Abstract
Two pathogenic species in the genus Listeria, Listeria monocytogenes and Listeria ivanovii, are characterized by the production of hemolysins belonging to cholesterol-dependent cytolysins, listeriolysin O (LLO) and ivanolysin O (ILO), respectively. LLO, produced by L. monocytogenes, is able to induce gamma interferon (IFN-γ) production and contributes to the generation of Th1-dependent protective immunity. On the other hand, nothing is known about the role of ILO, produced by L. ivanovii, in this regard. In this study, we immunized mice with 0.1 50% lethal dose (LD50) of L. monocytogenes and L. ivanovii. Protective immunity against a challenge with 10 LD50 was generated in mice infected with L. monocytogenes, whereas L. ivanovii infection did not induce protection. After immunization, the level of IFN-γ in serum samples was increased in mice given L. monocytogenes but not in those given L. ivanovii. To determine the IFN-γ-inducing activity of cytolysins, recombinant protein was constructed. Recombinant ILO exhibited significantly lower IFN-γ-inducing activity than LLO. By comparing the IFN-γ-inducing activity of a chimera incorporating LLO and ILO, it was found that domains 1 to 3 of LLO were critical for IFN-γ-inducing activity while the counterpart in ILO was unable to induce cytokine production. These results suggested that the weak ability of ILO to induce IFN-γ production is responsible for the failure of L. ivanovii to generate effective protective immunity.
Among the bacterial species belonging to the genus Listeria, two species, Listeria monocytogenes and Listeria ivanovii, are known to be pathogenic (42). L. monocytogenes causes serious infections in newborns, pregnant women, immunocompromised individuals (9, 11, 24), and animals (25). L. ivanovii is pathogenic to ruminants (25) but rarely causes human disease (6, 23).
These two species possess a similar central virulence gene cluster consisting of prfA, plcA, hly, mpl, actA, and plcB, the transcription of which is positively regulated by PrfA (13, 21). Both listeriolysin O (LLO), an hly gene product in L. monocytogenes, and ivanolysin O (ILO), an ilo gene product in L. ivanovii, are 58-kDa secretory proteins that are the major virulence determinants in each (12, 20, 40). They show 80% homology in amino acid sequence (14, 16) and belong to a family of cholesterol-dependent cytolysins (CDCs) characterized by the presence of a highly conserved undecapeptide sequence (ECTGLAWEWWR) located near the C terminus (7, 42). The CDCs are known to bind cholesterol on the cell surface and form oligomers, resulting in a ring-shaped pore on the cytoplasmic membrane. The cytolytic activity of CDC is easily blocked by treatment with small amounts of free cholesterol (2). Rossjohn et al. analyzed the three-dimensional structure of perfringolysin O, a member of the CDC family produced by Clostridium perfringens, and reported that perfringolysin O consists of four domains (34). The continuous domains 1 to 3 are involved in oligomerization and insertion of the oligomer into the cytoplasmic membrane. Domain 4 is considered critical for initial binding of the toxin to cholesterol on the cell surface.
In the course of a sublethal infection with L. monocytogenes, Th1-mediated immunity is generated, and then the bacteria are rapidly cleared from the organs. Gamma interferon (IFN-γ) produced from antigen-specific αβ T cells appears to play an essential role in acquired resistance by enhancing macrophage microbicidal activity. At the early stage of infection with the innate immune response, IFN-γ is also produced from NK cells and γδ T cells and contributes to the primary host defense. We have reported that IFN-γ is an important cytokine for the development of Th1 cells mediating acquired resistance against L. monocytogenes and Mycobacterium bovis BCG, as neutralization of IFN-γ with a specific antibody caused a decrease in the number of antigen-specific IFN-γ-producing T cells and in the level of host resistance (45, 46).
Our previous studies also showed that the IFN-γ-inducing ability of Listeria spp. is associated with virulence (28, 43). Virulent L. monocytogenes strains were able to induce strong IFN-γ production, but avirulent strains exhibit only weak IFN-γ-inducing activity (44). We further found that IFN-γ-inducing ability was related to the production of LLO and that purified LLO can induce the production of cytokines, including IFN-γ, in vitro (19, 29, 30, 39, 47). These results indicate that LLO not only acts as the major virulence factor of L. monocytogenes but also plays an important role in the generation of host resistance by inducing IFN-γ production during the initial period of infection.
In contrast to the number of studies on L. monocytogenes, there are only a limited number of reports on the generation of protective immunity and cytokine production in mice infected with L. ivanovii. Khun et al. (22) indicated that interleukin-1 alpha (IL-1α) and IL-6 but not tumor necrosis factor alpha are produced from P388D1 macrophage cell lines after infection with L. ivanovii in vitro. However, it is not clear whether Th1 cytokines such as IL-12, IL-18, and IFN-γ are produced upon infection with L. ivanovii or whether ILO contributes to cytokine production. Because ILO is highly homologous to LLO, it appears that ILO also exerts cytokine-inducing activity and contributes to the generation of protective immunity as LLO does. In the present study, we compared the ability of L. ivanovii to induce protective immunity in vivo and that of recombinant ILO to induce IFN-γ production in vitro with L. monocytogenes and recombinant LLO.
MATERIALS AND METHODS
Mice.
Female C3H/He mice were purchased from Charles River Japan (Kanagawa, Japan). Mice were kept in specific-pathogen-free conditions and used for the experiments at 7 to 10 weeks of age.
Bacteria.
