Abstract
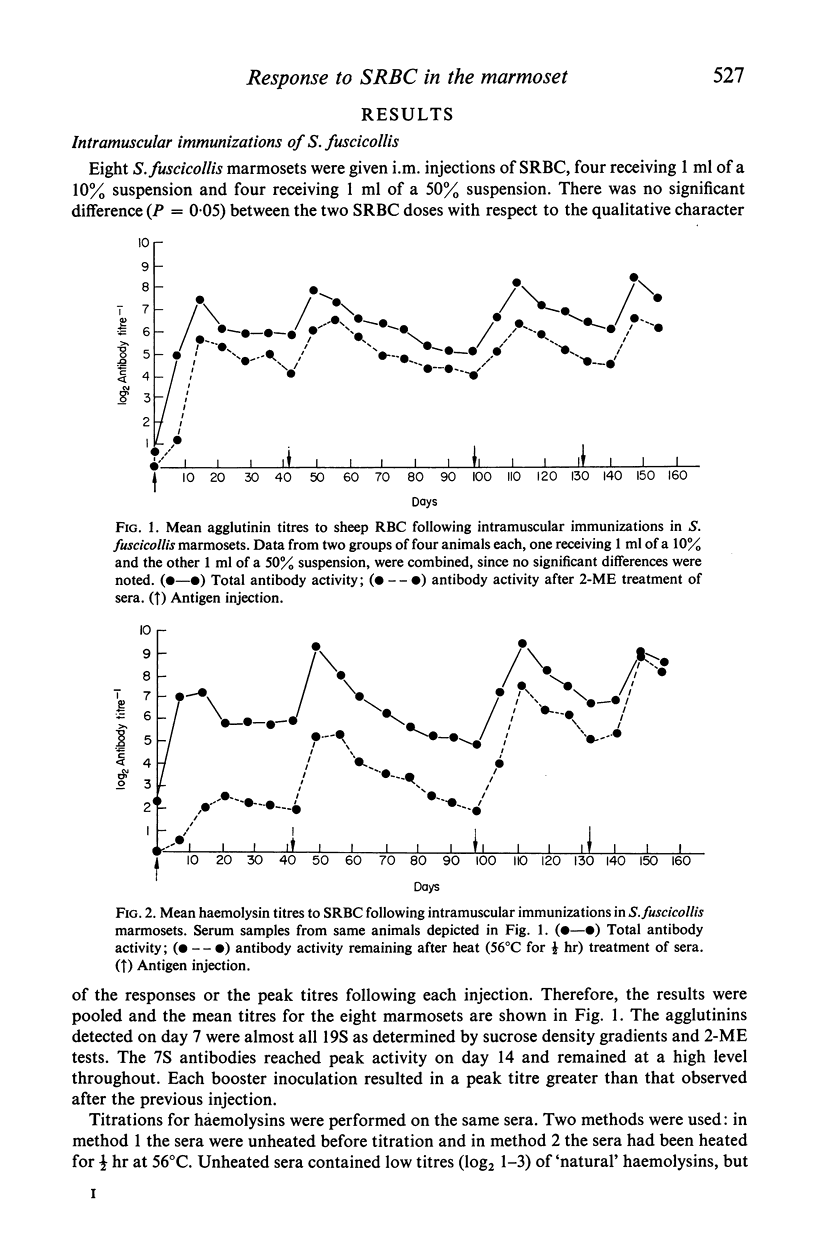
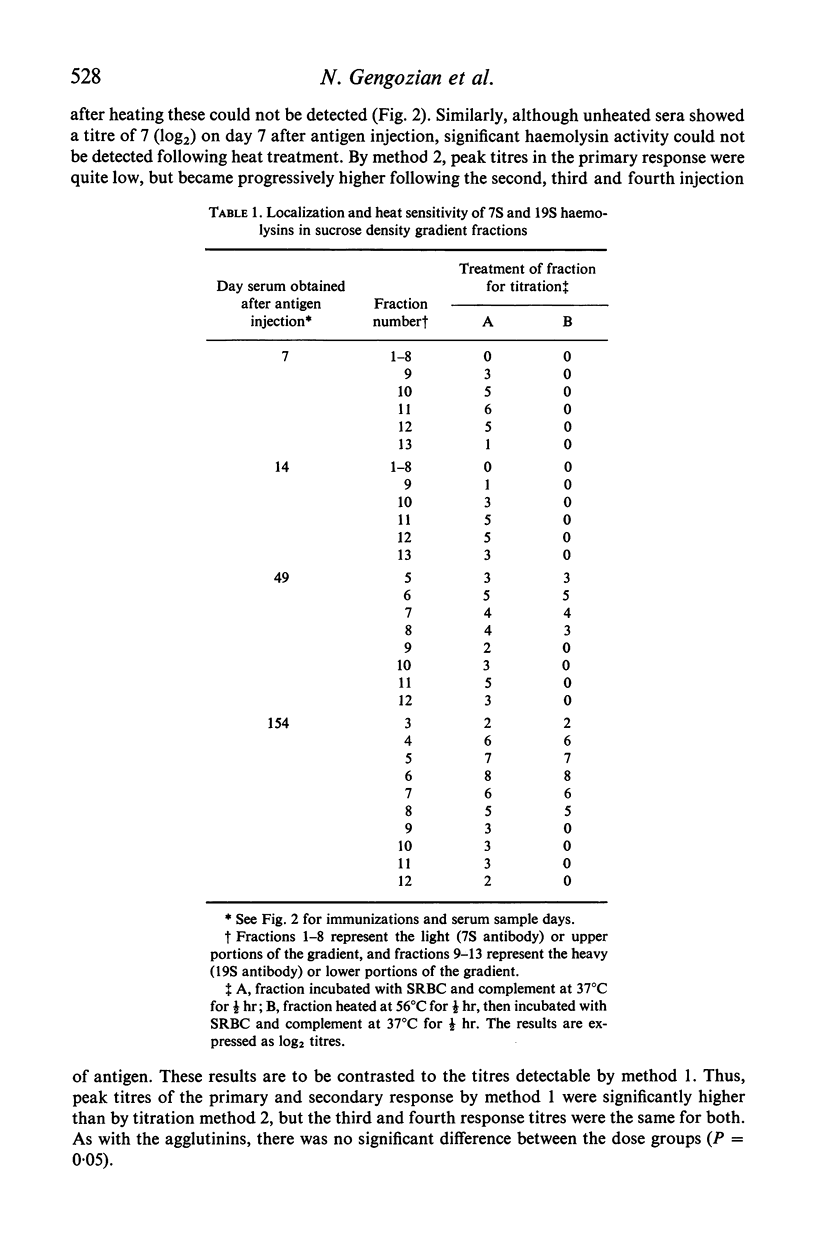
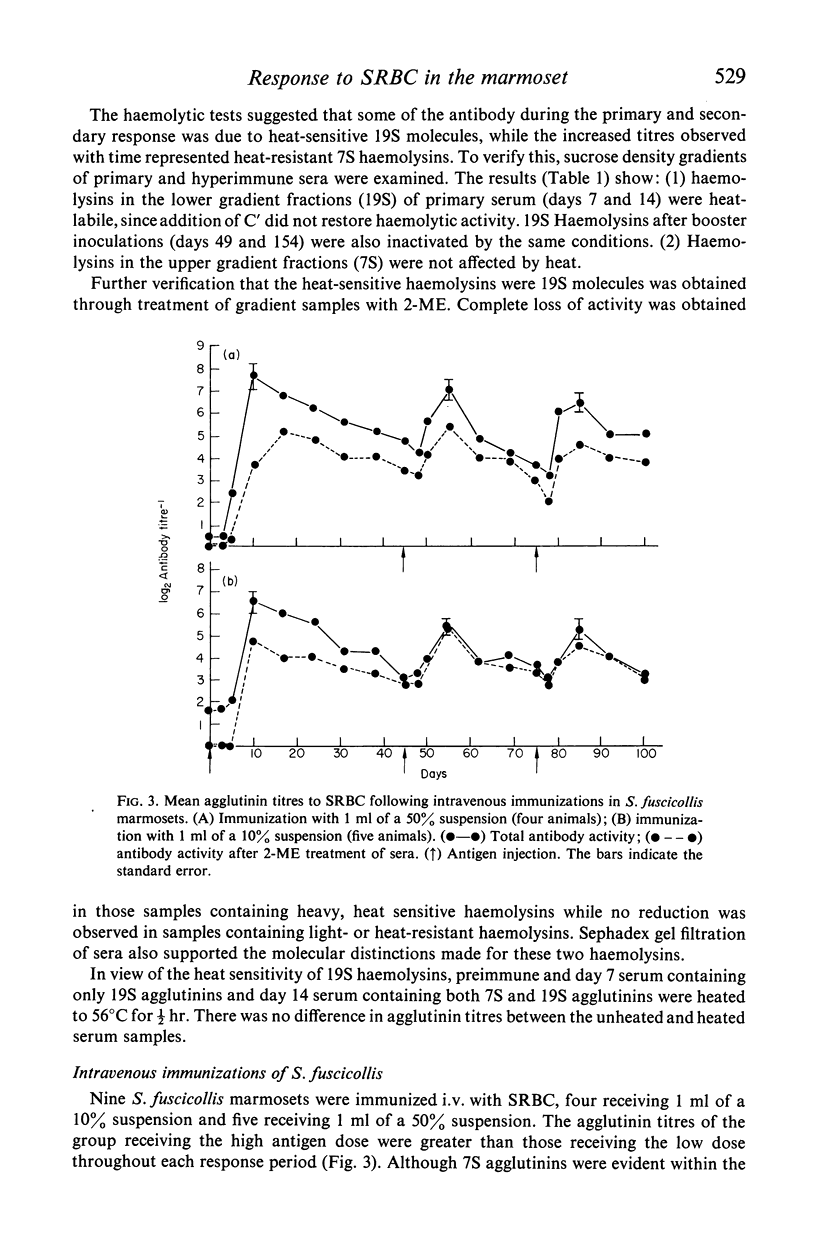
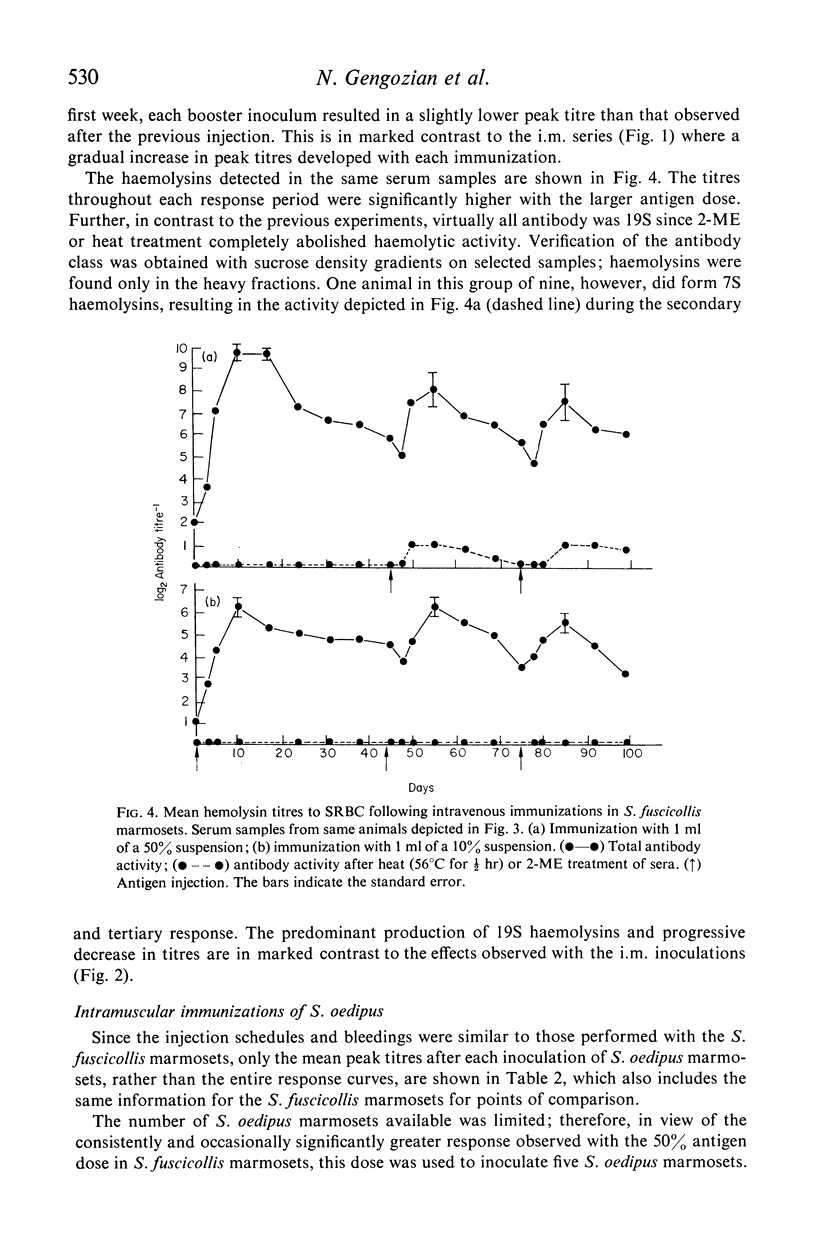
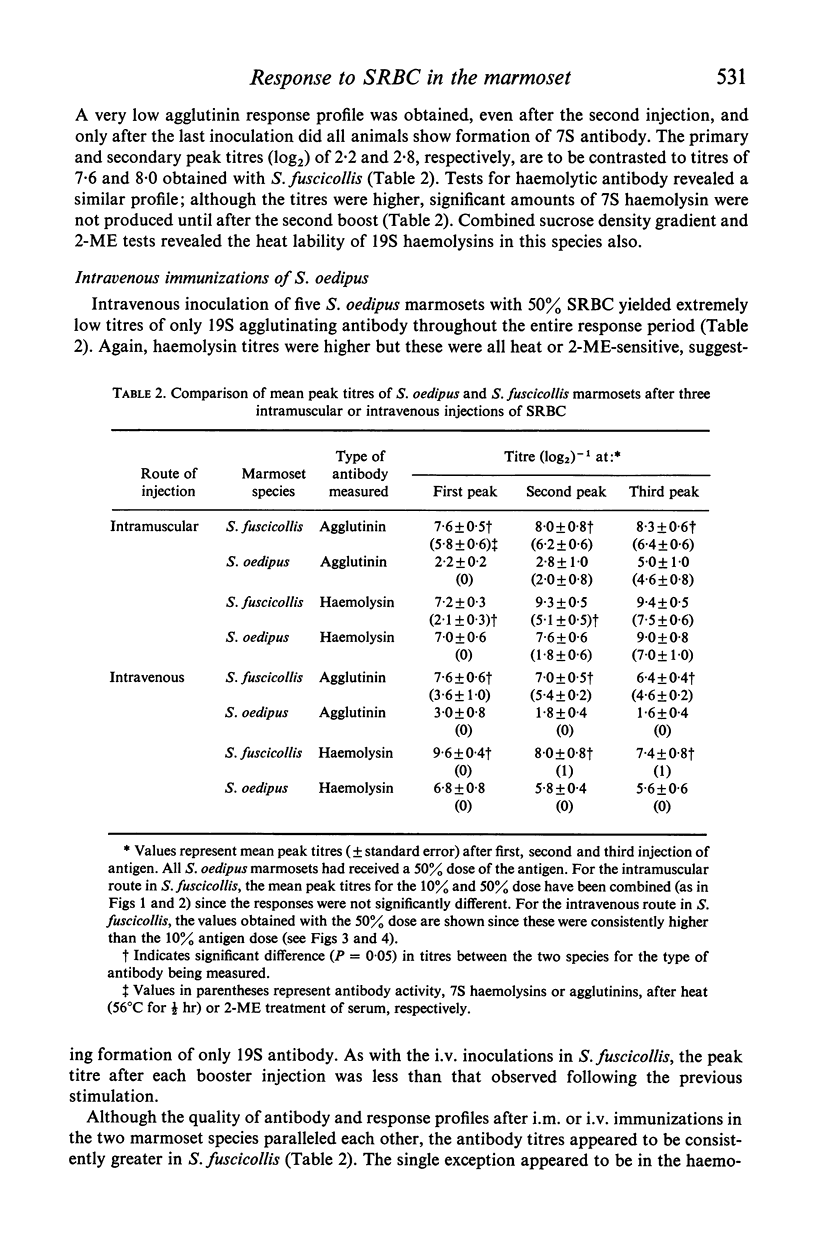
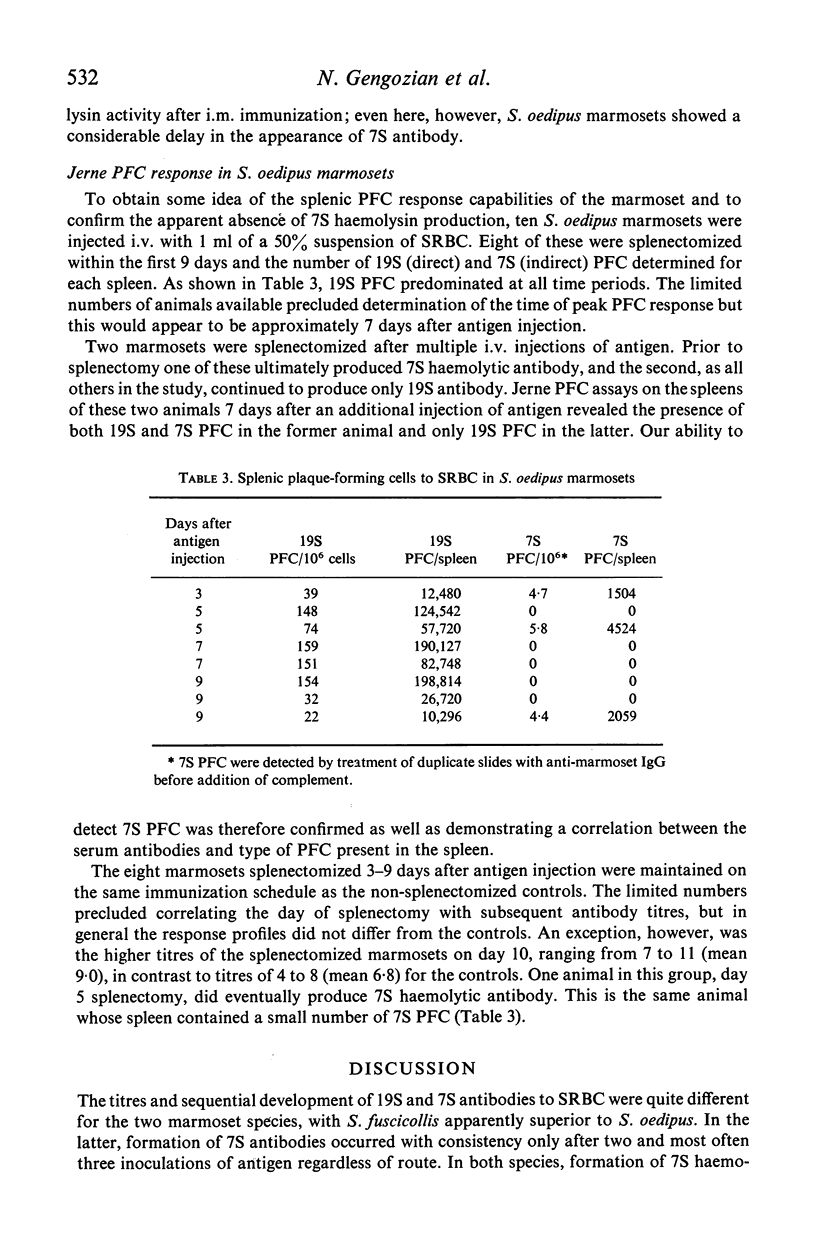
The immune competence of two species of marmosets, S. fusciollis and S. oedipus, was evaluated by the intravenous (i.v.) and intramuscular (i.m.) injection of sheep red blood cells (SRBC). In S. fusciollis marmosets, 1 ml of a 50% suspension yielded titres of haemolysin and agglutinating antibodies equal to or greater than 1 ml of a 10% dose of antigen. In both species, the i.v. route, while resulting in formation of 19S and 7S agglutinins, yielded only 19S haemolysins, even after multiple antigen injections. Repeated i.v. injections resulted in a progressive decrease in peak titres, in contrast to the i.m. route, where booster inoculations gave a typical anamnestic response. Jerne plaque-forming cells (PFC) in the spleens of S. oedipus marmosets showed predominately 19S plaques after a primary