Abstract
During development, the regulated expression of tissue-specific genes can be preceded by their potentiation, that is, by chromatin activation in progenitor cells. For example, the human β-like globin genes are potentiated in a gene- and developmental-specific manner in hematopoietic progenitors. Developmental regulation of human β-gene expression in erythroid cells is mostly determined by transcriptional activators; however, it is not clear how gene-specific potentiation is set in hematopoietic progenitors. Using human and transgenic multipotent hematopoietic progenitors, we demonstrate that human β-globin locus activation is characterized by TBP, NF-E2, CBP and BRG1 recruitment at both the Locus Control Region and human β-gene promoter. Our results further indicate that in hematopoietic progenitors, EKLF influences chromatin organization at the human β-globin locus and is instrumental for human β-gene potentiation. Thus, we show that lineage-specific transcriptional activators expressed at basal levels in progenitor cells can participate in gene potentiation.
Keywords: chromatin, epigenetics, globins, hematopoietic progenitors, transcriptional activators
Introduction
The expression of lineage-specific genes can be potentiated before transcriptional activation (Bonifer, 1999). Gene potentiation is characterized by an active chromatin organization in multipotent progenitor cells (Jimenez et al, 1992; Bottardi et al, 2003) and can be accompanied by partial occupancy of gene regulatory regions by transcriptional activators. Gene expression in differentiated cells requires synergy among activators, co-activators, general transcription factors, chromatin remodeling and histone modifying complexes (Struhl, 2005). In hematopoietic stem cells (Ye et al, 2003) and in multipotent hematopoietic progenitor cells (HPC) (Hu et al, 1997), the ‘promiscuous' expression of lineage-specific factors such as PU.1, C/EBPα, PAX5, SCL, GATA-1, GATA-2 and GATA-3 does not alter the biological potential of these cells, but ostensibly provides a proper environment for stochastic lineage commitment mainly by repressing or activating groups of genes (Cantor and Orkin, 2002; Graf, 2002). However, it is not known if promiscuously expressed transcriptional activators influence gene potentiation in multipotent progenitor cells.
In mammals, the human β-(huβ-) globin locus is a well-characterized multigenic locus, and therefore a good model to identify mechanisms that control epigenetic regulation of gene expression during hematopoiesis. The huβ-globin locus consists of five developmentally regulated genes (ɛ–Gγ–Aγ–δ–β), whose high-level expression in erythroid cells (EryC) depends upon the Locus Control Region (βLCR) comprised of four erythroid-specific DNaseI hypersensitive sites (HS). The βLCR enhances gene transcription through direct interaction with globin promoters (Carter et al, 2002; Tolhuis et al, 2002; Palstra et al, 2003). Even though DNaseI sensitivity at the endogenous murine locus is not lost when the βLCR is deleted (Bender et al, 2000), in EryC the human βLCR is a major determinant of chromatin organization at the huβ-globin locus (Grosveld et al, 1987). In progenitor cells, the β-globin locus is in an active chromatin organization (Jimenez et al, 1992) and potentiation of huβ-like genes, most likely mediated by as yet unidentified transcriptional activators, is developmental-specific (Bottardi et al, 2003).
EKLF, GATA-1 and the p45 subunit of NF-E2 (p45) are among the best-characterized transcriptional activators involved in huβ-gene regulation in EryC. EKLF is essential for adult β-globin gene transcription and EKLF knockout (KO) mice suffer from severe anemia and die at approximately 14.5 d.p.c. (days postcoitus) (Nuez et al, 1995; Perkins et al, 1995). EKLF binds to βLCR and globin promoters (Bieker, 2001) and it is required for βLCR-β major (βmaj) gene direct interaction (Drissen et al, 2004). EKLF recruits the erythroid-specific SWI/SNF chromatin remodeling complex 1 (E-RC1) to the β-globin locus (Armstrong et al, 1998) and the absence of EKLF leads to reduced DNaseI HS formation at the βmaj and huβ-promoters (Wijgerde et al, 1996). GATA-1 binds to βLCR HSs and globin promoters and is critical for primitive and definitive erythroid cell differentiation (Pevny et al, 1991; Fujiwara et al, 1996). It interacts with CBP, SWI/SNF, ACF/WCRF and NuRD complexes (Blobel et al, 1998; Kadam et al, 2000; Hong et al, 2005; Rodriguez et al, 2005). GATA-1 is also required for βLCR-βmaj direct interaction (Vakoc et al, 2005). NF-E2 is comprised of a ubiquitously expressed subunit, MafK, and the hematopoietic-specific activator p45 (Andrews et al, 1993), and it is recruited to mouse and human βLCR and promoter regions (Daftari et al, 1999; Forsberg et al, 2000; Sawado et al, 2001; Leach et al, 2003). NF-E2 is necessary for globin gene expression in differentiated MEL (mouse erythroleukemia) cells but p45 KO mice exhibit only mild effects on erythropoiesis and no significant influence on globin gene expression (Shivdasani and Orkin, 1995).
