Abstract
Infection by Kaposi's sarcoma-associated herpesvirus (KSHV) is central to the pathogenesis of the endothelial neoplasm Kaposi's sarcoma (KS) and is also linked to the rare B-cell tumor known as primary effusion lymphoma (PEL). Latently infected PEL cell lines can be induced to enter the lytic cycle and produce KSHV virions. However, such cells do not support de novo infection or serial propagation of KSHV. These limitations have prevented the development of systems for the genetic analysis of KSHV and have impeded a deeper understanding of KS pathogenesis. Here we show that human dermal microvascular endothelial cells immortalized by expression of telomerase can be readily infected by KSHV virions produced by PEL cells. Infection is predominantly latent, but a small subpopulation enters the lytic cycle spontaneously. Phorbol ester (tetradecanoyl phorbol acetate [TPA]) treatment of latently infected cells leads to enhanced induction of lytic KSHV replication, resulting in foci of cytopathic effect. There is no cytopathic effect or viral DNA expansion when infected TIME cells (telomerase-immortalized microvascular endothelial cells) are TPA induced in the presence of phosphonoacetic acid (PAA), an inhibitor of herpesvirus replication. Supernatants from phorbol-induced cultures transfer latent KSHV infection to uninfected cells, which can likewise be induced to undergo lytic replication by TPA treatment, and the virus can be further serially transmitted. Serial passage of the virus in TIME cells is completely inhibited when TPA treatment is done in the presence of PAA. Latently infected endothelial cells do not undergo major morphological changes or growth transformation, and infection is lost from the culture upon serial passage. This behavior faithfully recapitulates the behavior of spindle cells explanted from primary KS biopsies, strongly supporting the biological relevance of this culture system. These findings suggest that either the stability or the growth-deregulatory potential of the KSHV latency program in endothelial cells is more limited than might be predicted by analogy with other oncogenic viruses.
Infection by Kaposi's sarcoma-associated herpesvirus (KSHV) (also called human herpesvirus 8) is now widely acknowledged to be essential for the development of Kaposi's sarcoma (KS), a curious endothelial proliferation that until recently was the leading neoplasm of AIDS patients. Initially identified by the cloning of fragments of its genome from KS tumors (4), KSHV was first recovered as a virus by cultivation of latently infected cells from another KSHV-associated neoplasm, primary effusion lymphoma (PEL) (30). As isolated from patients, PEL cells are predominantly latently infected by KSHV, although at any given time a low percentage of cells are undergoing lytic reactivation. Treatment of PEL cells in vitro with phorbol esters or sodium butyrate induces the virus to enter the lytic replicative cycle, leading to release of viral progeny (3, 14, 15, 20, 24, 26, 30, 32). While PEL-based systems are very useful for KSHV research, they have serious limitations. First, since PEL cells are lymphoid cells, they cannot be used to study the impact of viral replication on endothelial function or proliferation. Second, neither they nor many other standard established cell lines support efficient de novo infection and serial propagation by KSHV, although some lines (e.g., 293 cells) are reported to support a low level of infection with limited release of infectious progeny (12, 29).
Prior to this work, a few reports have examined KSHV infection of cultured endothelial cells. Flore et al. (11) exposed primary vascular endothelial cells to PEL-derived KSHV and reported that such cells gave rise to long-lived cell subpopulations. Such cells took weeks to grow out, and less that 5% of the resulting cell population proved to harbor viral DNA, suggesting that rare infected cells might be able to promote the survival of uninfected cells via an as-yet-unspecified paracrine pathway. Although this is interesting in view of the growing suspicion that paracrine signaling may play a role in KS (8-10, 21), the proportion of infected cells in such cultures is too low for most virologic studies. Moses et al. (27) reported that endothelial cells expressing the human papillomavirus E6 and E7 oncogenes could support a more efficient de novo infection by PEL-derived KSHV. Initially a low percentage of cells were latently infected, but over a period of weeks a large percentage of the cells became infected. However, these cells can be difficult to maintain in culture as a continuously proliferating population (M. Lagunoff and D. Ganem, unpublished data) and have not been widely used. Recently, Ciufo et al. (5) reported that primary dermal microvascular endothelial cells could be infected with KSHV; again, initially only a small percentage of cells were infected, but there was expansion of infection with time, such that by 1 month postinfection most of the cells maintained KSHV in the latent state. In these experiments, fresh, uninfected cells had to be added periodically to perpetuate infection, which appeared to be sustained by a mix of latently and lytically infected cells. This system has the virtue of employing cells that are phenotypically closer to normal vascular endothelial cells than most established lines, but the fact that such cells are difficult to prepare and are of limited availability makes this system a challenging one for routine use.
The ability of many primary cell types, including endothelial cells (34), to be immortalized by expression of the telomerase reverse transcriptase subunit (hTERT) has recently been recognized. Venetsanakos et al. (33) have derived a new cell line, called TIME cells (for telomerase-immortalized microvascular endothelial cells) by transduction of dermal microvascular endothelial cells with a retrovirus expressing hTERT. TIME cells grow continuously in culture and maintain many properties of primary endothelial cells. Here we show that incubation of TIME cells with KSHV virions results in efficient latent infection, and we report on the phenotypic properties of the latently infected endothelial cultures. KSHV in such cultures can be readily induced to lytic growth by phorbol ester treatment, generating infectious progeny that can be serially propagated in vitro.
MATERIALS AND METHODS
Cells and media.