L. monocytogenes EGD has been maintained in our laboratory, and L. ivanovii ATCC 19119 was obtained from the American Type Culture Collection. Both species of bacteria were injected into mice and recovered from the spleen homogenate 2 days later. After another cycle of in vivo passage, bacteria were grown in brain heart infusion broth (BHI) (Becton Dickinson, Sparks, Md.) at 37°C overnight with shaking. One volume of the overnight culture was added to 100 volumes of fresh BHI medium. Bacteria were cultured for a further 4 h, washed, suspended in phosphate-buffered saline (PBS) supplemented with 10% glycerol, and stored in aliquots at −80°C. The concentration of listeriae was enumerated by plating 10-fold serially diluted suspension on BHI plate and counting the number of colonies. To determine the 50% lethal dose (LD50) of the two Listeria species, 10 mice per group were injected intraperitoneally with various doses of each species, and mortality was monitored for 14 days. The LD50 was calculated by the method of Reed and Muench (33) and was 6 × 104 CFU for L. monocytogenes and 108 CFU for L. ivanovii.
Generation of host resistance after infection with L. monocytogenes and L. ivanovii.
Mice were intraperitoneally infected with 6 × 103 CFU (0.1 LD50) of L. monocytogenes or 107 CFU (0.1 LD50) of L. ivanovii in a volume of 0.2 ml of PBS. Control mice were given 0.2 ml of PBS. Seven days after the infection, mice were intraperitoneally challenged with 6 × 105 CFU (10 LD50) of L. monocytogenes or 109 CFU (10 LD50) of L. ivanovii. Mortality was monitored for 10 days.
Construction and purification of recombinant proteins.
Chromosomal DNA was extracted from L. monocytogenes and L. ivanovii as reported previously (19). The region of the gene encoding mature LLO was amplified by PCR with a forward primer containing a BamHI site (italic), 5′-CGATGGATCCTGATGCATCTGCATTCAATAAAG-3′ (hly forward primer), and a reverse primer containing a PstI site, 5′-ACGCCTGCAGTTCGATTGGATTATCTACACTATTAC-3′ (hly reverse primer). The region of the gene encoding mature ILO was also amplified by PCR with a forward primer containing a BamHI site, 5′-CGATGGATCCTGATGCCTCAGTATATAGTTAC-3′ (ilo forward primer), and a reverse primer containing a SalI site, 5′-ACGCGTCGACTTACTTATTGGATTATCTACAG-3′ (ilo reverse primer).
To obtain a chimeric protein containing domains 1 to 3 of LLO and domain 4 of ILO (LLO1-3/ILO4), we amplified the part of gene encoding domains 1 to 3 of LLO (D26 to Y427) with the hly forward primer and a reverse primer, 5′-TTGAATTGAGCTACGTATCCT-3′. The gene coding for domain 4 of ILO (V427 to K528) was amplified with a forward primer, 5′-GTGCCTACGTAGCGAGATT-3′, and the ilo reverse primer. Because both PCR products carry a SnaBI site (italic), they were digested with the restriction enzyme and ligated with Ligation High (Toyobo Co., Ltd., Osaka, Japan). The DNA fragment was amplified with the hly forward primer and the ilo reverse primer.
To construct the gene coding for a chimeric protein of domains 1 to 3 of ILO (D25 to Y426) with domain 4 of LLO (V428 to E529), part of the ilo gene was amplified with the ilo forward primer and a reverse primer, 5′-GAATCTCGCTACGTAGGCA-3′, and part of the hly gene was amplified with a forward primer, 5′-AGGATACGTAGCTCAATTCA-3′, and the hly reverse primer. Those PCR products were digested with SnaBI, ligated, and PCR amplified with the ilo forward primer and the hly reverse primer. All PCR products were digested with restriction enzymes and ligated into the pQE31 expression plasmid (Qiagen, Tokyo, Japan). The recombinant plasmid was electroporated into Escherichia coli SG13009. The sequences of all PCR products were analyzed with an ABI Prism 310 genetic analyzer (Perkin-Elmer Applied Biosystems, Foster City, Calif.) to confirm that no mutations had been introduced.
The recombinant E. coli clone was cultured in tryptic soy broth (Becton Dickinson) in the presence of 1.0 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Nacalai Tesque, Kyoto, Japan) for 8 h at 25°C to produce recombinant His-tagged protein. The protein was purified with nickel-nitrilotriacetic acid-agarose column (Qiagen) according to the manufacturer's protocol and passed through PD-10 desalting columns (Amersham Pharmacia Biotech AB, Uppsala, Sweden) to exchange the buffer for PBS. To eliminate contaminating lipopolysaccharide (LPS) from the preparation, recombinant protein was passed through a Detoxi-Gel endotoxin-removing gel column (Pierce Chemical Company, Rockford, Ill.) several times until the level of LPS was less than 10 pg per ml. Protein concentration was measured with the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.). The recombinant cytolysins and the chimeric proteins were identified by immunoblotting with anti-His tag antibody (penta-His antibody; Qiagen) after sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-PAGE), and the purity was determined by Coomassie blue staining.
Hemolytic activity.
Hemolytic activity of the recombinant proteins was determined as described previously (15, 28). Briefly, cytolysins were twofold diluted with PBS and incubated with an equal volume of a 1% suspension of sheep erythrocytes (SRBC) for 1 h at 37°C. Degree of hemolysis was determined by the absorbance of hemoglobin released into the supernatant at 415 nm. One hemolytic unit (HU) was defined as the amount of cytolysin required for 50% hemolysis of SRBC.
IFN-γ production.