Interestingly EKLF, GATA-1 and p45 are also promiscuously expressed in hematopoietic progenitors (Hu et al, 1997). p45 could be involved in priming/potentiation of the mouse α-globin locus (Anguita et al, 2004), whereas PU.1–GATA-1 interaction influences lineage commitment (Cantor and Orkin, 2002; Graf, 2002). However, it has never been investigated whether these factors play roles in developmental-specific potentiation of the huβ-gene and globin locus activation in multipotent HPC.
To understand the molecular events leading to huβ-like globin gene potentiation, we studied the influence of promiscuously expressed transcriptional activators on recruitment of general transcription factors as well as chromatin remodeling and histone modifying activities at βLCR HS2, huγ- and huβ-promoter in primary multipotent HPC. Our results, obtained with human cells and transgenic mice expressing the huβ-globin locus, suggest that EKLF is instrumental for globin gene potentiation in HPC, facilitating p45, CBP, BRG1 and TBP recruitment at the huβ-promoter. Finally, we provide insight into the cooperative role of EKLF and p45 for promoting appropriate chromatin activation in HPC, as well as for Pol II recruitment and subsequent transcriptional elongation in EryC. Based on these results, we suggest a model whereby EKLF is a key regulator of huβ-gene potentiation in HPC.
Results
HPC purification and characterization
Mouse HPC were isolated from 13.5 d.p.c. fetal livers of line 2 mice (ln2 HPC), which are transgenic for the entire huβ-globin locus and express the huβ-globin genes normally (Strouboulis et al, 1992). Mouse HPC corresponds to a Ter119−, Gr-1−, B220−, CD31high population, which represents 1–2% of the total fetal liver and does not contain mature or late committed cells (see below). The sorted population was always ⩾98% pure. The hematopoietic potential of these cells was ascertained by in vitro clonal assays in methylcellulose (Table I, ln2 HPC). On average, out of 100 colonies, 85 originated from progenitors with multilineage potential (CFU-GEMM: colony forming unit-granulocyte, erythrocyte, megakaryocyte, macrophage; CFU-GM: colony forming unit-granulocyte, megakaryocyte), whereas 15 showed unilineage commitment (BFU-E: Burst forming unit-erythrocyte). No CFU-E (colony forming unit-erythrocyte) was detected.
Table 1.
Clonal assays in methylcellulose: 150 HPC purified from 13.5 d.p.c. ln2 or ln2 EKLF−/− fetal livers were plated onto methylcellulose plates
| Clonogenic ability of Ter119−, Gr-1−, B220−, CD31high cells | ||||
|---|---|---|---|---|
| CFU- GEMM (%) | CFU-GM (%) | BFU-E (%) | Colonies/103 cells | |
| ln2 HPC | 38.9 | 44.8 | 16.3 | 210±40 |
| ln2 EKLF−/− HPC | 34 | 48.2 | 17.8 | 240±35 |
To further characterize this population, ln2 HPC cDNA was used to study the expression of marker genes. SCL, GATA-1, p45, GATA-2, EKLF, C/EBPα and globin transcripts are all expressed, whereas transcripts corresponding to GATA-3 are not detected (Figure 1A; ln2 HPC). This expression pattern suggests that the Ter119−, Gr-1−, B220−, CD31high cell population is mainly composed of multipotent HPC (Akashi et al, 2000), namely common myeloid and megakaryocyte/erythrocyte lineage-restricted progenitors, a finding supported by our in vitro clonal assays (see above). As evaluated by quantitative real-time reverse transcriptase (RT)–PCR, the levels of p45, EKLF and huβ-globin mRNA are, respectively, ∼7-, ∼6- and ∼100-fold higher in EryC than in HPC (Supplementary Figure 1). Accordingly, Western blot analyses revealed that GATA-1, p45 and EKLF protein levels are approximately, 12-, 6- and 11-fold higher in EryC than in HPC (Figure 1B).
Figure 1.
