Abstract
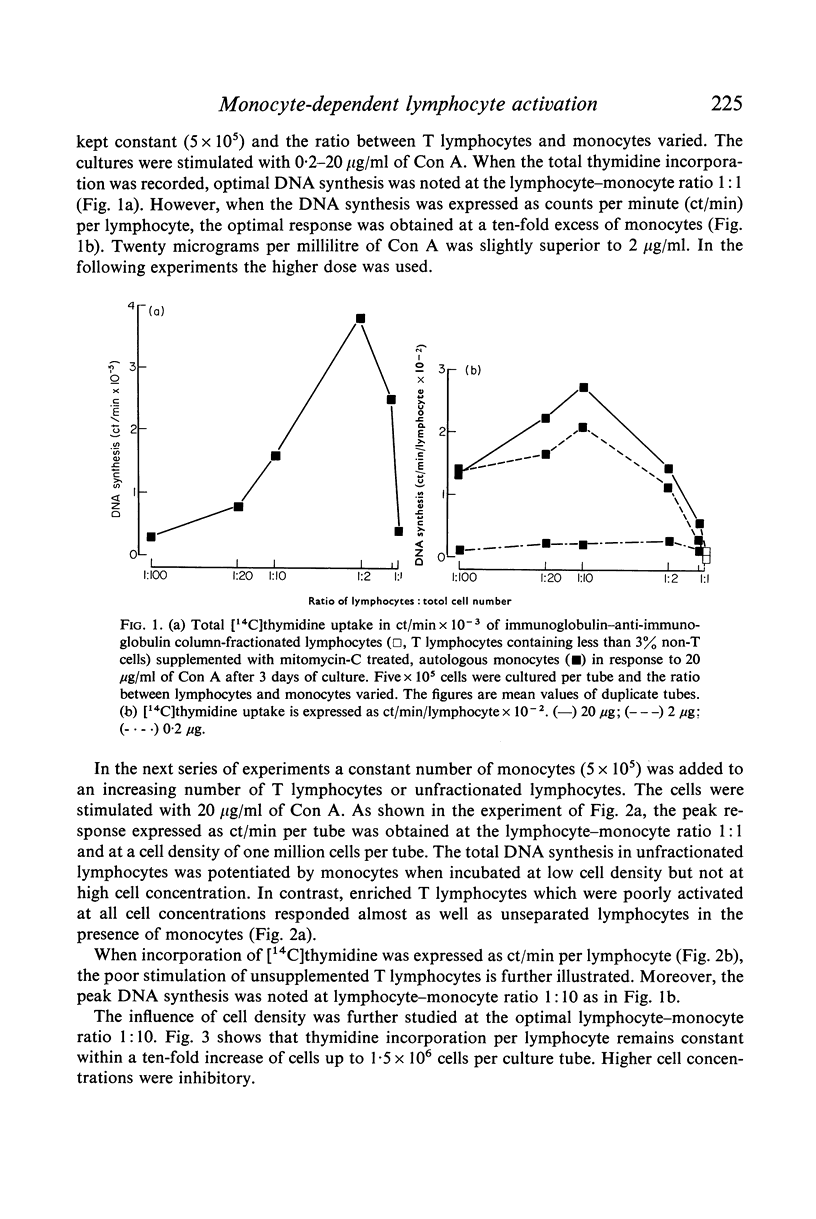
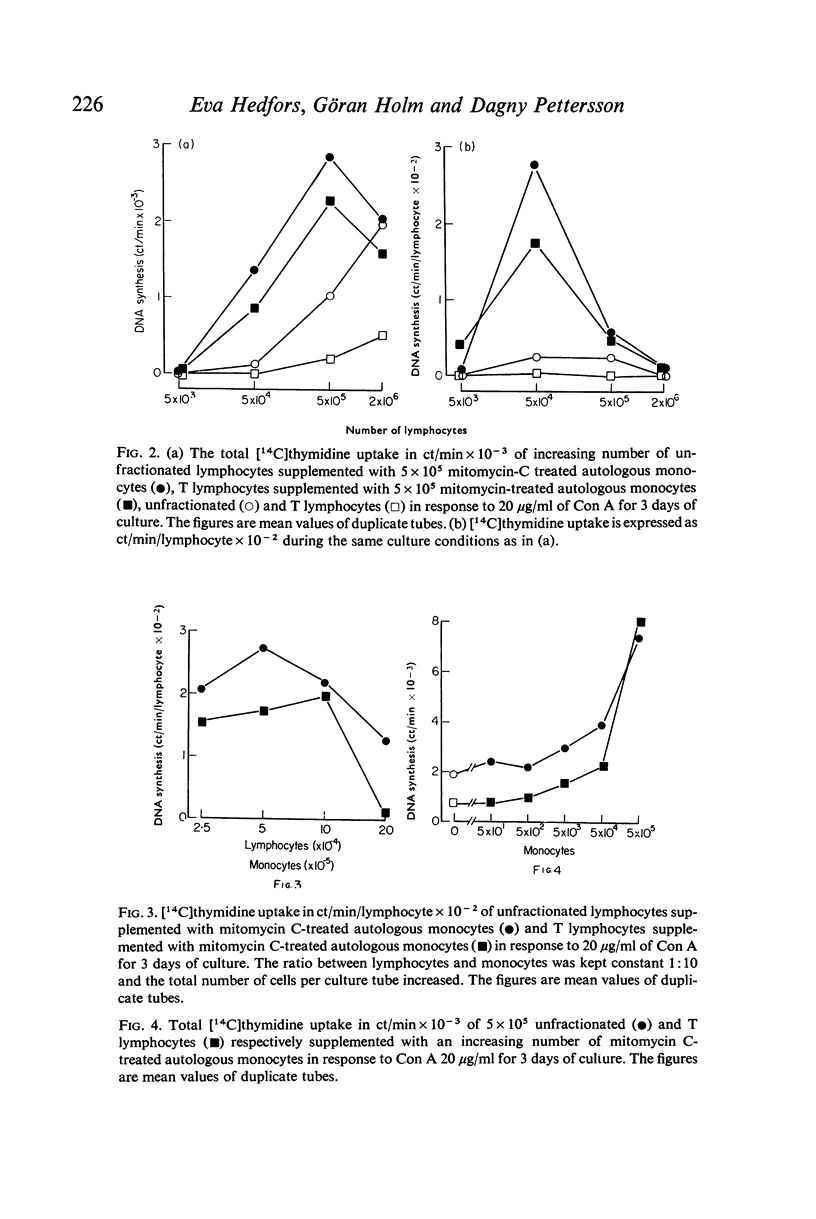
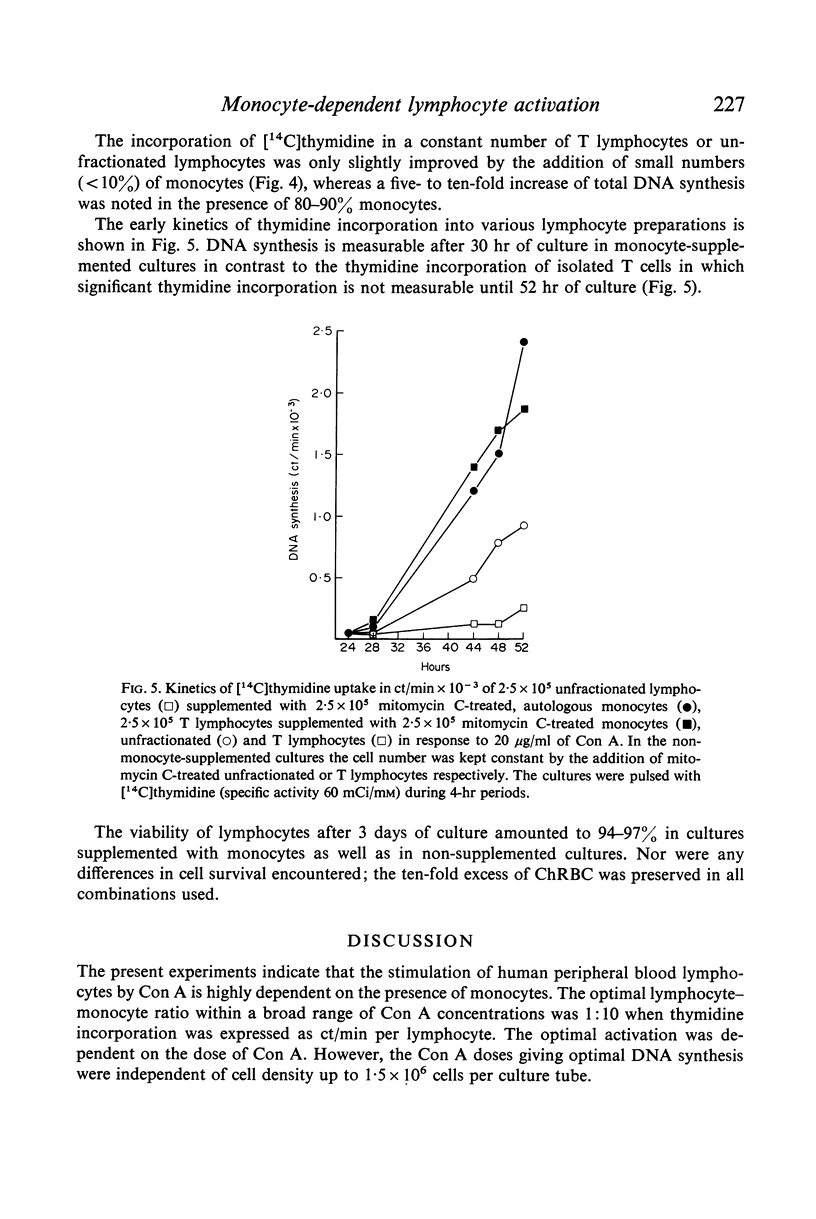
The activation of human peripheral blood lymphocytes or isolated T lymphocytes by concanavalin A (Con A) is hightly potentiated by the presence of autologous, mitomycin C-treated monocytes. The optimal lymphocyte: monocyte ratio within a broad dose range is 1:1 when the incorporation of [14C]thymidine is expressed as total incorporation per culture tube and 1:10 when expressed per lymphocyte. A five-to-ten-fold increase of total DNA synthesis is noted in the presence of 10-90% monocytes. The data may help to explain the wide variations in Con A responsiveness of human peripheral lymphocytes which may be partly related to differences in purification which give rise to cell preparations containing varying amounts of monocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Sjöberg O., Möller G. Mitogens as probes for immunocyte activation and cellular cooperation. Transplant Rev. 1972;11:131–177. doi: 10.1111/j.1600-065x.1972.