Spleens were aseptically removed from normal C3H/HeN mice. After removal of erythrocytes by treatment with 0.83% ammonium chloride in 170 mM Tris-HCl (pH 7.65), cells were washed and suspended at 107 cells per ml in culture medium which consisted of RPMI 1640 medium (Gibco BRL, Life Technologies, Rockville, Md.) supplemented with 10% heat-inactivated fetal bovine serum (Gibco-BRL) and 5 μg of gentamicin (Gibco-BRL) per ml. The cells were plated at 5 × 106 cells per well in a 48-well flat-bottomed tissue culture plate. Recombinant LLO (rLLO) and rILO (250 nM) were treated with 10 μg of cholesterol per ml overnight to block the cytolytic activity, and cells were stimulated with 50 nM rLLO or rILO. Culture supernatant was collected after 24 h of culture, and the level of IFN-γ in the culture supernatant was determined by enzyme-linked immunosorbent assay (ELISA) as described previously (1, 19). Briefly, the wells of a Nunc immunoplate (Nalge Nunc International, Rochester, N.Y.) were coated with rat anti-mouse IFN-γ antibody (Endogen, Woburn, Mass.). Fifty microliters of samples and biotin-conjugated anti-mouse IFN-γ antibody (Endogen) were added sequentially to each well within a 1-h interval. Wells were washed, and horseradish peroxidase-conjugated streptavidin was added (Endogen). After incubation for 30 min, IFN-γ was detected by addition of 3,3′,5,5′-tetramethylbenzidine dihydrochloride dihydrate (TMB; Nacalai) solution (50 μg per ml) containing 0.01% H2O2, and the absorbance was measured at 450 nm.
In vitro survival of L. monocytogenes and L. ivanovii.
Spleen cells were plated at 2 × 106 cells per well in a 48-well tissue culture plate and infected with L. monocytogenes or L. ivanovii at 0.2 CFU per cell in the absence of antibiotics. One hour after infection, gentamicin (5 μg per ml) was added, and the number of bacteria was counted at 2-h intervals.
RESULTS
Generation of protective immunity after infection with L. monocytogenes and L. ivanovii.
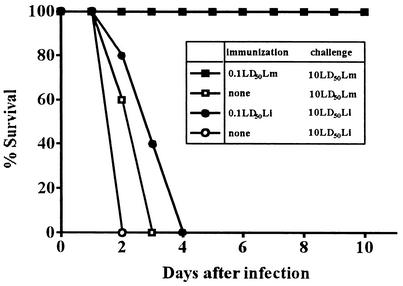
We first compared the ability of L. monocytogenes and L. ivanovii to generate protective immunity. Mice were immunized with 6 × 103 CFU (0.1 LD50) of L. monocytogenes or 107 CFU (0.1 LD50) of L. ivanovii. Seven days later, mice were challenged with 10 LD50 of each bacterium, and survival was monitored. All the nonimmunized mice were dead by 3 days after the challenge infection with either L. monocytogenes or L. ivanovii (Fig. 1). Mice immunized with 0.1 LD50 of L. monocytogenes were completely resistant to the challenge infection with a lethal dose. In contrast, 0.1 LD50 of L. ivanovii did not confer protection against the challenge infection.
FIG. 1.
Survival rate of mice immunized with L. monocytogenes or L. ivanovii after challenge infection. Mice were intraperitoneally immunized with 0.1 LD50 of L. monocytogenes (Lm) (6 × 103 CFU) or L. ivanovii (Li) (107 CFU). Seven days after immunization, mice were challenged with 10 LD50 of L. monocytogenes (6 × 105 CFU) or L. ivanovii (109 CFU). Survival was monitored for 10 days. Each experimental group consisted of five mice.
IFN-γ-inducing ability of L. monocytogenes and L. ivanovii.
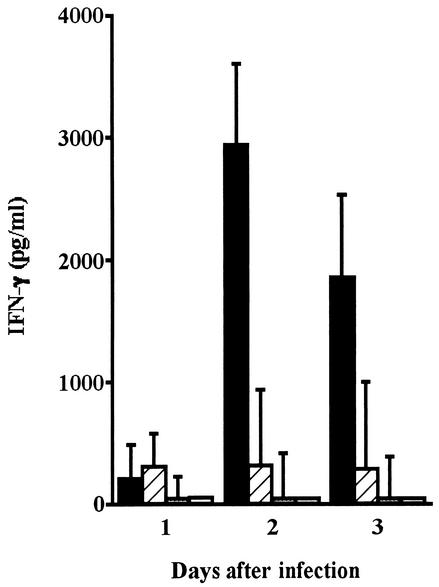
We have reported that IFN-γ produced at the first stage of infection with L. monocytogenes is critical for the generation of host resistance (43). In order to test whether IFN-γ was produced after infection with L. ivanovii, mice were intraperitoneally injected with 6 × 103 CFU of L. monocytogenes or 107 CFU or 6 × 10 3 CFU of L. ivanovii, and the concentration of IFN-γ was measured in serum samples. A low level of IFN-γ was detected 1 day after infection with L. monocytogenes (Fig. 2). The level reached 3,000 pg/ml on day 2 and decreased slightly on day 3. In contrast, IFN-γ was not produced in mice infected with 107 CFU of L. ivanovii after 3 days, and IFN-γ was not detected in the serum samples of mice infected with the lower dose of L. ivanovii (6 × 103 CFU). These data raised the possibility that L. ivanovii is not able to induce specific resistance because of insufficient production of IFN-γ at the stage of innate immune response to primary infection.
FIG. 2.
Level of IFN-γ in serum samples from mice infected with L. monocytogenes and L. ivanovii. Mice were intraperitoneally infected with 6 × 103 CFU of L. monocytogenes (solid bars), 107 CFU of L. ivanovii (hatched bars), or 6 ×103 CFU of L. ivanovii (dotted bars). Serum samples were collected daily from the infected mice and from control mice (open bars) for 3 days, and the level of IFN-γ was determined by ELISA. Data represent the mean ± standard deviation for five mice.
Different in vivo fates of L. monocytogenes and L. ivanovii.
