||||
|---|---|---|---|---|---|---|---|
| Blood
|
Lymph node
|
||||||
| DNA PCR | RT-PCR | DNA PCR | Virus isolation | ||||
| 98P031 | 1:123 | No | No | No | No | ||
| 98P049 | 1:123 | No | No | No | No | ||
| 98P024 | 1:40 | No | No | No | No | ||
| 98P043 | 1:40 | No | No | No | No | ||
| 98P023 | 1:15 | No | No | No | No | ||
| 98P046 | 1:18 | Yesc | Yesc | Yes | Yes | ||
| 98P045 | 1:18 | No | No | No | No | ||
| 98P021 | 1:13 | No | No | No | No | ||
| 98P061 | 1:12 | No | No | No | No | ||
| 98P035 | 1:7 | Yes | Yes | Yes | Yes | ||
| 98P042 | 1:6 | No | No | No | No | ||
| 96P082 | 1:7 | Yesc | Yesc | Yes | Yes | ||
| 96P076 | 1:3 | Yes | Yes | Yes | Yes | ||
| 96P081 | 1:4 | Yesc | Yesc | Yes | Yes | ||
| 96P088 | 1:4 | No | No | No | No | ||
| 94P011b | 1:8 | No | NDa | ND | ND | ||
| 94P040b | 1:5 | No | ND | ND | ND | ||
| 94P028b | 1:4 | Yes | ND | ND | ND | ||
| 94P027b | 1:3 | Yes | ND | ND | ND | ||
| 94P029b | 1:3 | Yes | ND | ND | ND | ||
| 94P032b | 1:3 | Yes | ND | ND | ND | ||
| 98P033 | Control IgG | Yes | Yes | Yes | Yes | ||
| 98P056 | Control IgG | Yes | Yes | Yes | Yes | ||
ND, not done.
Data from reference 32.
Significantly low virus load and/or delayed kinetics compared with that of control.
TABLE 3.
Inoculation of pooled lymph node suspensions or whole blood into naïve pigtailed macaques
| Lymph node cell and blood donor | Naïve macaque | Infectiona |
|---|---|---|
| 98P031 | 93P030 | None detected |
| 98P049 | ||
| 98P042 | ||
| 98P024 | 1242 | None detected |
| 98P043 | ||
| 98P023 | ||
| 98P045 | 1165 | None detected |
| 98P021 | ||
| 98P061 |
DNA PCR and RT-PCR were performed multiple times between weeks 1 and 26 postinfection.
Calculation of the protective in vivo neutralization titer.
In determining the titer of antivirus neutralizing antibody capable of protecting pig-tailed macaques against an intravenous challenge of SHIVDH12, we have combined the results obtained from the 15 animals described in this study with the 6 monkeys, passively immunized with a similar chimpanzee IgG, reported earlier (32). No evidence of SHIVDH12 infection was found in 12 of these 22 macaques (Table 2). Using the Reed and Muench approach to analyze the endpoint of protection attending the administration of the neutralizing chimpanzee IgG, we calculated the protective plasma titer to be 1:6.5 (Table 4). This is the concentration of neutralizing antibody in macaque plasma that will prevent infection of 50% of virus-inoculated monkeys. Because a vaccine capable of protecting only 50% of exposed individuals would not be considered effective, the data shown in Table 4 were used to determine the titer in plasma resulting in 99% protection. The titer calculated to protect 99% of inoculated monkeys was 1:38.
TABLE 4.