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Boldt S., Skinner A. M., Kornfeld S. Studies of two subpopulations of human lymphocytes differing in responsiveness to concanavalin A. J Clin Invest. 1972 Dec;51(12):3225–3234. doi: 10.1172/JCI107149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- COULSON A. S., CHALMERS D. G. SEPARATION OF VIABLE LYMPHOCYTES FROM HUMAN BLOOD. Lancet. 1964 Feb 29;1(7331):468–469. doi: 10.1016/s0140-6736(64)90799-8. [DOI] [PubMed] [Google Scholar]
- Hedfors E. Activation of peripheral T cells of sarcoidosis patients and healthy controls. Clin Exp Immunol. 1974 Nov;18(3):379–390. [PMC free article] [PubMed] [Google Scholar]
- Holm G., Hammarström S. Haemolytic activity of human blood monocytes. Lysis of human erythrocytes treated with anti-A serum. Clin Exp Immunol. 1973 Jan;13(1):29–43. [PMC free article] [PubMed] [Google Scholar]
- Holm G. Lysis of antibody-treated human erythrocytes by human leukocytes and macrophages in tissue culture. Int Arch Allergy Appl Immunol. 1972;43(5):671–682. doi: 10.1159/000230883. [DOI] [PubMed] [Google Scholar]