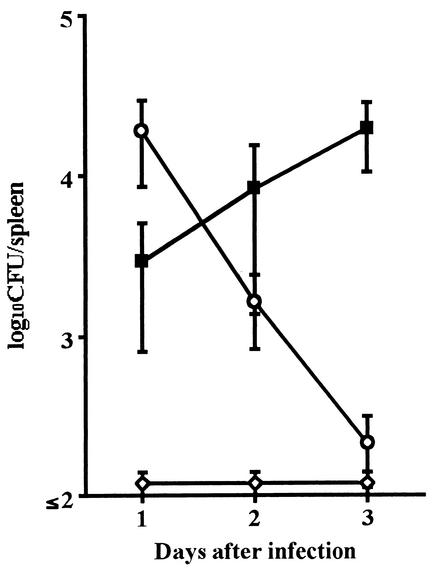
To address the question of why IFN-γ production was not induced by infection with L. ivanovii, bacterial growth in vivo was compared. Mice were injected with L. monocytogenes (6 × 103 CFU) or L. ivanovii (107 CFU or 6 × 103 CFU), and the number of viable bacteria in the spleen was counted for 3 days (Fig. 3). L. monocytogenes proliferated in vivo, and the number increased to 104 CFU per spleen on day 3. On the other hand, the viable count of L. ivanovii detected in the spleen was 2 × 104 CFU on day 1 and decreased to 102 CFU on day 3 after infection with 107 CFU. No bacteria were recovered in the spleen of mice infected with 6 × 103 CFU of L. ivanovii.
FIG. 3.
Number of L. monocytogenes and L. ivanovii in spleen after intraperitoneal infection. Mice were infected with 6 × 103 CFU of L. monocytogenes (solid squares), 107 CFU of L. ivanovii (open circles), or 6 × 103 CFU of L. ivanovii (open diamonds). The spleen was removed, and CFU were counted for 3 days. Data represent the mean ± standard deviation for five mice.
Comparison of in vitro growth of L. monocytogenes and L. ivanovii.
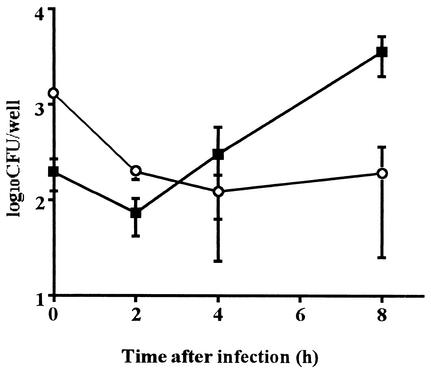
Next, we compared the intracellular survival of the two bacterial species in vitro. Spleen cells were suspended in RPMI 1640 medium containing 10% fetal bovine serum and infected with L. monocytogenes and L. ivanovii in vitro, and intracellular growth was monitored for 8 h. The number of L. monocytogenes decreased for the initial 2 h of incubation (Fig. 4), and then a gradual multiplication was observed at a later period. The number of CFU increased by a factor of 20 during the 8-h incubation. The number of L. ivanovii also decreased during the initial period. However, the bacterium could not multiply intracellularly afterward. The results indicated that L. ivanovii is not as capable of intracellular multiplication as L. monocytogenes, resulting in the accelerated clearance of L. ivanovii in vivo.
FIG. 4.
Intracellular survival of L. monocytogenes and L. ivanovii. Spleen cells (2 × 106 cells per ml) were suspended in RPMI 1640 medium without antibiotics and infected with L. monocytogenes (solid squares) and L. ivanovii (open diamonds) at 0.2 CFU per cell. After 1 h of incubation, the culture was treated with gentamicin (5 μg per ml) for 1 h to kill extracellular bacteria. Cells were lysed at the indicated times and plated on tryptic soy agar plates to count the number of viable bacteria. Data are representative of three independent experiments. Bars indicate standard deviations.
Difference in IFN-γ-inducing activity between ILO and LLO.
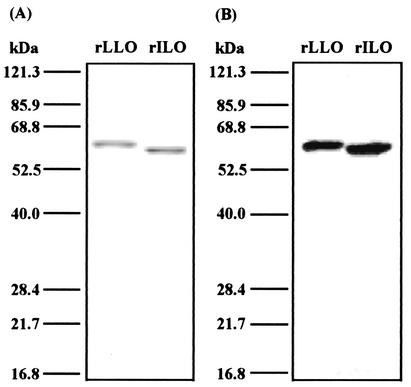
We have reported that LLO, the principal virulence factor of L. monocytogenes, plays a pivotal role in the generation of host resistance by induction of Th1 cytokines such as IL-12, IL-18, and IFN-γ (29, 30, 36). L. ivanovii is known to secrete ILO, which is highly homologous to LLO (20, 40). They have similar signal sequences (25 amino acids in LLO and 24 amino acids in ILO), and the secreted form of both proteins is 504 amino acids long (Fig. 5). The amino acid sequence of LLO shows an overall high degree of similarity to ILO, and the identity at amino acid level is 80% (14, 16). We examined whether ILO exhibits Th1 cytokine-inducing activity. To test the activity, recombinant His-tagged ILO (rILO) and rLLO were constructed. The final purified proteins were detected as single bands of the predicted molecular sizes on the SDS-PAGE gel by Coomassie brilliant blue staining and by immunoblot with anti-penta-His antibody (Fig. 6). The concentration of LPS was less than 10 pg per ml, a concentration that never induces IFN-γ in our in vitro system.
FIG. 5.
Alignment of amino acid sequences of LLO and ILO. A horizontal bar indicates the signal sequence. Stars indicate identical residues.
FIG. 6.
SDS-PAGE and Western blot analysis of recombinant proteins. Purified rLLO and rILO were applied to SDS-PAGE under reducing conditions. The gel was stained with Coomassie brilliant blue (A). The two recombinant proteins were electrophoresed and transferred to a polyvinylidene difluoride membrane. Western blotting was performed with an anti-His tag antibody (B). The positions of molecular size markers are indicated.