i.v. challenge; only 19S PFC were detected in the spleen of an animal that had been given multiple inoculations, the type of antibody produced reflecting that found in the serum. 19S but not 7S haemolysins of both species were sensitive to heating at 56 degrees C for 1/2 hr. The serum titres and splenic PFC data from the marmosets suggest these animals, particularly S. oedipus, respond poorly to SRBC when a comparison is made to similar studies in mice and rats.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER F. L. STUDIES ON MOUSE ANTIBODIES. I. THE RESPONSE TO SHEEP RED CELLS. J Immunol. 1965 Jul;95:26–38. [PubMed] [Google Scholar]
- ARNASON B. G., STCYRCDE V., SHAFFNER J. B. A COMPARISON OF IMMUNOGLOBULINS AND ANTIBODY PRODUCTION IN THE NORMAL AND THYMECTOMIZED MOUSE. J Immunol. 1964 Dec;93:915–925. [PubMed] [Google Scholar]
- ARNASON B. G., VAUXST-CYRC DE, RELYVELD E. H. ROLE OF THE THYMUS IN IMMUNE REACTIONS IN RATS. IV. IMMUNOGLOBULINS AND ANTIBODY FORMATION. Int Arch Allergy Appl Immunol. 1964;25:206–224. doi: 10.1159/000229522. [DOI] [PubMed] [Google Scholar]