Calculation of the endpoint protection titer by the Reed and Muench method
| Endpoint neutralization titer in plasma | No. of animals
|
Accumated value
|
Protected
|
|||
|---|---|---|---|---|---|---|
| Protected | Infected | Protecteda | Infectedb | Ratio | % | |
| 1:123 | 2 | 0 | 12 | 0 | 12/12 | 100 |
| 1:40 | 2 | 0 | 10 | 0 | 10/10 | 100c |
| 1:18 | 1 | 1 | 8 | 1 | 8/9 | 88.8c |
| 1:15 | 1 | 0 | 7 | 1 | 7/8 | 87.5 |
| 1:13 | 1 | 0 | 6 | 1 | 6/7 | 85.7 |
| 1:12 | 1 | 0 | 5 | 1 | 5/6 | 83.3 |
| 1:8 | 1 | 0 | 4 | 1 | 4/5 | 80 |
| 1:7 | 0 | 2 | 3 | 3 | 3/6 | 50d |
| 1:6 | 1 | 0 | 3 | 3 | 3/6 | 50d |
| 1:5 | 1 | 0 | 2 | 3 | 2/5 | 40 |
| 1:4 | 1 | 2 | 1 | 5 | 1/6 | 16.7 |
| 1:3 | 0 | 4 | 0 | 9 | 0/9 | 0 |
Sum from the bottom.
Sum from the top.
99% protective titer was calculated to be 1:38.
Endpoint protection titer (50% protective titer) was calculated to be 1:6.5.
DISCUSSION
Although we previously reported that high concentrations of HIV-1 envelope glycoprotein-specific neutralizing antibodies in plasma could completely block SHIV infections of macaque monkeys, the experimental design used at the time did not permit the calculation of a statistically valid neutralization endpoint for protection in vivo (32). The results obtained in the present study not only confirmed our previous conclusion that anti-HIV-1 antibodies, capable of completely neutralizing a virus infection in vitro, can confer sterilizing protection in vivo but provided a statistically powerful framework for predicting the efficacy of future prophylactic vaccine formulations designed to elicit protective neutralizing antibodies. While it could be argued that a low-level or transient infection did, in fact, occur in the sterilely protected recipients of the anti-HIV-1 IgG, this seems to be highly unlikely in view of the extremely stringent assays used to detect virus or viral nucleic acids in these animals, which included the transfer of 108 lymph node cells and 8 ml of whole blood from the exposed to naïve recipient macaques.
The sterilizing protection against intravenously inoculated virus, observed for 10 of 15 rhesus monkeys following passive transfer of the anti-HIV-1 IgG, shares several similarities with a recently published study (26) in which administration of the anti-HIV-1 MAb b12 protected macaques from a vaginal challenge with the R5 SHIV162P4 (16). Although it is neither possible nor valid to directly relate the concentration in plasma of b12 MAb conferring complete protection to that measured for anti-HIV-1 IgG, the administration of 5 mg of b12 per kg, which resulted in sterilizing protection of two of four animals, appears to be equivalent in potency to the amount of IgG eliciting a neutralization titer in plasma of approximately 1:6.5, the neutralizing activity that we calculated to protect 50% of exposed animals. Furthermore, Parren et al. reported that four of four macaques receiving fivefold-higher amounts of the b12 MAb (viz., 25 mg/kg) were completely protected from a subsequent virus infection (26). This would correspond to animals in our study with neutralization titers in plasma of 1:32.5 or greater. All four animals with such antibody levels remained virus free (Table 2). Thus, although the SHIV inocula used, the routes of administration, and the nature and targeted epitopes of the protective antibodies differed greatly in the two studies, the relative protective effects of the two neutralizing antibody preparations were surprisingly similar. It is also worth noting that, although five of the passively immunized macaques became infected following virus challenge, the onset of the viremia was delayed in three of these animals and the peak PBMC-associated viral DNA loads were markedly reduced (100- to 500-fold) compared to those for the two naïve control monkeys (Fig. 3H and Table 2). This is similar to the results reported for b12 MAb recipient animals which subsequently became infected and suggests that low levels of virus-neutralizing antibody may be an important component of protective immunity even though sterilizing protection was not achieved.