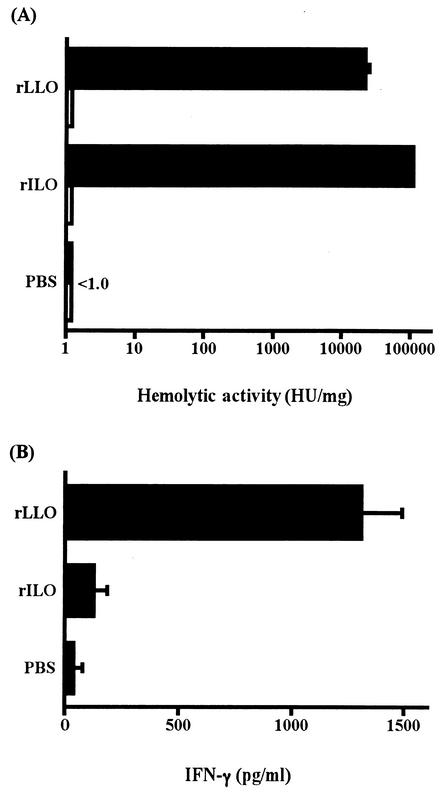
The hemolytic activity of rILO and rLLO was 112,156 HU/mg and 20,723 HU/mg, respectively, and was blocked by treatment with cholesterol (Fig. 7A). To determine the IFN-γ-inducing activities, spleen cells from normal mice were stimulated with cholesterol-treated rILO and rLLO for 24 h. The level of IFN-γ in the culture supernatant was assayed by ELISA. A high level of IFN-γ production was observed by stimulation with rLLO, but rILO did not induce cytokine production (Fig. 7B) despite the higher hemolytic activity of rILO than of rLLO.
FIG. 7.
Hemolytic and IFN-γ-inducing activities of rLLO and rILO. rLLO and rILO which had been treated (open bars) or not (solid bars) with cholesterol were serially twofold diluted with PBS and mixed with an equal volume of 1% SRBC. After incubation for 1 h at 37°C, the hemolytic activity was measured by the release of hemoglobin into the supernatant, and HU were calculated as described in Materials and Methods (A). Normal spleen cells were stimulated with 50 nM rLLO and rILO treated with cholesterol, and the level of IFN-γ production was measured by ELISA 24 h later (B). The data are representative of three independent experiments. Bars indicate standard deviations.
IFN-γ-inducing activity of ILO-LLO chimera.
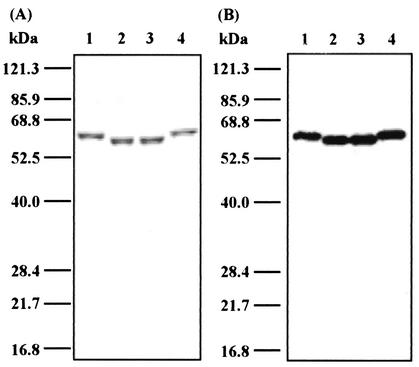
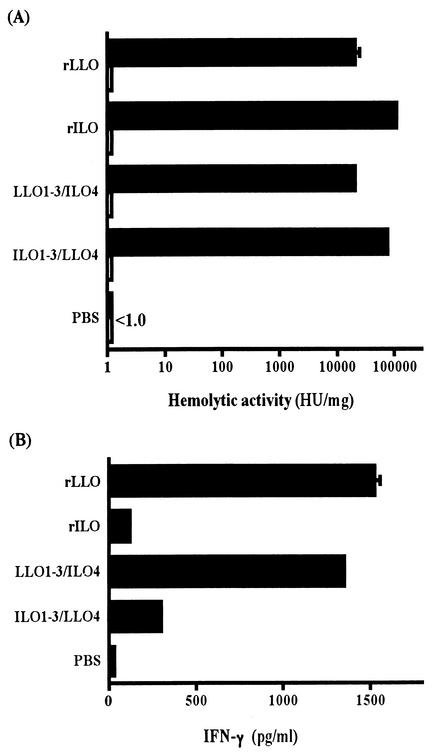
We have reported that domains 1 to 3 of LLO are the portion responsible for cytokine induction, while domain 4 is critical for cholesterol binding and resulting cytolytic activity (19). To confirm the inability of ILO to induce IFN-γ production and to know whether the difference in the IFN-γ-inducing activity between the two CDCs is due to any functional change in domains 1 to 3, we generated a chimeric protein carrying domains 1 to 3 of LLO and domain 4 of ILO (LLO1-3/ILO4) and, conversely, a protein consisting of domains 1 to 3 of ILO plus domain 4 of LLO (ILO1-3/LLO4). These chimeric proteins were purified by Ni-agarose column chromatography, and LPS was extensively eliminated. The purified proteins were detected as single bands on SDS-PAGE, and the bands were reactive with anti-penta-His antibody (Fig. 8). All the proteins exhibited a high level of hemolytic activity that was completely blocked by cholesterol (Fig. 9A).
FIG. 8.
SDS-PAGE and Western blot analysis of rLLO, rILO, and chimeric proteins. rLLO, rILO, and the chimeric proteins were analyzed on SDS-PAGE, and the gel was stained with Coomassie brilliant blue (A). The four recombinant proteins were electrophoresed and transferred to a polyvinylidene difluoride membrane. Western blotting was performed with an anti-His tag antibody (B). The positions of molecular size markers are indicated. Lanes: 1, rLLO; 2, rILO; 3, LLO1-3/ILO4; 4, ILO1-3/LLO4.
FIG. 9.
Hemolytic and IFN-γ-inducing activities of four recombinant proteins. Recombinant proteins that had been treated (open bars) or not (solid bars) with cholesterol were serially twofold diluted with PBS and mixed with an equal volume of 1% SRBC. After incubation for 1 h at 37°C, the hemolytic activity was measured by the release of hemoglobin into the supernatant, and HU were calculated (A). Normal spleen cells were stimulated with 50 nM rLLO, rILO, and the chimeric proteins that had been treated with cholesterol, and the level of IFN-γ production was measured by ELISA 24 h later. The data are representative of three independent experiments. Bars indicate standard deviations.