- Barnhart D. D., Gengozian N. An evaluation of the mixed lymphocyte culture reaction in marmosets. Transplantation. 1975 Aug;20(2):107–115. doi: 10.1097/00007890-197508000-00003. [DOI] [PubMed] [Google Scholar]
- Bechtol K. B., Wegmann T. G., Freed J. H., Grumet F. C., Chesebro B. W., Herzenberg L. A., McDevitt H. O. Genetic control of the immune response to (T,G)-A--L in C3H in equilibrium C57 tetraparental mice. Cell Immunol. 1974 Aug;13(2):264–277. doi: 10.1016/0008-8749(74)90244-5. [DOI] [PubMed] [Google Scholar]
- Benirschke K., Anderson J. M., Brownhill L. E. Marrow Chimerism in Marmosets. Science. 1962 Oct 26;138(3539):513–515. doi: 10.1126/science.138.3539.513. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A. Immunologic complementation between thymus and marrow cells--a model for the two-cell theory of immunocompetence. Transplant Rev. 1969;1:92–113. doi: 10.1111/j.1600-065x.1969.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Coe J., Peel L., Smith R. F. The immune response in the hamster. V. Biologic activities of 7S-gamma-1 and 7S-gamma-2 globulins. J Immunol. 1971 Jul;107(1):76–82. [PubMed] [Google Scholar]