While these types of experiments provide proof of the concept that preexisting neutralizing antibodies alone are sufficient to block primate lentivirus infections in vivo, the real challenge is to identify immunogens and/or vaccine strategies capable of eliciting high levels of neutralizing antibodies in naïve animals. In a previously published attempt to generate a broader neutralizing antibody response, pig-tailed monkeys were immunized with monovalent or polyvalent envelope glycoprotein vaccines (recombinant vaccinia virus prime plus protein boost) and then challenged with SHIVs bearing homologous or heterologous gp120s (4). Although the increase in the breadth of neutralizing activity was directed, disappointingly, almost entirely against viral strains present in the vaccine, sterilizing protection against an intravenous virus challenge was, in fact, achieved in the homologous arm of the experiment. No virus was detected in the plasma (RT-PCR) or lymph node cells (DNA PCR) in two vaccinated monkeys with neutralization titers of 1:81 (measured by 100% neutralization assay) at the time of virus challenge (4). In contrast, only one of the two immunized animals with neutralization titers in plasma of 1:9 resisted infection. Thus, the protective humoral immune responses elicited by an experimental prime-boost vaccine protocol, albeit in the contrived context of a homologous virus challenge, were completely consistent with the results obtained by passively transferring neutralizing anti-HIV-1 IgG (Tables 2 and 4).
As noted earlier, prior to the development of an effective poliovirus vaccine, the administration of human IgG in controlled clinical trials significantly reduced the incidence of paralytic poliomyelitis (14). The IgG used was prepared from a pool of plasma collected from a general population that had been exposed to yearly encounters with wild-type strains of poliovirus. In those trials, children received intramuscular injections of Red Cross gamma globulin (now designated immune globulin [IG]) at an empirically chosen dose of “0.14 cc per pound of body weight.” Assuming that the IG formulation used in the poliovirus passive immunization trial is similar to the present-day standardized human IG (165 mg/ml), the children were actually given 51 mg/kg (Table 5).
TABLE 5.
Dosage for prophylactic passive immunization against viral diseasea
| Virus | Immunoglobulin | Route | Dose (mg/kg) |
|---|---|---|---|
| Poliovirus | Human pooled IG | i.m. | 51b |
| Hepatitis A virus | IG | i.m. | 3.3c |
| Measles virus | IG | i.m. | 40c |
| Hepatitis B virus | Hepatitis B IG | i.m. | 10c |
| Rabies virus | Rabies IG | i.m. | 22c |
| Varicella-zoster virus | Varicella-zoster IG | i.m. | 30c |
| CMV | CMV IG | i.v. | 150c |
| RSV | RSV IG | i.v. | 750c |
| SHIVDH12 | Chimpanzee purified IgG | i.v. | 19d |
Passive immunization with human IG is currently used for hepatitis A virus and measles virus prophylaxis at the dosages shown in Table 5. Specific hyperimmune IGs, prepared from individuals known to have high titers of antibodies directed against other viral pathogens, are also available for human use. The recommended prophylactic dosage for these preparations is indicated in Table 5. When the polyclonal anti-HIV-1 chimpanzee IgG, evaluated in this study, is included with this group of IGs, its protective dose falls well within the range used for the other hyperimmune IGs. However, unlike other approved hyperimmune IGs, the protective anti-HIV-1 IgG evaluated here is highly specific for a single HIV-1 strain (HIV-1DH12) and is ineffective against other HIV-1 isolates (32, 35). Nonetheless, as proof of the concept that antibodies alone can be protective in vivo, the results obtained indicate that a vaccine capable of generating neutralization titers in plasma of 1:38 or greater against a wide range of HIV-1 isolates would confer almost complete protection against a virus challenge of 75 TCID50. Thus, the challenge ahead is clear: to identify immunogens capable of eliciting broadly reactive neutralizing antibodies which achieve sustained and protective titers in the plasma.
Acknowledgments
We are indebted to Carol Clarke, Brent Morse, and Wes Thornton for their diligence and assistance in the care and maintenance of our animals.