BCBL-1 cells are an Epstein-Barr virus-negative primary effusion lymphoma cell line and have been previously described (30). BCBL-1 cells were carried in RPMI 1640 supplemented with 10% fetal bovine serum, penicillin, streptomycin, glutamine, and β-mercaptoethanol. TIME cells are a dermal microvascular endothelial cell line immortalized with hTERT and are described elsewhere (33). They were maintained in an EBM-2 medium bullet kit (Clonetics) that contains vascular endothelial growth factor, basic fibroblast growth factor, insulin-like growth factor 3, epidermal growth factor, and hydrocortisone and grown in standard tissue culture flasks.
Viruses and infection.
Virus inoculum was obtained by inducing BCBL-1 cells, split to 2 × 105 to 3 × 105 cells per ml the day before, with 20 ng of tetradecanoyl phorbol acetate (TPA) per ml and 500 ng of ionomycin per ml. After 16 to 20 h the cells were pelleted and resuspended in the same amount of medium without TPA and ionomcycin. At 5 days postinduction cells were pelleted, the supernatant was filtered through a 0.45 μm-pore-size filter, and the virions were pelleted at 15,000 rpm for 2 h in an SW28 rotor. A portion of the pellet was used to isolate virion DNA as described previously (22). The virion DNA was then digested with BamHI and separated on an agarose gel beside a known dilution series of a plasmid containing a 1-kb BamHI fragment of KSHV. The amount of viral DNA was then estimated by comparing the intensities of Southern hybridization of the KSHV DNA to the diluted plasmid. From this the number of total virion particles per milliliter of BCBL-1 supernatant was back calculated. For infections, the viral pellet was resuspended in the indicated medium and used to inoculate TIME cell cultures for 2 h, and then the cell monolayer was washed one time and overlaid with fresh complete medium. For TIME cell inductions, the medium was subsequently replaced with fresh medium containing TPA or TPA with phosphonoacetic acid (PAA). When used, PAA was added to 0.5 mM.
Immunofluorescence and immunohistochemistry.
Immunofluoresence was done as previously described (24). The primary antibodies used were an anti-LANA peptide polyclonal antiserum from rabbits (provided by A. Polson) or mouse monoclonal antibodies that recognize either K8.1 or ORF 59 (kind gifts of L. Wu and B. Forghani). The primary antibody was diluted 1:500 in the block solution. Cells were mounted in medium containing 4",6"-diamidino-2-phenylindole (DAPI) to stain the nuclei and examined with a fluorescence microscope. The immunohistochemistry was done essentially as previously described (17).
Southern hybridization.
Uninfected and KSHV-infected TIME cells growing in 75-cm2 flasks were trypsinized and lysed in sodium dodecyl sulfate buffer (100 mM Tris, 150 mM NaCl, 10 mM EDTA, 0.1% sodium dodecyl sulfate) containing 100 μg of proteinase K per ml. DNA was extracted with phenol-chloroform, ethanol precipitated, and digested with BamHI, and 8 μg of DNA for each sample was separated by agarose gel electrophoresis, transferred to a nylon membrane, and hybridized to a radiolabeled probe from the 1-kb BamHI fragment containing the viral G-protein-coupled receptor (GPCR). The membrane was washed and exposed to Kodak XAR5 film for 5 days.
Tubule formation in Matrigel.
The Matrigel matrix cellware (Fisher) was thawed for 12 h at 4°C and then incubated at 37°C for 30 min prior to use. Cells were trypsinized and counted on a hemacytometer, and 25,000 mock- and KSHV-infected TIME cells were plated into each well of a 24-well Matrigel matrix cellware. At 6 to 8 h after plating, tubule formation was visualized by light microscopy.
RESULTS
Infection of TIME cells by KSHV.
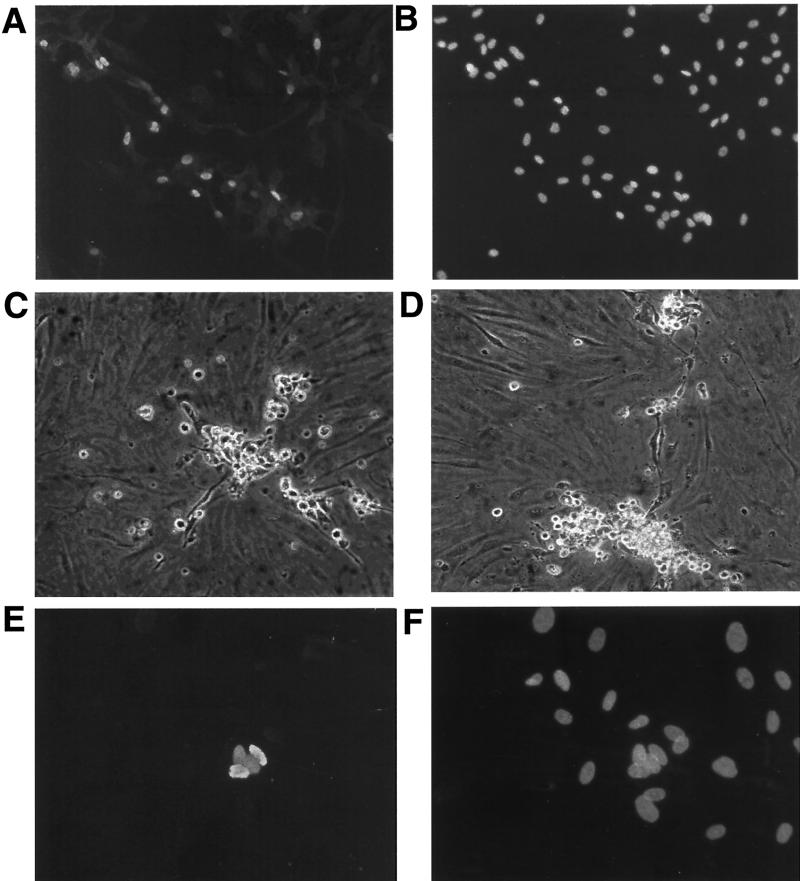
TIME cells are telomerase-immortalized microvascular endothelial cells that retain many functions of differentiated vascular endothelium (see below). They grow well (doubling time of 36 to 48 h) as monolayer cultures and, though immortalized, do not show laboratory signs of transformation, inasmuch as they do not form foci in vitro or form tumors in SCID mice (33; C. Stoddart, M. Lagunoff, M. McCune, and D. Ganem, unpublished data). KSHV virions were prepared by phorbol ester-mediated induction of the PEL cell line BCBL-1. Concentrated supernatants bearing virus harvested from 10 ml of BCBL-1 (estimated particle number, 107 per ml) (see Materials and Methods) were incubated with approximately 104 TIME cells in serum-free medium for 2 h and then removed by washing, and the cells were incubated in complete endothelial growth medium for 48 h. Cells were examined by immunofluorescence with antibodies specific for LANA, the viral latency-associated nuclear antigen encoded by KSHV ORF 73. As shown in Fig. 1A and B, under these conditions nearly all cells on the dish displayed LANA expression, indicative of latent KSHV infection. Higher magnification revealed that LANA is distributed in characteristic intranuclear dots (Fig. 1C and D) identical to those observed in authentic KSHV infection of KS spindle cells (13, 18, 19, 28). Such dots are thought to represent foci of LANA bound to latent viral episomes, likely attached to cellular chromatin (2, 6).