- DEUTSCH H. F., MORTON J. I. Dissociation of human serum macroglobulins. Science. 1957 Mar 29;125(3248):600–601. doi: 10.1126/science.125.3248.600. [DOI] [PubMed] [Google Scholar]
- Eidinger D., Pross H. F. The immune response to sheep erythrocytes in the mouse. I. A study of the immunological events utilizing the plaque technique. J Exp Med. 1967 Jul 1;126(1):15–33. doi: 10.1084/jem.126.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENGOZIAN N., BATSON J. S., EIDE P. HEMATOLOGIC AND CYTOGENETIC EVIDENCE FOR HEMATOPOIETIC CHIMERISM IN THE MARMOSET, TAMARINUS NIGRICOLLIS. Cytogenetics. 1964;3:384–393. doi: 10.1159/000129828. [DOI] [PubMed] [Google Scholar]
- Gengozian N. A blood factor in the marmoset, Saguinus fusciollis--its detection, mode of inheritance, and species specificity. J Med Primatol. 1972;1(5):272–286. doi: 10.1159/000460398. [DOI] [PubMed] [Google Scholar]
- Gengozian N., Batson J. S., Greene C. T., Gosslee D. G. Hemopoietic chimerism in imported and laboratory-bred marmosets. Transplantation. 1969 Nov;8(5):633–652. doi: 10.1097/00007890-196911000-00009. [DOI] [PubMed] [Google Scholar]
- Gengozian N. Marmosets: their potential in experimental medicine. Ann N Y Acad Sci. 1969 Jul 3;162(1):336–362. doi: 10.1111/j.1749-6632.1969.tb56381.x. [DOI] [PubMed] [Google Scholar]
- Harvey J. S., Jr, Felsburg P. J., Heberling R. L., Kniker W. T., Kalter S. S. Immunological competence in non-human primates: differences observed in four species. Clin Exp Immunol. 1974 Feb;16(2):267–277. [PMC free article] [PubMed] [Google Scholar]
- Hübner K. F., Gengozian N. Critical variables of the Jerne plaque technique as applied to rodent antibody-forming systems responding to heterologous red cell antigens. J Immunol. 1969 Jan;102(1):155–167. [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- Niblack G. D., Gengozian N. T and B lymphocytes in the marmoset: a natural haemopoietic chimera. Clin Exp Immunol. 1976 Mar;23(3):536–543. [PMC free article] [PubMed] [Google Scholar]