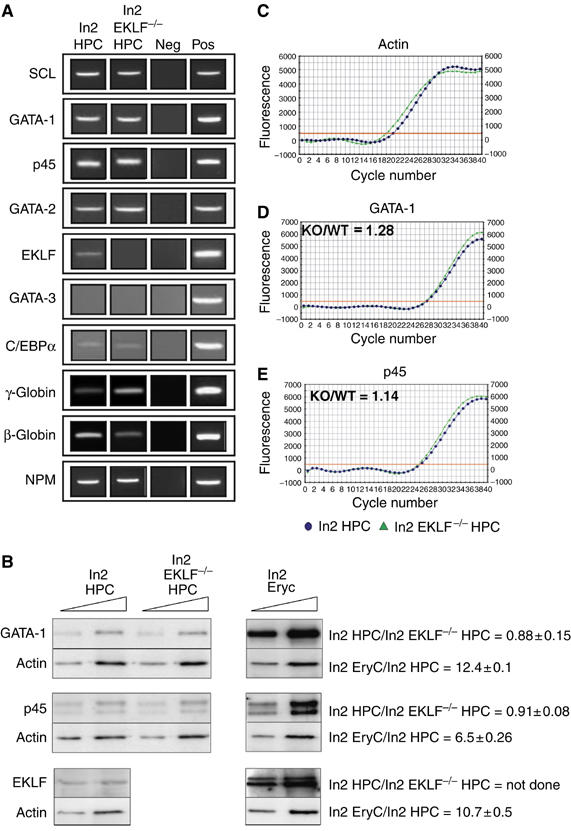
Expression of marker genes in ln2 HPC and ln2 EKLF−/− HPC. (A) Semiquantitative RT–PCR performed on equal amounts of RNA purified from ln2 HPC or ln2 EKLF−/− HPC. PCR samples were resolved onto agarose gel. γ-Globin: fetal human (γ) and mouse embryonic (βH1) transcripts; β-globin: adult human (β and δ) and mouse (βmin and βmaj) globin transcripts; NPM: ubiquitously expressed nucleophosmin transcript, used as internal control; Neg: negative control; Pos: positive control; (B) Western blot analysis of ln2 HPC, ln2 EKLF−/− HPC and ln2 EryC; 4 and 8 μg of whole-cell protein extract were loaded in each lane of a 10% SDS–PAGE. Anti-GATA-1 and -p45 antibodies were purchased from SantaCruz; anti-actin antibodies were purchased from LabVision; anti-EKLF serum is a generous gift of S Philipsen. Protein levels in ln2 HPC versus ln2 EKLF−/− HPC or ln2 EryC versus ln2 HPC were calculated using actin as internal control and they are shown on the right side of each panel together with their standard error of means; (C–E) representative examples of quantitative real-time RT–PCR; the relative level of GATA-1 or p45 gene expression in ln2 EKLF−/− HPC versus ln2 EKLF HPC were calculated according to Pfaffl (2001), using mouse actin as internal control and expressed as KO/WT ratio; x axis: cycle number; y axis: derivative of SYBR Green fluorescence. Blue dots: ln2 HPC; green triangles: ln2 EKLF−/− HPC.
Since an EKLF KO background is extensively used in this study, we investigated whether the absence of EKLF causes a general failure of cellular differentiation during hematopoiesis because EKLF regulates other genes crucial for final EryC differentiation (Drissen et al, 2005). HPC purified from 13.5 d.p.c. ln2 EKLF KO fetal livers (ln2 EKLF−/− HPC) represent 1–2% of total fetal liver (as observed in ln2 HPC) and, as expected (Nuez et al, 1995; Perkins et al, 1995), give rise to erythroid colonies in methylcellulose (Table I). Furthermore, the gated populations and marker gene expression analyses (with the exception of globin genes) do not reveal any apparent difference between ln2 HPC and ln2 EKLF−/− HPC (Figure 1A). In particular, GATA-1 and p45 expression levels, as evaluated by quantitative real-time RT–PCR and Western blot analysis, do not change significantly among the two populations (Figure 1B–E). Thus, the absence of EKLF does not preclude normal hematopoietic differentiation of HPC.
Transcriptional activators recruitment at the huβ-globin locus in HPC
Our previous results suggested that in bone marrow-derived ln2 HPC and human CD34+ cells, chromatin at the huβ-globin LCR is in an active state, and the huβ-promoter is potentiated for transcriptional activation (Bottardi et al, 2003). We now investigate how histone covalent modification and chromatin remodeling activities are recruited at HS2, huβ- and huγ-promoters during hematopoiesis. Chromatin immunoprecipitation (ChIP) assays were performed on 13.5 d.p.c. fetal liver-derived hematopoietic cells. In 13.5 d.p.c. fetal livers, the huβ-gene is expressed and huγ-genes are mostly silent as judged by the fact that huγ-transcripts represent ∼10% of total huβ-globin level (Strouboulis et al, 1992). HS2 was chosen because it is important for high-level huβ-gene expression in EryC (Morley et al, 1992), and moreover HS2 deletion can abrogate epigenetic regulation of globin genes in EryC (Milot et al, 1996) as well as locus activation in HPC (Bottardi et al, 2005).