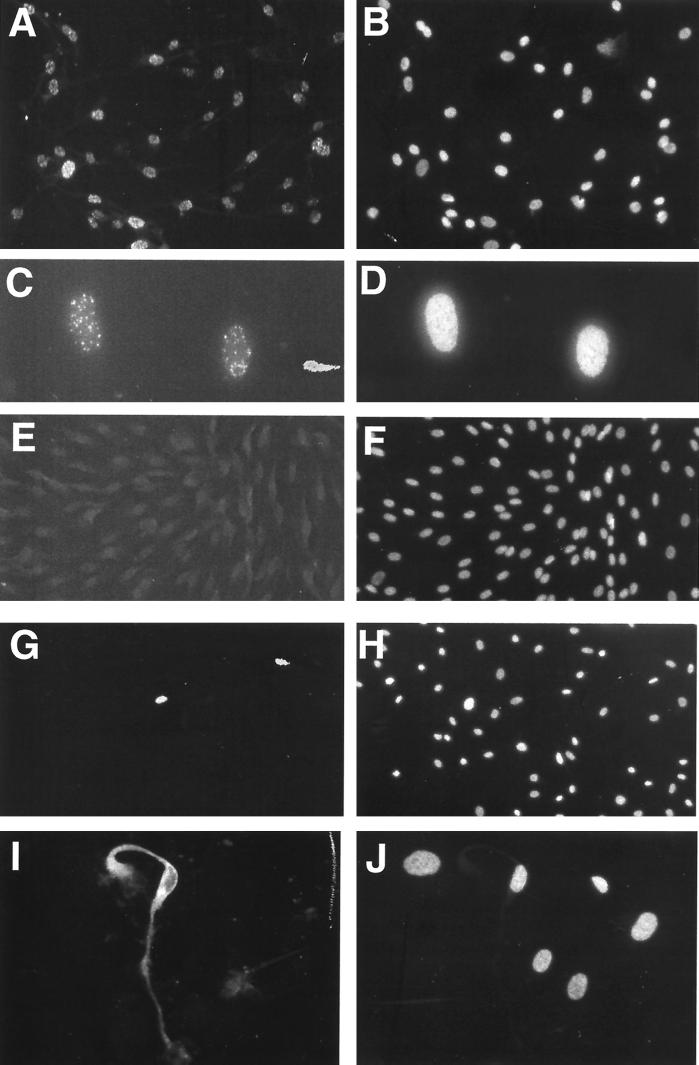
FIG. 1.
Immunofluoresence of KSHV-infected and uninfected TIME cells. (A) KSHV-infected TIME cells stained with anti-LANA antibody 48 h after infection. (B) DAPI nuclear stain of panel A. (C) Higher magnification of two TIME cells, stained with anti-LANA antibody, 48 h after infection. (D) DAPI stain of panel C. (E) Uninfected TIME cells stained with anti-LANA. (F) DAPI stain of panel E. (G and I) Infected TIME cells stained with anti-ORF 59 and anti-K8.1, respectively. (H and J) DAPI stains of panels G and I, respectively.
Under our standard infection conditions, latent infection appears to predominate. Only a small subset (ca. 1%) of cells stain for lytic-cycle markers, such as the product of ORF 59, a delayed-early gene involved in viral DNA replication (Fig. 1G and H). This is highly reminiscent of the situation of authentic KS, in which a similar proportion of spindle cells can be shown to be in the lytic cycle (31). When staining for a late lytic marker (in this case, the K8.1 envelope glycoprotein) is performed, a similarly small number of cells are detected, but these cells often have more dramatically abnormal morphologies consistent with viral cytopathic effect (Fig. 1I and J). This is not unexpected, since K8.1 scoring preferentially identifies cells with advanced lytic infection (35).