- OVARY Z., BARTH W. F., FAHEY J. L. THE IMMUNOGLOBULINS OF MICE. 3. SKIN SENSITIZING ACTIVITY OF MOUSE IMMUNOGLOBULINS. J Immunol. 1965 Mar;94:410–415. [PubMed] [Google Scholar]
- OVARY Z., BENACERRAF B., BLOCH K. J. Properties of guinea pig 7S antibodies. II. Identification of antibodies involved in passive cutaneous and systemic anaphylaxis. J Exp Med. 1963 Jun 1;117:951–964. doi: 10.1084/jem.117.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. P., Gengozian N. Immunological responsiveness and tolerance of marmoset lymphoid tissue in vitro. Transplantation. 1973 Feb;15(2):221–230. doi: 10.1097/00007890-197302000-00006. [DOI] [PubMed] [Google Scholar]
- Porter R. P., Gengozian N. Immunological tolerance and rejection of skin allografts in hhe marmoset. Transplantation. 1969 Nov;8(5):653–665. doi: 10.1097/00007890-196911000-00010. [DOI] [PubMed] [Google Scholar]
- SASLAW S., CARLISLE H. N. ANTIBODY RESPONSE IN SPLENECTOMIZED MONKEYS. Proc Soc Exp Biol Med. 1964 Jul;116:738–742. doi: 10.3181/00379727-116-29360. [DOI] [PubMed] [Google Scholar]