The production of IFN-γ was induced by stimulation with rLLO but not with rILO. The chimeric LLO1-3/ILO4 protein was capable of inducing IFN-γ production at a level as high as that by rLLO (Fig. 9B). However, cytokine production was not induced by ILO1-3/LLO4 (Fig. 9B). The dose of recombinants (50 nM) was optimal for rLLO and LLO1-3/ILO4, and IFN-γ induction was not observed with rILO or ILO1-3/LLO4 within the range from 1 to 100 nM (data not shown). This result clearly indicated that domains 1 to 3 of ILO do not possess IFN-γ-inducing activity.
DISCUSSION
L. monocytogenes is capable of intracellular replication in a variety of mammalian cells, including macrophages, by means of various virulence gene products (42). After being phagocytosed in macrophages, L. monocytogenes disrupts the phagosomal membrane with LLO (10) and phosphatidylinositol-phospholipase C and escapes into the cytosol from the phagosome (4, 41). Inside the cytosol, hexose phosphate translocase appears to enable this bacterium to utilize glucose 1-phosphate as a carbon source (5). Intracellular movement is possible by virtue of actin polymerization induced by ActA (18, 37). Moreover, the bacterium spreads to neighboring cells by disruption of a double-membrane vacuole by phosphatidylcholine-phospholipase C (35). The orchestrated action of these virulence determinants allows the intracellular survival of L. monocytogenes in macrophages.
It is well-known that a Th1-type immune response is induced in mice upon infection with L. monocytogenes and that antigen-specific T cells play a central role in protective immunity against challenge infection. There is a consensus that protective T cells are generated only by infection with viable and virulent bacteria and that avirulent or killed bacteria are not capable of inducing antigen-specific, effective immunity (38). This suggests that the virulence factor of L. monocytogenes may contribute directly to the induction of protective immunity. On the basis of this finding, we have been studying the contribution of LLO to the induction of protective immunity. We found that an LLO-deficient L. monocytogenes strain is not only less virulent but also less capable of inducing host resistance (36, 43). LLO was shown to possess the ability to induce various cytokines, including IFN-γ, which is important for the generation of protective T cells (29, 43, 44). Moreover, we showed that administration of liposome-encapsulated LLO confers protection on mice immunized with the avirulent Listeria strain (36). These results indicate that LLO plays an important role in the generation of protective immunity against L. monocytogenes infection.
L. ivanovii is a pathogenic species for animals but rarely for humans. Being similar to L. monocytogenes, L. ivanovii carries a central virulence gene cluster comprising prfA, plcA, hly (ilo), mpl, actA, and plcB genes (42). In this study, however, we found that L. ivanovii was less virulent than L. monocytogenes and the LD50 was almost 1,000-fold higher than that of L. monocytogenes. In an in vitro study, L. ivanovii was shown to be less capable of intracellular multiplication. Previous studies also showed that L. ivanovii is less virulent to mice (26, 32). There was no reliable explanation for the difference in virulence between these two Listeria species. Our preliminary studies showed that both Listeria species secreted comparable amounts of each cytolysin into the broth and that the culture supernatants showed similar cytotoxicity for macrophages (data not shown). We have been unable to ascribe the difference in virulence to either the production or the activity of their cytolysins. Although they carry similar virulence gene clusters, the actual DNA sequence of each virulence gene shows 73 to 78% similarity, and the actA genes of the two Listeria species are not homologous (42). It is probable that some of the virulence gene products of L. ivanovii are incompetent for intracellular survival and that the genetic diversity may reflect the functional difference. Alternatively, there may be some difference in genes other than the virulence genes known to date which additionally contribute to intracellular survival.
To analyze the inability of L. ivanovii to induce protective immunity, we determined the level of IFN-γ in serum samples from mice infected with listeriae because IFN-γ is the most important cytokine for the generation of protective immunity (3, 8, 27, 45). The level of IFN-γ in L. ivanovii-infected mice was considerably lower than that in L. monocytogenes-infected mice (Fig. 2). L. ivanovii secrets ILO, a CDC protein highly homologous to LLO, into the culture supernatant. Because LLO exhibits IFN-γ-inducing activity and contributes to the generation of protective immunity, it was expected that ILO would also exhibit similar activity. However, only a low level of cytokine production was detected after infection with L. ivanovii.
Because the in vivo virulence of this bacterium was lower than that of L. monocytogenes, it is postulated that the amount of ILO produced intracellularly is lower than that of LLO. Although there is no quantitative study on the intracellular production of ILO, Karunasagar et al. showed that the growth of L. ivanovii in the J774.1 murine macrophage cell line is similar to that of L. monocytogenes (17). This suggests that L. ivanovii must secrete a good amount of ILO inside the phagosome to escape into the cytoplasm. To clarify the activity of ILO in inducing IFN-γ production, we prepared recombinant ILO and LLO and compared their activities. Although LLO induced the production of IFN-γ, ILO was not able to induce cytokine production. Thus, it appears that the inability of ILO to induce IFN-γ production accounts at least in part for the failure of L. ivanovii to generate protective immunity.
It is unlikely that the inability of ILO to induce cytokine production is due to the absence of biological function of rILO. Because ILO and LLO belong to the same CDC family of proteins that are believed to form ring-shaped pores and causes cell lysis, three-dimensional structure appears to be basically important for cytolytic activity (2). All recombinant proteins used in this study showed strong hemolytic activity, suggesting that they were properly folded and maintained the natural three-dimensional structure. Furthermore, it seems unlikely that the inability of rILO to induce IFN-γ production is due to cytotoxicity to assay cells. Actually, rILO showed stronger hemolytic activity than rLLO. In this study, rILO and rLLO were treated with cholesterol to completely inhibit the cytotoxicity of each protein before its addition to the cell culture. After treatment with cholesterol, rILO and rLLO caused neither hemolysis nor lactate dehydrogenase release from normal spleen cells any longer (data not shown). We were able to rule out the contribution of contaminating LPS to IFN-γ induction because LPS was extensively removed and IFN-γ production induced by rLLO was not affected by the addition of 0.5 μg of polymyxin B per ml (data not shown). Accordingly, our data indicate that IFN-γ-inducing activity is present in LLO but not in ILO.