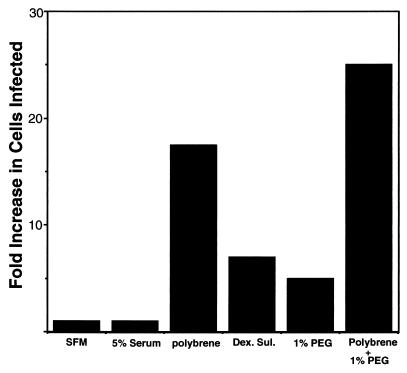
When infection is carried out in standard complete medium, rather large amounts of virus supernatant are required to infect most cells in the culture. To improve the efficiency of infection, we tested the effects of various compounds on KSHV infectivity. Polyanions like Polybrene and dextran sulfate are known to enhance the infectivity of many classes of animal viruses; fusogens like polyethylene glycol (PEG) have also been shown to enhance the infectivity of some types of viruses even at concentrations well below those needed to fuse cellular membranes (16). Accordingly, we tested the ability of several of these compounds to enhance the infectivity of KSHV for TIME cells. We infected cells with serial dilutions of KSHV in the presence or absence of different drugs and calculated the fold increases in infection rates based on comparing the dilution at which the treated inoculum yielded 50% infection to the dilution at which the untreated inoculum yielded 50% infection. Figure 2 shows that while the presence or absence of 5% fetal calf serum did not significantly affect infectivity, addition of Polybrene at 8 μg/ml enhanced the yield of LANA-positive cells nearly 20-fold. Dextran sulfate and 1% PEG enhanced infection five- to sevenfold, and the combination of Polybrene plus PEG was additive.
FIG. 2.
Enhancement of KSHV infection of TIME cells by different factors. TIME cells were infected for 2 h with serial dilutions of KSHV in the absence or presence of serum, Polybrene (8 μg/ml), dextran sulfate (Dex. Sul.), PEG, or Polybrene and PEG as indicated. At 48 h after infection the number of LANA-positive cells for each dilution was assessed, and the fold increase in the number of cells expressing LANA was plotted. SFM, serum-free medium.
Phenotype of latently infected TIME cells.
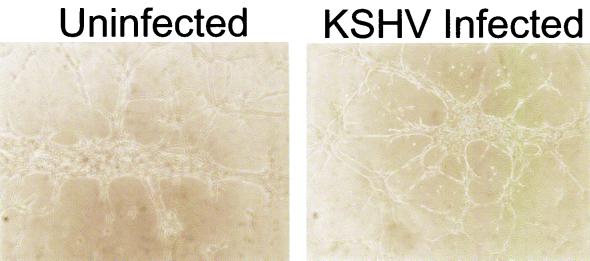
When latently infected cells were examined morphologically, only small differences from uninfected TIME cells were observed. Immortalization of the endothelial cells by the hTERT retrovirus induced spindle-like morphology in the absence of KSHV. Addition of KSHV caused some initial further extension of the cells, but only a small percentage of the cells elongated more dramatically (data not shown). To determine if KSHV latency affects the differentiated endothelial phenotype, we analyzed the expression of a variety of endothelial and other markers in uninfected and infected TIME cells by immunohistochemistry. Table 1 lists the markers tested and indicates the patterns of expression seen in TIME cells. KSHV infection did not substantially alter the expression of any of the endothelial cell-specific proteins tested that were positive in the parental cells, although we cannot rule out the possibility that some markers’ expression might have been slightly affected. KSHV latency was not associated with the expression of markers from hematopoietic, epithelial, or neural cell lineages. As further evidence that the differentiated endothelial phenotype of the cells was not grossly altered, we examined the ability of infected cells to form tube-like structures on Matrigel. As shown in Fig. 3, by 8 h after plating on Matrigel, uninfected and infected cells showed comparable levels of tube formation.
TABLE 1.
Marker expression in TIME cells
| Antigena | Comment | Pattern |
|---|---|---|
| Factor VIII, vWF | Endothelium | Cytoplasmic |
| CD31 | Endothelium | Cytoplasmic |
| Ulex europeus | Endothelium binding lectin | Cytoplasmic |
| CD36 | Endothelium, epithelium, lymphoid | Cytoplasmic |
| CD68 | Macrophages, monocytes | Negative |
| S-100 | Neural crest | Negative |
| CD45 | Pan-leukocyte | Negative |
| Keratin | Epithelium | Negative |
| αvβ3 | Integrin | Cytoplasmic |
| αvβ5 | Integrin | Cytoplasmic |
| VEGF-R1, flt-1 | Endothelium | Membrane |
| VEGF-R2, flk-1 | Endothelium | Membrane |
| CD123 (IL-3R) | Endothelium, dendritic cells | Negative |
VEGF-R1 and -R2, vascular endothelial growth factor receptors 1 and 2; IL-3R, interleukin-3 receptor.
FIG. 3.
Tubule formation by TIME cells on Matrigel medium. Uninfected and KSHV-infected TIME cells (25,000 cells) were plated on Matrigel matrix plugs in each well of a 24-well plate for 6 to 8 h, and tubule formation was examined by light microscopy.