Histone acetylation of the huβ-globin locus in fetal liver ln2 HPC (Figure 2B; Supplementary Figure 2A) is similar to the pattern observed in bone marrow HPC (Bottardi et al, 2003), suggesting that βLCR and huβ-promoter are in an active/potent chromatin organization also in 13.5 d.p.c. fetal liver HPC. As shown in Figure 2C, GATA-1 is not significantly crosslinked at HS2, huβ- or huγ-promoters while, as reported previously (Anguita et al, 2004), HS-12 of the mouse α-globin locus is slightly enriched (Supplementary Figure 3A). p45 is detected at HS2 and huβ-promoter in ln2 HPC and in CD34+ cells (Figure 2D; Supplementary Figure 3B). p45 is also detected at huγ-promoters in ln2 HPC (Figure 2D). Thus, p45 is recruited to the huβ-globin locus at early stages of hematopoiesis.
Figure 2.
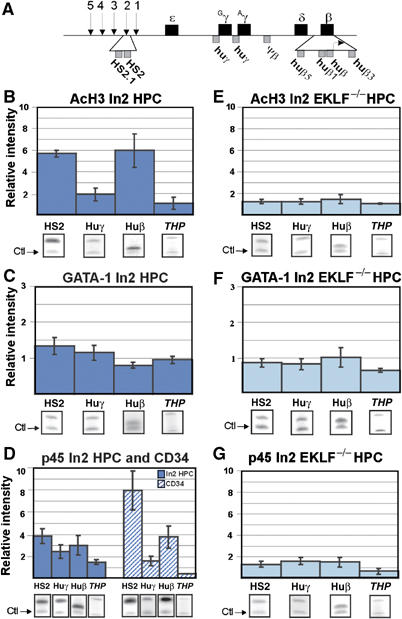
Histone H3 acetylation, GATA-1 and p45 recruitment at the huβ-globin locus in HPC. (A) A map of the huβ-globin locus; genes are shown as black boxes and the location of βLCR HSs is indicated by arrows. Amplified regions used for ChIP analyses are indicated by grey boxes. (B–G) ChIP assays: immunoprecipitated and unbound (input) chromatin samples were subjected to duplex radioactive PCR with one primer set specific for huβ-globin regions and another primer set specific for mouse zfp37 (ZFP, zinc-finger protein 37) or human pax6 (pax6, paired box protein 6) regulatory regions (‘Ctl', indicated by arrows), two genes that are not expressed in hematopoietic cells. All PCR reactions were performed in parallel under conditions of linear amplification. Products were quantified by PhosphorImager. The level of enrichment of globin regions relative to the control and input samples is represented by bars, with their corresponding standard deviations. A value of 1 indicates no enrichment. Mouse THP/ZFP or human THP/pax6 controls are included (THP, kidney-specific Tamm-Horsfall gene) to confirm that no enrichment is detected at regulatory regions of non-hematopoietic genes. To obviate for weak signals, THP/ZFP PCR reactions were run longer but always in conditions of linear amplification. Representative gel images are shown below each panel; AcH3: anti-acetylated histone H3 antibodies; dark blue bars: ln2 HPC; dark blue hatched bars: human CD34+ cells; light blue bars: ln2 EKLF−/− HPC.
Since EKLF is expressed at basal levels in HPC, we investigated whether it can influence huβ-gene potentiation in HPC. Commercially available antibodies against EKLF are not suitable for ChIP analysis (Feng and Kan, 2005). Thus, we used a genetic approach in comparing ln2 HPC with ln2 EKLF−/− HPC. As shown in Figure 2E, histone H3 acetylation at the huβ-globin locus decreases significantly in ln2 EKLF−/− HPC relative to ln2 HPC, whereas no major differences are detected at HS-29 of the mouse α-globin locus (Supplementary Figure 3C). (Unless specified, ‘significant' refers to a statistically significant difference between wild type and KO cells, based on unpaired student's t-test P-values <0.05). As in ln2 HPC, GATA-1 is not detected at HS2 or globin promoters in ln2 EKLF−/− HPC (Figure 2F). p45 binding to HS2 and huβ-promoter is significantly affected in ln2 EKLF−/− HPC (Figure 2G), suggesting that EKLF can influence p45 recruitment to these regions and that p45 as well as EKLF participate in locus organization in HPC.
Recruitment of histone modifying and chromatin remodeling activities at the huβ-globin locus in HPC
Based on the above results, we investigated whether the role of p45 and EKLF in globin potentiation includes their capacity to recruit histone modifying and chromatin remodeling activities to the globin locus.
