Abstract
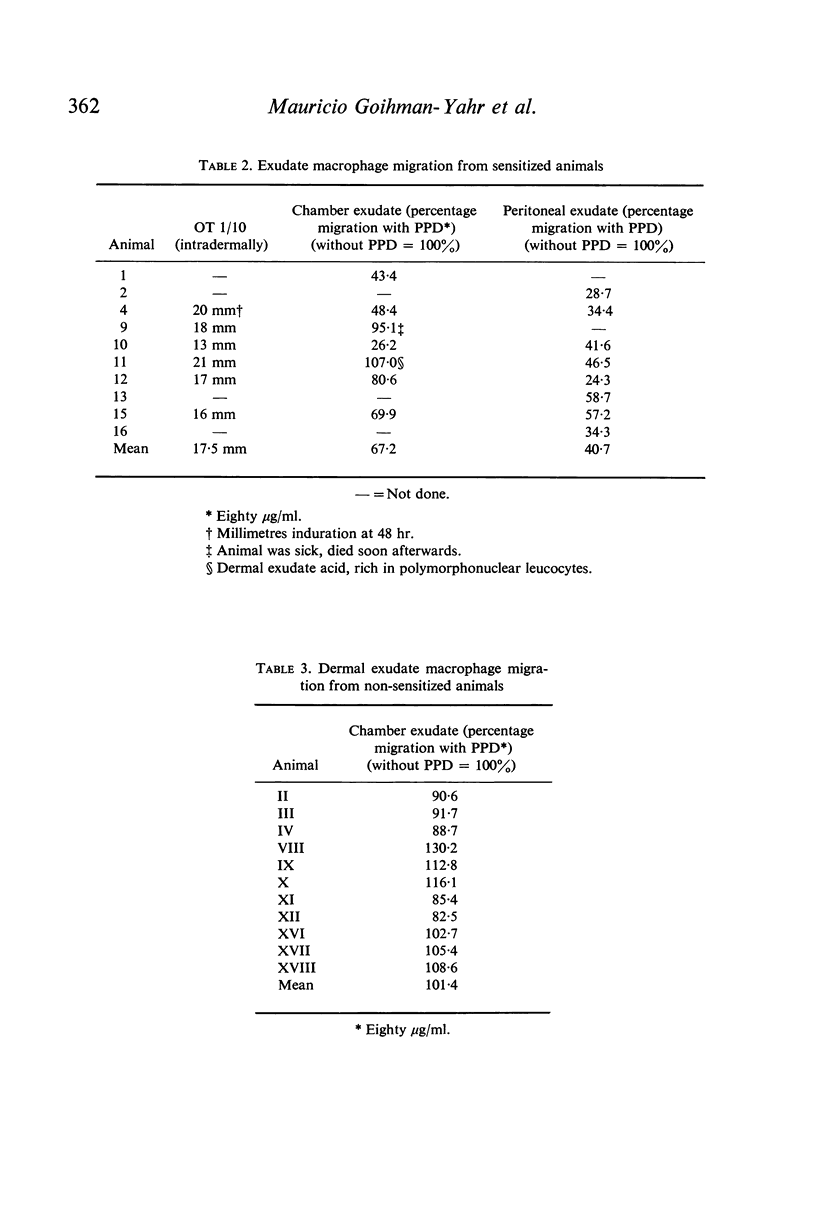
Chambers were implanted in the dorsum of guinea-pigs at the dermal-subcutaneous junction. Exudates were induced and harvested. Macrophages obtained were able to migrate in vitro. If procured from sensitized donors, macrophage migration was inhibited by the corresponding antigen. Dermal exudate macrophages are therefore subject to the effect of lymphokines. The chamber model may be useful for in vivo studies of cell to cell and cell-parasite interactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando M., Dannenberg A. M., Jr, Shima K. Macrophage accumulation, division, maturation and digestive and microbicidal capacities in tuberculous lesions. II. Rate at which mononuclear cells enter and divide in primary BCG lesions and those of reinfection. J Immunol. 1972 Jul;109(1):8–19. [PubMed] [Google Scholar]
- Arko R. J. Neisseria gonorrhoeae: experimental infection of laboratory animals. Science. 1972 Sep 29;177(4055):1200–1201. doi: 10.1126/science.177.4055.1200. [DOI] [PubMed] [Google Scholar]
- Cabello Díaz B., Alberto Bernaola O. Aplicación de plástico líquido sintético en las heridas producidas por la castración en cerdos de 21 días (evaluación preliminar. Acta Cient Venez. 1973;24(3):86–87. [PubMed] [Google Scholar]
- Convit J., Pinardi M. E., Rodríguez Ochoa G., Ulrich M., Avila J. L., Goihman M. Elimination of Mycobacterium leprae subsequent to local in vivo activation of macrophages in lepromatous leprosy by other mycobacteria. Clin Exp Immunol. 1974 Jun;17(2):261–265. [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Ando M., Shima K. Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. 3. The turnover of macrophages and its relation to their activation and antimicrobial immunity in primary BCG lesions and those of reinfection. J Immunol. 1972 Nov;109(5):1109–1121. [PubMed] [Google Scholar]
- Ferraresi R. W., Dedrick C. T., Raffel S., Goihman-Yahr M. Studies of the macrophage inhibition test. I. Comparison of the skin and cell migration reactions during the course of development of delayed hypersensitivity. J Immunol. 1969 Apr;102(4):852–858. [PubMed] [Google Scholar]
- Godal T., Myklestad B., Samuel D. R., Myrvang B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin Exp Immunol. 1971 Dec;9(6):821–831. [PMC free article] [PubMed] [Google Scholar]
- Guyton A. C., Granger H. J., Taylor A. E. Interstitial fluid pressure. Physiol Rev. 1971 Jul;51(3):527–563. doi: 10.1152/physrev.1971.51.3.527. [DOI] [PubMed] [Google Scholar]
- Leu R. W., Eddleston A. L., Hadden J. W., Good R. A. Mechanism of action of migration inhibitory factor (MIF). I. Evidence for a receptor for MIF present on the peritoneal macrophage but not on the alveolar macrophage. J Exp Med. 1972 Sep 1;136(3):589–603. doi: 10.1084/jem.136.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS H. J., TERRYBERRY J. E. Counting actively metabolizing tissue cultured cells. Exp Cell Res. 1957 Oct;13(2):341–347. doi: 10.1016/0014-4827(57)90013-7. [DOI] [PubMed] [Google Scholar]
- Waldron J. A., Jr, Horn R. G., Rosenthal A. S. Antigen-induced proliferation of guinea pig lymphocytes in vitro: obligatory role of macrophages in the recognition of antigen by immune T-lymphocytes. J Immunol. 1973 Jul;111(1):58–64. [PubMed] [Google Scholar]