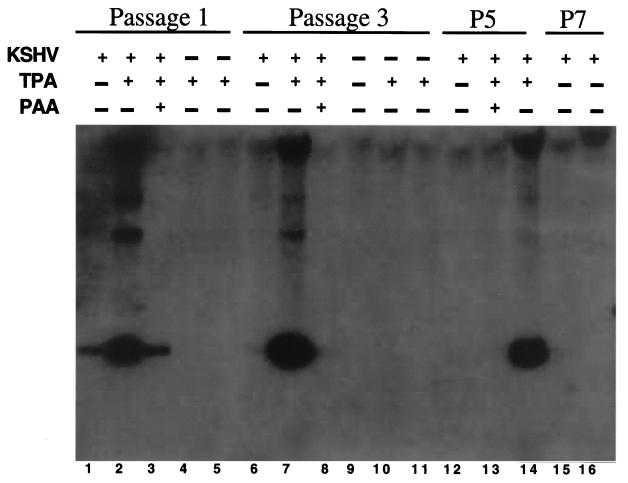
To examine the effects of KSHV latency on cellular growth, cultures bearing 99% LANA-positive cells were serially passaged and the fraction of LANA-positive cells was assayed at each passage level. At cell passage 1, 95% of the cells were LANA positive, but by cell passage 3, only 50% of the cells were LANA positive, by cell passage 5, 5 to 10% of the cells were positive, and by cell passage 7, only 0.1% of the cells continued to harbor detectable latent infection. To confirm that the loss of LANA expression reflected loss of latent KSHV infection, total DNA was also isolated from infected TIME cells at various passages, and viral DNA was detected by Southern hybridization. DNA could clearly be seen after one passage (see Fig. 5, lane 1) and decreased with serial passage (lanes 6, 12, and 15), although treatment with TPA continued to allow lytic reactivation in the remaining latently infected cells, as described below.
FIG. 5.
TIME cell DNA digested and hybridized to a radiolabeled fragment of KSHV. Total DNA was isolated from TIME cells at the indicated cell passage after infection. Cells were left untreated throughout the passages or were treated with TPA for 3 days (passage [P] 1), with TPA for 6 days (passages 3 and 5), or with TPA and PAA for 3 to 6 days after the indicated passage. Uninfected cells were passaged and treated identically to the infected cells. After isolation, DNA was digested with BamHI, separated, and probed with a 1-kb BamHI fragment from the GPCR region of KSHV.
The progressive loss of KSHV infection indicates that, contrary to what might have been expected, KSHV latency did not confer a growth advantage over uninfected TIME cells in vitro. This result exactly parallels the behavior of authentic KS spindle cells when explanted into culture; namely, there is a progressive decrease in viral copy number with each passage until, at steady state, virtually all proliferating cells lack viral infection (1, 7, 23). This correspondence strongly suggests that this phenotype, which arguably is the opposite of what conventional tumor viruses would be expected to engender, is biologically relevant, and its potential meanings are considered in Discussion.
Lytic induction and serial passage of KSHV infection.
To demonstrate that TIME cells support the entire cycle of KSHV infection, we tested whether TPA could induce lytic replication in infected TIME cells. TIME cell cultures bearing >80% LANA-positive cells were treated with TPA for 4 days and then assayed for lytic induction by immunofluorescence staining for the lytic markers ORF 59 and K8.1. TPA treatment resulted in a large percentage of cells undergoing lytic replication compared to untreated cells (20 to 30% versus 0.1 to 2%) (Fig. 4A and B), with a concurrent increase in the amount of viral DNA present (Fig. 5, lanes 1, 2, 6, 7, 12, and 14). Groups of rounded-up cells, often containing multinucleated cells piling up in discrete foci, were observed (Fig. 4E and F). Many cells in these foci detached readily from the plates and so were difficult to stain, but immunofluorescence from cultures induced for a shorter time showed smaller plaques whose cells remained on the plate, and these cells stained brightly for lytic markers (e.g., ORF 59 [Fig. 4E]). These structures were never seen in infected cells treated with TPA in the presence of PAA, a broad-spectrum inhibitor of herpesvirus DNA replication, indicating that viral replication was necessary for plaque formation (data not shown). PAA also inhibited induction of DNA replication by KSHV (Fig. 5, lanes 3, 8, and 13). Thus, these plaque-like elements were foci of lytic replication. Interestingly, when a culture of TIME cells that was only approximately 1% latently infected was induced with TPA for 5 to 6 days, there were only a few large plaques in a 25-cm2 plate (Fig. 4C and D). This indicates that plaques were formed due to localized cell-to-cell spread of virus reactivated from a rare latently infected cell and renders unlikely the model that in more densely infected cultures, plaques might arise from the localized switching of clusters of latently infected cells to the lytic cycle.
FIG. 4.
KSHV lytic induction and plaque formation in TIME cells treated with TPA. (A) Anti-ORF 59 immunofluorescence of KSHV-infected TIME cells induced for 3 days with TPA. (B) DAPI stain of panel A. (C and D) Light microscope pictures of infected TIME cells (1% LANA positive) 5 days after TPA induction. (E) Anti-ORF 59 immunofluorescence of infected TIME cells 3 days after TPA induction. (F) DAPI staining of panel E.