CBP is detected at HS2 and huβ-promoter in ln2 HPC and CD34+ cells (Figure 3A; Supplementary Figure 3D). However, CBP binding is significantly reduced at huβ-promoter in ln2 EKLF−/− HPC (Figure 3B). p45 (Hung et al, 2001) and EKLF (Zhang and Bieker, 1998) interact with and are acetylated by CBP, but unlike p45 (Johnson et al, 2001), the ability of EKLF to recruit CBP to any region of the globin locus has never been demonstrated. The fact that in ln2 EKLF−/− HPC H3 acetylation and p45 recruitment are affected at both HS2 and huβ-promoter (Figure 2E and G), whereas CBP binding is reduced only at huβ-promoter, suggests that the absence of EKLF could either: (i) directly impede CBP recruitment at the huβ-promoter; or (ii) preclude p45 and CBP recruitment at the huβ-promoter with consequent hypoacetylation. Therefore, the role of p45 in locus acetylation was verified in HPC purified from 13.5 d.p.c. ln2 p45 KO fetal livers (ln2 p45−/− HPC). The absence of p45 does not significantly change histone H3 acetylation level at HS2 (data not shown), whereas H3 acetylation and CBP recruitment at the huβ-promoter significantly decrease in ln2 p45−/− HPC (Supplementary Figure 4). Thus, it appears that p45-mediated recruitment of CBP, which is facilitated by EKLF, contributes to histone acetylation of the huβ-promoter in HPC. The detection of histone H3 acetylation at HS2 in ln2 p45−/− HPC suggests that other activator/s capable of interacting with histone acetyltransferases can bind HS2 in HPC.
Figure 3.
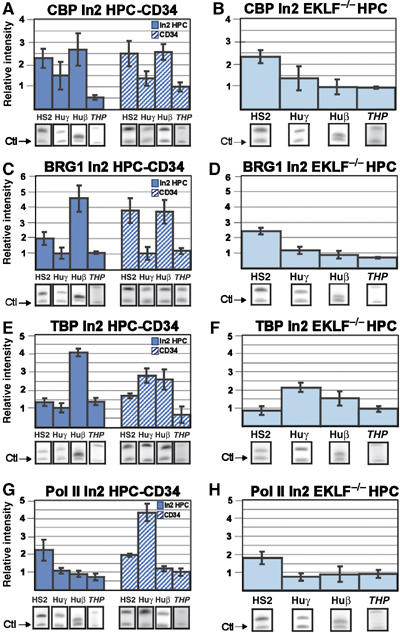
CBP, BRG1, TBP and Pol II recruitment at the huβ-globin locus in HPC. Ln2 HPC, human CD34+ cells, or ln2 EKLF−/− HPC were subjected to ChIP analyses with anti-CPB (A, B), -BRG1 (C, D), -TBP (E, F), or -Pol II (G, H) antibodies. Analysis and quantification of immunoprecipitated samples were performed as described in Figure 2. Dark blue bars: ln2 HPC; dark blue hatched bars: human CD34+ cells; light blue bars: ln2 EKLF−/− HPC.
The remodeling complex E-RC1 contains, among other proteins, the yeast homologue BRG1 and EKLF (Armstrong et al, 1998). We therefore wondered whether EKLF might be part of a remodeling complex in HPC. Since the EKLF-BRG1 interaction is sufficient to remodel a chromatin-assembled β-globin promoter (Kadam et al, 2000), and BRG1 is the first among the SWI/SNF components to be recruited to promoter regions, we verified whether EKLF could influence huβ-gene potentiation through BRG1 recruitment and hence chromatin remodeling. The comparison between ln2 HPC or CD34+ cells and ln2 EKLF−/− HPC (Figure 3C and D) reveals a significant reduction of BRG1 binding at huβ-promoter in ln2 EKLF−/− HPC. As expected, BRG1 binding at the c-myc promoter (Nagl et al, 2006) is similar in ln2 HPC versus ln2 EKLF−/− HPC (Supplementary Figure 3E). Based on these results, we propose that in HPC EKLF facilitates chromatin remodeling of the huβ-promoter through BRG1 recruitment.
Transcription preinitiation complex assembly and globin gene potentiation in HPC
The preinitiation complex (PIC) includes Pol II, general transcription factors, for example TBP, and additional cofactors. Basal levels of huβ-gene expression in HPC (Figure 1A) prompted us to ask whether gene potentiation in these cells requires PIC assembly. TBP was detected at huβ-promoter in ln2 HPC and CD34+ cells (Figure 3E). Even though bound to the huγ-promoter in CD34+ cells (see Discussion), TBP is not found at huγ- (Figure 3E) or huɛ- (data not shown) promoters in ln2 HPC. Thus, globin potentiation in ln2 HPC is developmental-specific and is associated with TBP recruitment at the huβ-promoter before the onset of high-level transcriptional activity. This is supported by our results obtained in EKLF KO cells. In EKLF KO mice, the switch from γ-to-β-globin expression is delayed; indeed, around 13.5 d.p.c. γ-globins are expressed in a greater number of fetal liver EryC derived from these mice relative to the situation for wild-type counterparts (Perkins et al, 1996; Wijgerde et al, 1996). Consequently, in ln2 EKLF−/− HPC the TBP binding significantly decreases at huβ-promoter, whereas it increases at huγ-promoters (Figure 3F).