It has been reported that domain 4 of CDCs is essential for the initial binding to cholesterol on the cell surface, and domains 1 and 3 are required for the oligomerization and insertion of cytolysin into the cell membrane (31). On the basis of the IFN-γ-inducing activity of the chimeric proteins, it became clear that domains 1 to 3 are critical for cytokine induction and that changing the amino acids in the region causes the heterogeneity in IFN-γ-inducing activity. This finding is consistent with our recent report that domains 1 to 3 of LLO alone are sufficient for the expression of IFN-γ-inducing activity (19).
The present results strongly suggests that the level of protective immunity induced in infected mice is highly relevant to the distinct ability of secreted CDCs to induce a cytokine response. To confirm the pivotal role of LLO in cytokine-dependent Th1 induction in vivo, it will be necessary to construct a panel of recombinant strains of L. monocytogenes producing truncated and mutant LLOs or ILOs and also of L. ivanovii producing LLO. This line of study is under way, and bacterial growth and cytokine response in vivo will be determined with special reference to the production of protective immunity in mice.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research on Priority Areas (13226042) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the “Research for the Future” program (97L00706) and a Grant-in-Aid for Scientific Research (B/14370092 and C/13670270) from the Japan Society for the Promotion of Science.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Baba, H., I. Kawamura, C. Kohda, T. Nomura, Y. Ito, T. Kimoto, I. Watanabe, S. Ichiyama, and M. Mitsuyama. 2002. Induction of gamma interferon and nitric oxide by truncated pneumolysin that lacks pore-forming activity. Infect. Immun. 70:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billington, S. J., B. H. Jost, and J. G. Songer. 2000. Thiol-activated cytolysins: structure, function and role in pathogenesis. FEMS Microbiol. Lett. 182:197-205. [DOI] [PubMed] [Google Scholar]
- 3.Buchmeier, N. A., and R. D. Schreiber. 1985. Requirement of endogenous interferon-γ production for resolution of Listeria monocytogenes infection. Proc. Natl. Acad. Sci. USA 82:7404-7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chico-Calero, I., M. Suarez, B. Gonzalez-Zorn, M. Scortti, J. Slaghuis, W. Goebel, and J. A. Vazquez-Boland. 2002. Hpt, a bacterial homolog of the microsomal glucose-6-phosphate translocase, mediates rapid intracellular proliferation in Listeria. Proc. Natl. Acad. Sci. USA 99:431-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummins, A. J., A. K. Fielding, and J. McLauchlin. 1994. Listeria ivanovii infection in a patient with AIDS. J. Infect. 28:89-91. [DOI] [PubMed] [Google Scholar]
- 7.Dramsi, S., and P. Cossart. 2002. Listeriolysin O: a genuine cytolysin optimized for an intracellular parasite. J. Cell Biol. 156:943-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn, P. L., and R. J. North. 1991. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect. Immun. 59:2892-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaillard, J. L., P. Berche, J. Mounier, S. Richard, and P. Sansonetti. 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 55:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gellin, B. G., and C. V. Broome. 1989. Listeriosis. JAMA 261:1313-1320. [PubMed] [Google Scholar]
- 12.Geoffroy, C., J. L. Gaillard, J. E. Alouf, and P. Berche. 1987. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect. Immun. 55:1641-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gouin, E., J. Mengaud, and P. Cossart. 1994. The virulence gene cluster of Listeria monocytogenes is also present in Listeria ivanovii, an animal pathogen, and Listeria seeligeri, a nonpathogenic species. Infect. Immun. 62:3550-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas, A., M. Dumbsky, and J. Kreft. 1992. Listeriolysin genes: complete sequence of ilo from Listeria ivanovii and of lso from Listeria seeligeri. Biochim. Biophys. Acta 1130:81-84. [DOI] [PubMed] [Google Scholar]
- 15.Ito, Y., I. Kawamura, C. Kohda, H. Baba, T. Kimoto, I. Watanabe, T. Nomura, and M. Mitsuyama. 2001. Difference in cholesterol-binding and cytolytic activities between listeriolysin O and seeligeriolysin O: a possible role of alanine residue in tryptophan-rich undecapeptide. FEMS Microbiol. Lett. 203:185-189. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs, T., A. Darji, S. Weiss, and T. Chakraborty. 1999. Listeriolysin, the thiol-activated haemolysin of Listeria monocytogenes, p. 511-521. In J. E. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial protein toxins, 2nd ed. Academic Press, London, United Kingdom.
- 17.Karunasagar, I., G. Krohne, and W. Goebel. 1993. Listeria ivanovii is capable of cell-to-cell spread involving actin polymerization. Infect. Immun. 61:162-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kocks, C., E. Gouin, M. Tabouret, P. Berche, H. Ohayon, and P. Cossart. 1992. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68:521-531. [DOI] [PubMed] [Google Scholar]
- 19.Kohda, C., I. Kawamura, H. Baba, T. Nomura, Y. Ito, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2002. Dissociated linkage of cytokine-inducing activity and cytotoxicity to different domains of listeriolysin O from Listeria monocytogenes. Infect. Immun. 70:1334-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreft, J., D. Funke, A. Haas, F. Lottspeich, and W. Goebel. 1989. Production, purification and characterization of hemolysins from Listeria ivanovii and Listeria monocytogenes Sv4b. FEMS Microbiol. Lett. 57:197-202. [DOI] [PubMed] [Google Scholar]
- 21.Kreft, J., J. A. Vazquez-Boland, E. Ng, and W. Goebel. 1999. Virulence gene clusters and putative pathogenicity islands in listeriae, p. 219-232. In J. B. Kapeer and J. Hacker (ed.), Pathogenicity islands and other mobile virulence elements. American Society for Microbiology, Washington, D.C.