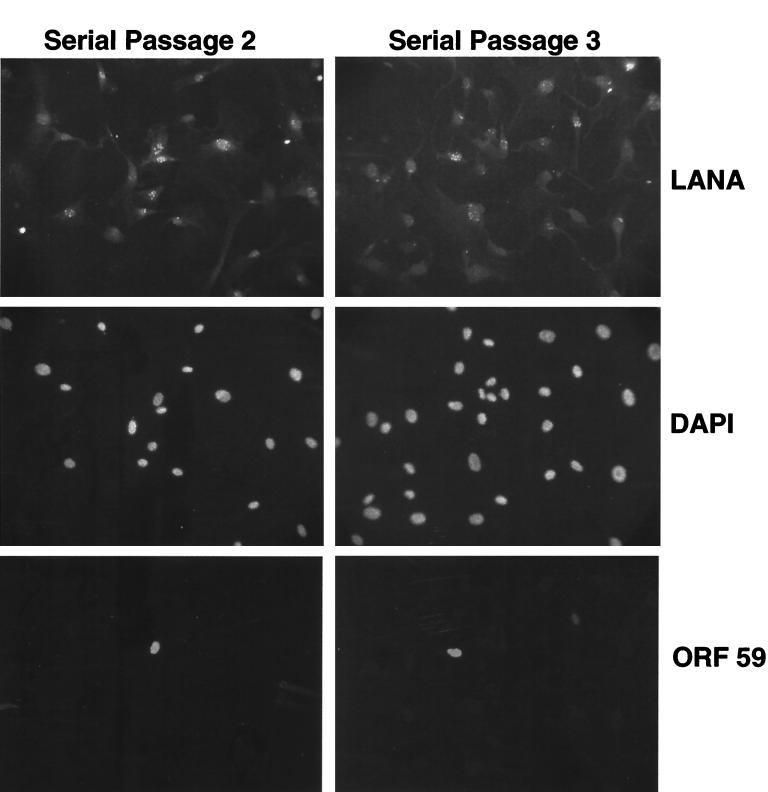
To demonstrate serial passage of KSHV infection, a 50% confluent 150-cm2 flask of TIME cells was infected with BCBL-1-derived KSHV so that nearly all cells in the culture expressed LANA (viral passage 1). Three days later, cells were treated with TPA (20 ng/ml) for 4 days; following this treatment, numerous plaque-like foci were visible. To document that infectious virus was indeed being released, all of the virus isolated from cell-free supernatants of one 150-cm2 flask was concentrated and used to inoculate a 50% confluent 75-cm2 dish of uninfected TIME cell monolayers. Three days later, the flasks were split 1:2 and a subset of cells were stained for LANA. Figure 6 shows that over 80% of the recipient cells became LANA positive, with (as expected) only a small subpopulation staining for ORF 59 (viral passage 2). The infected recipient culture was left untreated for 2 days, and one dish was induced with TPA for 6 days. A new 75-cm2 flask of uninfected TIME cells was inoculated with the virus concentrated from the cell-free supernatant of one 75-cm2 flask from passage 2, and these cells were split and stained for LANA 4 days after infection (Fig. 6, passage 3). These cells were 50 to 60% LANA positive; thus, the system allows completion of the full KSHV lytic cycle with the production of infectious progeny and serial passage of the virus. No transmission of KSHV infection was observed with supernatants from LANA-positive cultures that were TPA treated in the presence of PAA (or treated with TPA for only short times), indicating that phorbol exposure effectively amplified the copy number of infectious virions in the supernatant. This excludes the remote possibility that the observed passage of infection was due to carryover of infectious material from the BCBL-1-derived inoculum.
FIG. 6.
Serial passage of KSHV through TIME cells. Virions from BCBL-1 cells were used to infect TIME cells. The cells were subsequently split 1:2, and then one flask was induced with TPA for 4 days. Virions were harvested from the supernatant and used to infect fresh TIME cells. At 48 h after infection, a subset of cells were stained with anti-LANA (tetramethyl rhodamine isocyanate), DAPI, and anti-ORF 59 (fluorescein isothiocyanate) (serial passage 2). Virions from the second serial passage after one split and 5 days of TPA induction were used to infect fresh TIME cells, and 72 h later these cells were stained with anti-LANA (tetramethyl rhodamine isocyanate), DAPI, and anti-ORF 59 (fluorescein isothiocyanate) (serial passage 3).
DISCUSSION
We report here a simple and experimentally tractable system for the infection and serial propagation of KSHV in human microvascular endothelium. Our optimized conditions allow for reproducible infection of 60 to 95% of the cells in a standard culture, although we note that substantial quantities of virions are required for a high density of infection even after such optimization. It is possible that serial passage of BCBL-1-derived virus on TIME cells may select for variants adapted to more efficient and high-titer replication in endothelia, and efforts to select such variants are now under way. If successful, such efforts could remove one remaining limitation of the present system, namely, that yields of infectious virus are modest. Although we present clear documentation of serial transmission of infectivity, the system in its present form is more suited to studies on the analytical rather than the preparative scale.
Our system differs substantially in design from that of Flore et al. (11), so the two systems cannot be directly compared. Their system begins with primary (nonimmortalized) microvascular endothelium and scores for cell life span extension; ours begins with immortalized endothelium and scores for viral infection. Because the efficiency of latent infection is much higher in the TIME system, TIME cells are clearly preferable for studies of viral gene expression, viral stock preparation, and development of mutant viral strains. By contrast, the system of Flore et al. presents opportunities to explore possible roles of paracrine signaling that are not prominent features of the TIME system. Formally, our system is more analogous to that developed by Moses et al. (27), in that both use life-extended microvascular endothelium as infection targets and report higher frequencies of infection. However, the human papillomavirus E6/E7-expressing endothelial cell line used in their studies grows slowly and erratically, and stable long-term passage of the cells has been problematic in many laboratories. Nonetheless, the results in both systems are in agreement that the default pathway for KSHV infection in endothelia is latency, just as in infection of primary B cells by Epstein-Barr virus but in contrast to the case of alphaherpesviruses like herpes simplex virus, which promptly enter the lytic cycle in most cultured lines.
Our results have three important implications for KSHV research. First, they open several aspects of the viral life cycle to experimental scrutiny. The availability of a simple system for de novo infection should facilitate the study of the early events of infection, such as envelope-receptor interactions, membrane fusion, and nuclear delivery. Related to this is the fact that the system should be readily adaptable to the development of assays for virus neutralization, either by patient antisera or by experimentally raised antiviral antibodies. Second, the ability to serially passage KSHV opens the door to the construction and analysis of mutant viruses in which individual KSHV genes have been disrupted by homologous recombination. Such systems have revolutionized our understanding of other herpesviruses and should now be possible for KSHV.