It has been proposed that NF-E2−TAFII130 interaction facilitates PIC assembly at globin promoters (Amrolia et al, 1997). Accordingly, in ln2 p45−/− HPC, TBP recruitment at the huβ-promoter is significantly lower than in wild-type cells (Supplementary Figure 4). Altogether, these results suggest that EKLF, either directly or through p45, contributes to huβ-gene potentiation also by modulating the developmental-specific recruitment of TBP at the huβ-promoter in HPC.
Ln2 HPC were then subjected to ChIP analyses with an antibody that binds Pol II in a phosphorylation-independent manner. Pol II is detected at HS2, but not at the huβ-, huγ- (Figure 3G and H) or βmaj- (Supplementary Figure 5) promoters, suggesting that HS2-bound Pol II is not efficiently transferred to globin promoters in ln2 HPC or ln2 EKLF−/− HPC. However, Pol II is crosslinked at huγ-promoters in CD34+ cells (see Discussion). Thus, the presence of TBP but not Pol II suggests that the PIC is partially assembled at the huβ-promoter and contributes to globin gene potentiation in HPC.
Basal levels of globin gene expression in HPC
Globins are expressed at basal levels in multipotent progenitors (Figure 1A) (Hu et al, 1997; Bottardi et al, 2003). EKLF is essential for huβ-gene expression in EryC, but it is not known whether EKLF is also important for basal levels of globin gene expression in HPC. By quantitative real-time RT–PCR (Figure 4), we show that huβ-gene expression is 10-fold lower and huγ-gene expression is 4-fold higher in ln2 EKLF−/− HPC than in ln2 HPC. Thus, fetal liver-derived HPC express huβ- and huγ-globin genes and the absence of EKLF favors huγ- to the detriment of huβ-gene basal levels of expression.
Figure 4.

Quantitative real-time RT–PCR of ln2 HPC and ln2 EKLF−/− HPC. Representative examples of huβ- (A) and huγ-gene (B) expression were evaluated by real-time RT–PCR. The relative levels of globin gene expression in ln2 EKLF−/− HPC versus ln2 HPC were calculated according to Pfaffl (2001), using mouse actin as internal control (as described in Figure 1) and expressed as KO/WT ratios. Blue dots: ln2 HPC; green triangles: ln2 EKLF−/− HPC.
Chromatin organization at the huβ-globin locus in EryC
EKLF is required for enucleation and final erythrocyte formation during in vitro culture of definitive erythrocytes, whereas EKLF is dispensable for the first steps of erythroid cell differentiation (Table I; Drissen et al, 2005). Wright–Giemsa staining of ln2 EKLF wild type or ln2 EKLF KO 13.5 d.p.c. fetal livers revealed that, as expected (Perkins et al, 1995), late erythroblasts and enucleated red cells are less represented in 13.5 d.p.c. EKLF KO than wild-type fetal liver. However, only minor variations in the ratio of erythroid versus nonerythroid cells and in the percentage of primitive erythroid cells are observed among the two backgrounds (Supplementary Figure 6). In view of the above, ln2 EKLF wild type (ln2 EryC) as well as ln2 EKLF KO (ln2 EKLF−/− EryC) 13.5 d.p.c. fetal livers were used for ChIP analyses. First, compared with ln2 EryC, H3 lysine 4 (K4) dimethylation and H3 acetylation levels in ln2 EKLF−/− EryC (Figure 5A and B), though still considerable (except for K4 dimethylation at huβ-promoter), are lower at HS2, huγ- and huβ-promoters. GATA-1 and p45 are crosslinked to HS2 and huβ-promoter in ln2 EryC (Figure 5C and D). However, in ln2 EKLF−/− EryC, GATA-1 recruitment at the huβ-promoter (Figure 5C) and p45 recruitment at HS2 and huβ-promoter (Figure 5D) are reduced. Likewise, in ln2 EKLF−/− EryC CBP, BRG1, TBP and Pol II recruitment are affected at HS2 and huβ-promoter (Figure 5E–H). Pol II binding is also reduced at the βmaj promoter in ln2 EKLF−/− EryC (Supplementary Figure 5). Interestingly, in ln2 EKLF−/− EryC, TBP (Figure 5G) and Pol II (Figure 5H) binding to huγ-promoters is greater than in ln2 EryC. These results are in agreement with previous observations indicating that γ-genes are expressed at higher levels in EKLF KO than EKLF wild-type 13.5 d.p.c. fetal livers (Perkins et al, 1996; Wijgerde et al, 1996). To ascertain the significance (P<0.05) of the variations in histone acetylation as well as p45, CBP, and BRG1 recruitment in ln2 EryC versus ln2 EKLF−/− EryC, other regions were analyzed (Supplementary Figure 7A–D). Since no significant differences are observed at any of the additional regions analyzed, it is likely that the EKLF effect at the β-globin locus is not related to a global defect in transcriptional activation. Thus, the defect in huβ-gene expression documented in ln2 EKLF−/− EryC is most likely due to abnormal recruitment of chromatin proteins and histone modifying/chromatin remodeling activities, transcriptional activators, TBP, and Pol II at the βLCR, and, in particular, at the huβ-globin promoter.