- 22.Kuhn, M., and W. Goebel. 1994. Induction of cytokines in phagocytic mammalian cells infected with virulent and avirulent Listeria strains. Infect. Immun. 62:348-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lessing, M. P., G. D. Curtis, and I. C. Bowler. 1994. Listeria ivanovii infection. J. Infect. 29:230-231. [DOI] [PubMed] [Google Scholar]
- 24.Lorber, B. 1997. Listeriosis. Clin. Infect. Dis. 24: 1-11. [DOI] [PubMed] [Google Scholar]
- 25.Low, J. C., and W. Donachie. 1997. A review of Listeria monocytogenes and listeriosis. Vet. J. 153:9-29. [DOI] [PubMed] [Google Scholar]
- 26.Mainou-Fowler, T., A. P. MacGowan, and R. Postlethwaite. 1988. Virulence of Listeria spp.: course of infection in resistant and susceptible mice. J. Med. Microbiol. 27:131-140. [DOI] [PubMed] [Google Scholar]
- 27.Nakane, A., T. Minagawa, I. Yasuda, C. Yu, and K. Kato. 1988. Prevention by gamma interferon of fatal infection with Listeria monocytogenes in mice treated with cyclosporin A. Infect. Immun. 56:2011-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishibori, T., K. Cooray, H. Xiong, I. Kawamura, M. Fujita, and M. Mitsuyama. 1995. Correlation between the presence of virulence-associated genes as determined by PCR and actual virulence to mice in various strains of Listeria spp. Microbiol. Immunol. 39:343-349. [DOI] [PubMed] [Google Scholar]
- 29.Nishibori, T., H. Xiong, I. Kawamura, M. Arakawa, and M. Mitsuyama. 1996. Induction of cytokine gene expression by listeriolysin O and roles of macrophages and NK cells. Infect. Immun. 64:3188-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nomura, T., I. Kawamura, K. Tsuchiya, C. Kohda, H. Baba, Y. Ito, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2002. Essential role of interleukin-12 (IL-12) and IL-18 for gamma interferon production induced by listeriolysin O in mouse spleen cells. Infect. Immun. 70:1049-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer, M. 2001. The family of thiol-activated, cholesterol-binding cytolysins. Toxicon 39:1681-1689. [DOI] [PubMed] [Google Scholar]
- 32.Pine, L., R. E. Weaver, G. M. Carlone, P. A. Pienta, J. Rocourt, W. Goebel, S. Kathariou, W. F. Bibb, and G. B. Malcolm. 1987. Listeria monocytogenes ATCC 35152 and NCTC 7973 contain a nonhemolytic, nonvirulent variant. J. Clin. Microbiol. 25:2247-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 34.Rossjohn, J., S. C. Feil, W. J. McKinstry, R. K. Tweten, and M. W. Parker. 1997. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell 89:685-692. [DOI] [PubMed] [Google Scholar]
- 35.Smith, G. A., H. Marquis, S. Jones, N. C. Johnston, D. A. Portnoy, and H. Goldfine. 1995. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 63:4231-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanabe, Y., H. Xiong, T. Nomura, M. Arakawa, and M. Mitsuyama. 1999. Induction of protective T cells against Listeria monocytogenes in mice by immunization with a listeriolysin O-negative avirulent strain of bacteria and liposome-encapsulated listeriolysin O. Infect. Immun. 67:568-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilney, L. G., and D. A. Portnoy. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite Listeria monocytogenes. J. Cell Biol. 109:1597-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsukada, H., I. Kawamura, M. Arakawa, K. Nomoto, and M. Mitsuyama. 1991. Dissociated development of T cells mediating delayed-type hypersensitivity and protective T cells against Listeria monocytogenes and their functional difference in lymphokine production. Infect. Immun. 59:3589-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsukada, H., I. Kawamura, T. Fujimura, K. Igarashi, M. Arakawa, and M. Mitsuyama. 1992. Induction of macrophage interleukin-1 production by Listeria monocytogenes hemolysin. Cell. Immunol. 140:21-30. [DOI] [PubMed] [Google Scholar]
- 40.Vazquez-Boland, J. A., L. Dominguez, E. F. Rodriguez-Ferri, and G. Suarez. 1989. Purification and characterization of two Listeria ivanovii cytolysins, a sphingomyelinase C and a thiol-activated toxin (ivanolysin O). Infect. Immun. 57:3928-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vazquez-Boland, J. A., C. Kocks, S. Dramsi, H. Ohayon, C. Geoffroy, J. Mengaud, and P. Cossart. 1992. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect. Immun. 60:219-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiong, H., I. Kawamura, T. Nishibori, and M. Mitsuyama. 1994. Cytokine gene expression in mice at an early stage of infection with various strains of Listeria spp. differing in virulence. Infect. Immun. 62:3649-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiong, H., Y. Tanabe, S. Ohya, and M. Mitsuyama. 1998. Administration of killed bacteria together with listeriolysin O induces protective immunity against Listeria monocytogenes in mice. Immunology 94:14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang, J., I. Kawamura, and M. Mitsuyama. 1997. Requirement of the initial production of gamma interferon in the generation of protective immunity of mice against Listeria monocytogenes. Infect. Immun. 65:72-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang, J., and M. Mitsuyama. 1997. An essential role for endogenous interferon-gamma in the generation of protective T cells against Mycobacterium bovis BCG in mice. Immunology 91:529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshikawa, H., I. Kawamura, M. Fujita, H. Tsukada, M. Arakawa, and M. Mitsuyama. 1993. Membrane damage and interleukin-1 production in murine macrophages exposed to listeriolysin O. Infect. Immun. 61:1334-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]