Finally, our results have implications for the study of KSHV latency in endothelia and its relationship to cellular growth and survival. Clearly, the system can be used to enumerate the viral genes expressed in endothelial latency, which will allow instructive comparison with the better-studied latency program in B cells. Because TIME cells are already immortalized, inferences about the effects of latency on cell growth and survival must be made with circumspection (the primary microvascular culture system of Ciufo et al. [5] is superior for this sort of analysis). Certainly, infected TIME cells do not display the typical morphological changes described for malignantly transformed cells, nor do they lose contact inhibition or form foci in vitro. This accords well with the facts that KS spindle cells are diploid and that cultures derived from them also lack evidence of transformation, as they do not form colonies in soft agar or tumors in nude mice (10). While we cannot exclude the possibility that the latency program may be an immortalizing stimulus in endothelia, we note that in TIME cells, in primary endothelial cells, and in authentic KS spindle cells explanted in culture (1, 7, 23), latently infected cells are rapidly lost. We can envision two possible (and not mutually exclusive) explanations for this behavior. First, the plasmid maintenance function of LANA may be inefficient or nonfunctional in endothelial cells; if so, this would result in segregation of the viral episome with cell passage and the progressive accumulation of uninfected cells. Second, it is possible that the KSHV latency program may not lead to a sustained growth advantage under conditions employed in culture. Clearly, latently infected cells accumulate in KS tumors as they progress and therefore must have at least some competitive advantage over uninfected cells in vivo (31). However, such an advantage need not be at the level of increased cell proliferation. If, for example, infected cells were more resistant to growth inhibition by inflammatory mediators released in the tumor microenvironment, a similar enrichment for infected cells could result in vivo but be undetectable in vitro. In either case, the net result would be that, at the level of the single cell, latent endothelial infection is of finite duration.
Recently, Martin et al. (25) showed that treatment of patients with advanced AIDS with ganciclovir, a drug that blocks lytic but not latent KSHV infection, leads to a dramatic decrease in KS development. Thus, development of a KS lesion appears to require continuous lytic replication throughout the natural history of infection. Such a result is exactly what would be expected if the life span of an individual, latently infected spindle cell is not indefinite. Under such conditions, a tumor could form only if the rate of new latent infections generated by lytically produced virus exceeded the rate of disappearance of latently infected cells. Obviously, this clinical experiment can be interpreted in several other ways and by itself mandates no one model of tumorigenesis. However, at a minimum it can be said that nothing in the biology of KS requires that the latency program be transforming or even permanently life-extending, in the sense required to score in popular in vitro assays commonly used to define oncogenes. An important corollary of this is that searching for viral genes that score in such assays will not necessarily lead to identification of the critical determinants of KS pathogenesis.
Acknowledgments
We thank A. Polson for providing the anti-LANA antibody and L. Wu and B. Forghani for providing the anti-ORF 59 and anti-K8.1 monoclonal antibodies. We also thank E. Brazeau for excellent technical assistance.
M.L is supported by a special fellowship from the Leukemia and Lymphoma Society and by a new investigator award from the University of Washington Center for AIDS Research.
REFERENCES
- 1.Aluigi, M. G., A. Albini, S. Carlone, L. Repetto, R. De Marchi, A. Icardi, M. Moro, D. Noonan, and R. Benelli. 1996. KSHV sequences in biopsies and cultured spindle cells of epidemic, iatrogenic and Mediterranean forms of Kaposi's sarcoma. Res. Virol. 147:267-275. [DOI] [PubMed] [Google Scholar]
- 2.Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. [DOI] [PubMed] [Google Scholar]
- 3.Cannon, J. S., D. Ciufo, A. L. Hawkins, C. A. Griffin, M. J. Borowitz, G. S. Hayward, and R. F. Ambinder. 2000. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi's sarcoma herpesvirus-containing supernatant. J. Virol. 74:10187-10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 5.Ciufo, D. M., J. S. Cannon, L. J. Poole, F. Y. Wu, P. Murray, R. F. Ambinder, and G. S. Hayward. 2001. Spindle cell conversion by Kaposi's sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J. Virol. 75:5614-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter, M. A., II, and E. S. Robertson. 1999. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264:254-264. [DOI] [PubMed] [Google Scholar]
- 7.Dictor, M., E. Rambech, D. Way, M. Witte, and N. Bendsoe. 1996. Human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus) DNA in Kaposi's sarcoma lesions, AIDS Kaposi's sarcoma cell lines, endothelial Kaposi's sarcoma simulators, and the skin of immunosuppressed patients. Am. J. Pathol. 148:2009-2016. [PMC free article] [PubMed] [Google Scholar]
- 8.Ensoli, B., G. Barillari, and R. Gallo. 1992. Cytokines and growth factors in the pathogenesis of AIDS-associated Kaposi's sarcoma. Immunol. Rev. 127:147-155. [DOI] [PubMed] [Google Scholar]