Figure 5.
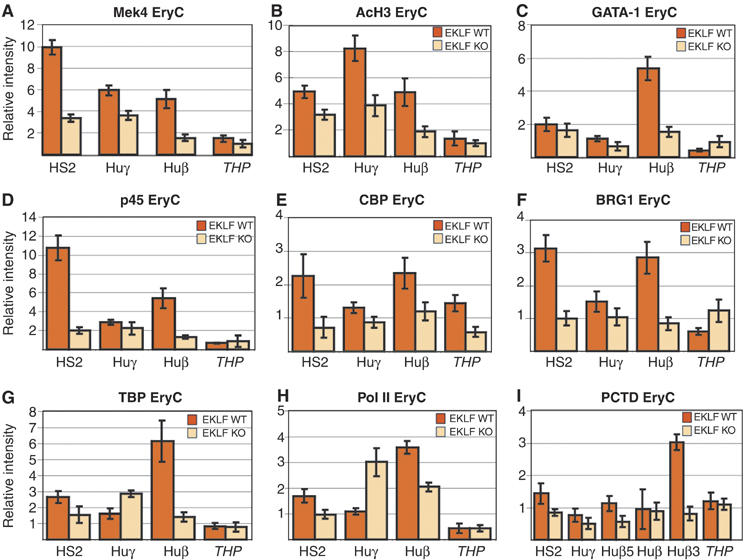
Chromatin organization at the huβ-globin locus in EryC. (A–I) ChIP analyses of ln2 EryC (dark orange bars) or ln2 EKLF−/− EryC (light orange bars). Analysis and quantification of immunoprecipitated samples, as well as antibodies used for ChIP assays are as described in Figures 2 and 3; MeK4: anti-lysine 4 dimethylated histone H3 antibodies; PCTD: anti-phospho-Ser2 Pol II CTD antibodies.
Pol II phosphorylation and transcriptional elongation
In the absence of EKLF, transgenic mice carrying the huβ-globin locus do not express mouse or human adult globin genes in EryC (Perkins et al, 1996; Wijgerde et al, 1996). Nonetheless, we detect Pol II at the huβ-promoter in ln2 EKLF−/− EryC (Figure 5H). To elucidate this conflicting result, ln2 EryC or ln2 EKLF−/− EryC were subjected to ChIP analyses with an antibody that recognizes phospho-Ser2 at the C-terminal domain (CTD) of Pol II (PCTD). It is known that the overall phosphorylation level of Pol II increases during transcriptional elongation. In fact, during promoter clearance and elongation, Pol II CTD is phosphorylated and this post-translational modification renders Pol II a transcription- and elongation-competent enzyme. As shown in Figure 5I, PCTD detection at the 3′end of the huβ-gene (Huβ3) is significantly lower in ln2 EKLF−/− EryC than in ln2 EryC. This result suggests that in EKLF−/− EryC, Pol II is not efficiently phosphorylated on Ser2 and is stalled at the huβ-promoter.
Discussion
Transcriptional activators involved in globin gene potentiation in HPC
Previous studies have shed light on the importance of EKLF during ontogeny (Xue et al, 2004), and for adult β-genes expression in EryC (Nuez et al, 1995; Perkins et al, 1995; Wijgerde et al, 1996). EKLF is not essential for early hematopoietic differentiation. However, observations here and elsewhere that EKLF is promiscuously expressed in multipotent HPC and progenitor cell lines (Hu et al, 1997; Figure 1) prompted us to explore whether EKLF is required for huβ-gene potentiation in HPC. In EryC, EKLF and BRG1 participate in the remodeling complex E-RC1 (Armstrong et al, 1998). Interestingly, EKLF−BRG1 interaction appears to be crucial since the Brg1 hypomorphic mutant mice exhibit abnormal definitive erythroid cell differentiation, which very much resembles to the phenotype observed in EKLF KO mice (Bultman et al, 2005). Since BRG1 recruitment to the huβ-promoter in HPC is EKLF-dependent, we postulate that the influence of EKLF on huβ-gene potentiation is linked to chromatin remodeling. Chromatin remodeling activity increases huβ-promoter accessibility for transcriptional activators as exemplified by the situation for p45, whose binding to the huβ-promoter depends upon EKLF.