REFERENCES
- 1.American Academy of Pediatrics. 1997. Report of the Committee on Infectious Diseases, 24th ed. American Academy of Pediatrics, Elk Grove Village, Ill.
- 2.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brander, C., and B. D. Walker. 1999. T lymphocyte responses in HIV-1 infection: implications for vaccine development. Curr. Opin. Immunol. 11:451-459. [DOI] [PubMed] [Google Scholar]
- 4.Cho, M. W., Y. B. Kim, M. K. Lee, K. C. Gupta, W. Ross, R. Plishka, A. Buckler-White, T. Igarashi, T. Theodore, R. Byrum, C. Kemp, D. C. Montefiori, and M. A. Martin. 2001. Polyvalent envelope glycoprotein vaccine elicits a broader neutralizing antibody response but is unable to provide sterilizing protection against heterologous simian/human immunodeficiency virus infection in pigtailed macaques. J. Virol. 75:2224-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Committee on Care and Use of Laboratory Animals. 1985. Guide for the care and use of laboratory animals. NIH 85-23. Department of Health and Human Services, Washington, D.C.
- 6.Dewar, R. L., H. C. Highbarger, M. D. Sarmiento, J. A. Todd, M. B. Vasudevachari, R. T. Davey, Jr., J. A. Kovacs, N. P. Salzman, H. C. Lane, and M. S. Urdea. 1994. Application of branched DNA signal amplification to monitor human immunodeficiency virus type 1 burden in human plasma. J. Infect. Dis. 170:1172-1179. [DOI] [PubMed] [Google Scholar]
- 7.Dittmer, U., D. M. Brooks, and K. J. Hasenkrug. 1999. Requirement for multiple lymphocyte subsets in protection by a live attenuated vaccine against retroviral infection. Nat. Med. 5:189-193. [DOI] [PubMed] [Google Scholar]
- 8.Dittmer, U., B. Race, and K. J. Hasenkrug. 1999. Kinetics of the development of protective immunity in mice vaccinated with a live attenuated retrovirus. J. Virol. 73:8435-8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emini, E. A., W. A. Schleif, J. H. Nunberg, A. J. Conley, Y. Eda, S. Tokiyoshi, S. D. Putney, S. Matsushita, K. E. Cobb, C. M. Jett, J. W. Eichberg, and K. K. Murthy. 1992. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature 355:728-730. [DOI] [PubMed] [Google Scholar]
- 10.Endo, Y., T. Igarashi, Y. Nishimura, C. Buckler, A. Buckler-White, R. Plishka, D. S. Dimitrov, and M. A. Martin. 2000. Short- and long-term clinical outcomes in rhesus monkeys inoculated with a highly pathogenic chimeric simian/human immunodeficiency virus. J. Virol. 74:6935-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gauduin, M. C., P. W. Parren, R. Weir, C. F. Barbas, D. R. Burton, and R. A. Koup. 1997. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat. Med. 3:1389-1393. [DOI] [PubMed] [Google Scholar]
- 12.Gauduin, M. C., J. T. Safrit, R. Weir, M. S. Fung, and R. A. Koup. 1995. Pre- and postexposure protection against human immunodeficiency virus type 1 infection mediated by a monoclonal antibody. J. Infect. Dis. 171:1203-1209. [DOI] [PubMed] [Google Scholar]
- 13.Haigwood, N. L., A. Watson, W. F. Sutton, J. McClure, A. Lewis, J. Ranchalis, B. Travis, G. Voss, N. L. Letvin, S. L. Hu, V. M. Hirsch, and P. R. Johnson. 1996. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol. Lett. 51:107-114. [DOI] [PubMed] [Google Scholar]
- 14.Hammon, W. M., L. L. Coriell, P. F. Wehrle, and J. Stokes. 1953. Evaluation of Red Cross gamma globulin as a prophylactic agent for poliomyelitis 4. Final report of results based on clinical diagnoses. JAMA 151:1272-1285. [PubMed] [Google Scholar]
- 15.Harada, S., Y. Koyanagi, and N. Yamamoto. 1985. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science 229:563-566. [DOI] [PubMed] [Google Scholar]