- Scott D. W., Howard J. C. Collaboration between thymus-derived and marrow-derived thoracic duct lymphocytes in the hemolysin response of the rat. Cell Immunol. 1972 Mar;3(3):430–441. doi: 10.1016/0008-8749(72)90248-1. [DOI] [PubMed] [Google Scholar]
- Urso P., Gengozian N. T cell deficiency in mouse allogeneic radiation chimeras. J Immunol. 1973 Sep;111(3):712–719. [PubMed] [Google Scholar]
- Urso P., Gengozian N. Variation in T and B cell deficiency in different mouse allogeneic radiation chimeras. J Immunol. 1974 Dec;113(6):1770–1779. [PubMed] [Google Scholar]
- Warner C. M., Fitzmaurice M., Maurer P. H., Merryman C. F., Schmerr M. J. The immune response of tetraparental mice to two synthetic amino acid polymers: "high-conjugation" 2,4 dinitrophenyl-glutamic acid57-lysine38-alanine5 (DNP-GLA5) and glutamic acid60 alanine30 tyrosine10 (GAT10). J Immunol. 1973 Dec;111(6):1887–1893. [PubMed] [Google Scholar]
- Weir D. M., Elson C. J. Heat labile antibody in rat serum. Clin Exp Immunol. 1968 Sep;3(7):725–731. [PMC free article] [PubMed] [Google Scholar]