- 9.Ensoli, B., S. Nakamura, S. Salahuddin, P. Biberfeld, L. Larsson, B. Beaver, F. Wong-Staal, and R. Gallo. 1989. AIDS-Kaposi's sarcoma derived cells express cytokines with autocrine and paracrine growth effect. Science 243:223-226. [DOI] [PubMed] [Google Scholar]
- 10.Ensoli, B., and M. Sturzl. 1998. Kaposi's sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 9:63-83. [DOI] [PubMed] [Google Scholar]
- 11.Flore, O., S. Rafii, S. Ely, J. J. O'Leary, E. M. Hyjek, and E. Cesarman. 1998. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature 394:588-592. [DOI] [PubMed] [Google Scholar]
- 12.Foreman, K. E., J. Friborg, Jr., W. P. Kong, C. Woffendin, P. J. Polverini, B. J. Nickoloff, and G. J. Nabel. 1997. Propagation of a human herpesvirus from AIDS-associated Kaposi's sarcoma. N. Engl. J. Med. 336:163-171. [DOI] [PubMed] [Google Scholar]
- 13.Gao, S. J., L. Kingsley, D. R. Hoover, T. J. Spira, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, P. Parry, Y. Chang, and P. S. Moore. 1996. Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N. Engl. J. Med. 335:233-241. [DOI] [PubMed] [Google Scholar]
- 14.Gao, S. J., L. Kingsley, M. Li, W. Zheng, C. Parravicini, J. Ziegler, R. Newton, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, Y. Chang, and P. S. Moore. 1996. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat. Med. 2:925-928. [DOI] [PubMed] [Google Scholar]
- 15.Gradoville, L., J. Gerlach, E. Grogan, D. Shedd, S. Nikiforow, C. Metroka, and G. Miller. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gripon, P., C. Diot, and C. Guguen-Guillouzo. 1993. Reproducible high level infection of cultured adult human hepatocytes by hepatitis B virus: effect of polyethylene glycol on adsorption and penetration. Virology 192:534-540. [DOI] [PubMed] [Google Scholar]
- 17.Herndier, B. G., A. Werner, P. Arnstein, N. W. Abbey, F. Demartis, R. L. Cohen, M. A. Shuman, and J. A. Levy. 1994. Characterization of a human Kaposi's sarcoma cell line that induces angiogenic tumors in animals. AIDS 8:575-581. [DOI] [PubMed] [Google Scholar]
- 18.Kedes, D. H., D. Ganem, N. Ameli, P. Bacchetti, and R. Greenblatt. 1997. The prevalence of serum antibody to human herpesvirus 8 (Kaposi sarcoma-associated herpesvirus) among HIV-seropositive and high-risk HIV-seronegative women. JAMA 277:478-481. [PubMed] [Google Scholar]
- 19.Kedes, D. H., M. L. Lagunoff, R. Renne, and D. Ganem. 1997. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi's sarcoma-associated herpesvirus. J. Clin. Investig. 100:2606-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kedes, D. H., E. Operskalski, M. Busch, R. Kohn, J. Flood, and D. Ganem. 1996. The seroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat. Med. 2:918-924. [DOI] [PubMed] [Google Scholar]
- 21.Kirshner, J. R., K. Staskus, A. Haase, M. Lagunoff, and D. Ganem. 1999. Expression of the open reading frame 74 (G-protein-coupled receptor) gene of Kaposi's sarcoma (KS)-associated herpesvirus: implications for KS pathogenesis. J. Virol. 73:6006-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagunoff, M., and D. Ganem. 1997. The structure and coding organization of the genomic termini of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8). Virology 236:147-154. [DOI] [PubMed] [Google Scholar]
- 23.Lebbe, C., P. de Cremoux, G. Millot, M. P. Podgorniak, O. Verola, R. Berger, P. Morel, and F. Calvo. 1997. Characterization of in vitro culture of HIV-negative Kaposi's sarcoma-derived cells. In vitro responses to alfa interferon. Arch. Dermatol. Res. 289:421-428. [DOI] [PubMed] [Google Scholar]
- 24.Lukac, D. M., R. Renne, J. R. Kirshner, and D. Ganem. 1998. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252:304-312. [DOI] [PubMed] [Google Scholar]
- 25.Martin, D. F., B. D. Kuppermann, R. A. Wolitz, A. G. Palestine, H. Li, and C. A. Robinson. 1999. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N. Engl. J. Med. 340:1063-1070. [DOI] [PubMed] [Google Scholar]
- 26.Miller, G., M. O. Rigsby, L. Heston, E. Grogan, R. Sun, C. Metroka, J. A. Levy, S. J. Gao, Y. Chang, and P. Moore. 1996. Antibodies to butyrate-inducible antigens of Kaposi's sarcoma-associated herpesvirus in patients with HIV-1 infection. N. Engl. J. Med. 334:1292-1297. [DOI] [PubMed] [Google Scholar]
- 27.Moses, A. V., K. N. Fish, R. Ruhl, P. P. Smith, J. G. Strussenberg, L. Zhu, B. Chandran, and J. A. Nelson. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rainbow, L., G. M. Platt, G. R. Simpson, R. Sarid, S. J. Gao, H. Stoiber, C. S. Herrington, P. S. Moore, and T. F. Schulz. 1997. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J. Virol. 71:5915-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 72:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 31.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venetsanakos, E., A. Mirza, C. Fanton, S. R. Romanov, T. Tlsty, and M. McMahon. Induction by glioblastoma cells of tubulogenesis in telomerase-immortalized human microvascular endothelial cells. Exp. Cell Res., 2002. 273:21-33. [DOI] [PubMed]
- 34.Yang, J., E. Chang, A. M. Cherry, C. D. Bangs, Y. Oei, A. Bodnar, A. Bronstein, C. P. Chiu, and G. S. Herron. 1999. Human endothelial cell life extension by telomerase expression. J. Biol. Chem. 274:26141-26148. [DOI] [PubMed] [Google Scholar]
- 35.Zoeteweij, J. P., S. T. Eyes, J. M. Orenstein, T. Kawamura, L. Wu, B. Chandran, B. Forghani, and A. Blauvelt. 1999. Identification and rapid quantification of early- and late-lytic human herpesvirus 8 infection in single cells by flow cytometric analysis: characterization of antiherpesvirus agents. J. Virol. 73:5894-5902. [DOI] [PMC free article] [PubMed] [Google Scholar]