- Lohrmann H. P., Graw C. M., Graw R. G., Jr Stimulated lymphocyte cultures: responder recruitment and cell cycle kinetics. J Exp Med. 1974 May 1;139(5):1037–1048. doi: 10.1084/jem.139.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrmann H. P., Novikovs L., Graw R. G., Jr Cellular interactions in the proliferative response of human T and B lymphocytes to phytomitogens and allogeneic lymphocytes. J Exp Med. 1974 Jun 1;139(6):1553–1567. doi: 10.1084/jem.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordling S., Andersson L. C., Häyry P. Separation of T and B lymphocytes by preparative cell electrophoresis. Eur J Immunol. 1972 Oct;2(5):405–410. doi: 10.1002/eji.1830020504. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D. Phytohemagglutin and concanavalin A: probes for murine 'T' cell activivation and differentiation. Transplant Rev. 1972;11:60–86. doi: 10.1111/j.1600-065x.1972.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Wigzell H., Sundqvist K. G., Yoshida T. O. Separation of cells according to surface antigens by the use of antibody-coated columns. Fractionation of cells carrying immunoglobulins and blood group antigen. Scand J Immunol. 1972;1(1):75–87. doi: 10.1111/j.1365-3083.1972.tb03737.x. [DOI] [PubMed] [Google Scholar]