- 16.Harouse, J. M., A. Gettie, R. C. Tan, J. Blanchard, and C. Cheng-Mayer. 1999. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284:816-819. [DOI] [PubMed] [Google Scholar]
- 17.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 20.McDougal, J. S., M. S. Kennedy, S. L. Orloff, J. K. Nicholson, and T. J. Spira. 1996. Mechanisms of human immunodeficiency virus type 1 (HIV-1) neutralization: irreversible inactivation of infectivity by anti-HIV-1 antibody. J. Virol. 70:5236-5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMichael, A. 1998. T cell responses and viral escape. Cell 93:673-676. [DOI] [PubMed] [Google Scholar]
- 22.Montefiori, D. C., G. Pantaleo, L. M. Fink, J. T. Zhou, J. Y. Zhou, M. Bilska, G. D. Miralles, and A. S. Fauci. 1996. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressors. J. Infect. Dis. 173:60-67. [DOI] [PubMed] [Google Scholar]
- 23.Moore, J. P., Y. Cao, J. Leu, L. Qin, B. Korber, and D. D. Ho. 1996. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J. Virol. 70:427-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy, B. R., and R. M. Chanock (ed.). 2001. Immunization against viral diseases, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 25.Parren, P. W., H. J. Ditzel, R. J. Gulizia, J. M. Binley, C. F. Barbas III, D. R. Burton, and D. E. Mosier. 1995. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS 9:F1-F6. [DOI] [PubMed] [Google Scholar]
- 26.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 28.Sabin, A. B. 1961. Effects of rapid mass immunization of a population with live oral poliovirus vaccine under conditions of massive enteric infection with other viruses, p. 342-349. In Fifth International Poliomyelitis Conference. J. B. Lippincott, Copenhagen, Denmark.
- 29.Salk, J. E. 1961. Persistence of immunity following administration of formalin-treated poliovirus vaccine, p. 157-175. In Fifth International Poliomyelitis Conference. J. B. Lippincott, Copenhagen, Denmark.
- 30.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 31.Shibata, R., M. D. Hoggan, C. Broscius, G. Englund, T. S. Theodore, A. Buckler-White, L. O. Arthur, Z. Israel, A. Schultz, H. C. Lane, and M. A. Martin. 1995. Isolation and characterization of a syncytium-inducing, macrophage/T-cell line-tropic human immunodeficiency virus type 1 isolate that readily infects chimpanzee cells in vitro and in vivo. J. Virol. 69:4453-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204-210. [DOI] [PubMed] [Google Scholar]
- 33.Shibata, R., F. Maldarelli, C. Siemon, T. Matano, M. Parta, G. Miller, T. Fredrickson, and M. A. Martin. 1997. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J. Infect. Dis. 176:362-373. [DOI] [PubMed] [Google Scholar]
- 34.Shibata, R., C. Siemon, T. A. Rizvi, T. Matano, W. C. Satterfield, H. C. Lane, and M. A. Martin. 1997. Reactivation of HIV type 1 in chronically infected chimpanzees following xenostimulation with human cells or with pulses of corticosteroid. AIDS Res. Hum. Retrovir. 13:377-381. [DOI] [PubMed] [Google Scholar]
- 35.Willey, R. L., R. Shibata, E. O. Freed, M. W. Cho, and M. A. Martin. 1996. Differential glycosylation, virion incorporation, and sensitivity to neutralizing antibodies of human immunodeficiency virus type 1 envelope produced from infected primary T-lymphocyte and macrophage cultures. J. Virol. 70:6431-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willey, R. L., D. H. Smith, L. A. Lasky, T. S. Theodore, P. L. Earl, B. Moss, D. J. Capon, and M. A. Martin. 1988. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J. Virol. 62:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]