We previously identified histone H3 hyperacetylation as a hallmark of developmental-specific potentiation of the huβ-globin gene in HPC (Bottardi et al, 2003, 2005). We now provide strong evidence that in transgenic and human HPC, histone acetylation at HS2 and huβ-promoter correlates with CBP recruitment, which is likely to be facilitated by transcriptional activators. CBP detection at the huβ-promoter is impaired in ln2 EKLF−/− HPC and in ln2 p45−/− HPC. Since EKLF affects p45 recruitment in HPC, we propose that the influence of EKLF on CBP recruitment at huβ-promoter is mediated by p45. Indeed, even if EKLF can interact with p300 and CBP, it would not be able to directly recruit CBP to the locus (Zhang and Bieker, 1998). However, in EryC p45–CBP interaction (Hung et al, 2001) favors histone acetylation at the βmaj promoter (Johnson et al, 2001), suggesting that p45 can interact with CBP while bound to the locus.
p45 interactions with CBP (Hung et al, 2001) and TFII130 (Amrolia et al, 1997) favor PIC assembly and β-globin gene expression in MEL cells (Johnson et al, 2001; Sawado et al, 2001). We demonstrate that TBP recruitment at huβ-promoter is markedly affected in p45−/− HPC. Additionally, disruption of globin gene potentiation in ln2 p45−/− HPC is associated with a significant decrease in huβ-gene expression in ln2 p45−/− EryC, which is, on average, three-fold lower than in ln2 EryC (Supplementary Figure 8). The influence of p45 on huβ-gene expression is particularly intriguing because adult mouse α- and β-globin gene expression are not significantly modified in p45 KO mice (Shivdasani and Orkin, 1995; Supplementary Figure 8). The different consequences of p45 KO on the regulation of adult mouse and human β-globin gene may be explained by: (i) variations in the epigenetic regulation of the two β-globin loci during hematopoiesis (Bottardi et al, 2003); and (ii) the fact that in human and transgenic ln2 EryC the huβ-globin locus appears to reside in a chromosomal environment more restrictive for transcription compared with its murine counterpart (Forrester et al, 1990; Milot et al, 1996; Epner et al, 1998). Thus, we suggest that p45 is important for huβ-gene potentiation and, since p45 is also detected at HS2 and huγ-promoters, possibly also for locus activation in HPC.
MafK can dimerize with different partners (Blank and Andrews, 1997). For instance, p45 is replaced by Bach1 to form a repressive Bach1/MafK complex in erythroid precursors (Brand et al, 2004), where p45 expression is mostly abolished (Anguita et al, 2004). In ln2 p45−/− HPC CBP recruitment and histone acetylation at huβ-promoter are impaired. Thus, MafK partners (if any) that replace p45 in these cells do not promote CBP recruitment and subsequent histone acetylation at the huβ-promoter.
GATA-1 and GATA-2 are expressed in HPC, but neither could be detected by ChIP at either HS2 or huβ-promoter in ln2 HPC (Figure 2C and data not shown). Thus, it appears that the aforementioned factors do not bind to these regions in HPC. However, in HPC, GATA-1 and/or GATA-2 could bind to other regions along the locus or be components of multiprotein complexes (Anguita et al, 2004; Rodriguez et al, 2005), which might impede their immunoprecipitation by ChIP.
Although the absence of EKLF severely impairs p45, CBP and BRG1 recruitment to the huβ-promoter in HPC, chromatin organization and transcriptional activator recruitment are also affected at the βLCR HS2, which was chosen because of its role in chromatin activation and huβ-gene potentiation in HPC (Bottardi et al, 2005). In EryC, HS2 requires the presence of EKLF to participate in the active chromatin hub (Drissen et al, 2004). However, in EKLF KO EryC, DNase I sensitivity is specifically affected at HS3 (Wijgerde et al, 1996). Therefore, we also evaluated the influence of EKLF at HS3 and observed that in ln2 EKLF−/− HPC and ln2 EKLF−/− EryC BRG1 recruitment is affected at HS3 as well (data not shown). Thus, EKLF action at HS2 might be either direct or indirect and requires the contribution of HS3, particularly in EryC, where HS2 and HS3 have been found to functionally interact.
It has been observed that general transcription factors can bind the mouse λ5 locus in embryonic stem cells and that the recruitment of specific transcription factors occurs in B cells-restricted progenitors (Szutorisz et al, 2005). Here, we show that lineage-specific transcriptional activators are recruited at the huβ-globin locus in multipotent HPC and they participate in the locus potentiation. Altogether, our results indicate that: (i) EKLF is critical for the recruitment of p45, CBP and BRG1 at the huβ-promoter in HPC; and (ii) these transcriptional activators contribute to locus chromatin activation and huβ-gene potentiation in HPC. Based on this we propose a model (Figure 6) where basal-level expression of erythroid-specific transcriptional activators, for example, EKLF and p45, either sequentially or simultaneously, allow appropriate huβ-gene potentiation in freshly isolated human and transgenic HPC. Reminiscent of what has been found in other systems with distinct transcriptional activators (Agalioti et al, 2002; de la Serna et al, 2005), EKLF, p45 (NF-E2) and TBP could cooperate to induce or stabilize a potent chromatin necessary for the recruitment of additional transcriptional activators during differentiation and to support huβ-gene expression in EryC.
Figure 6.
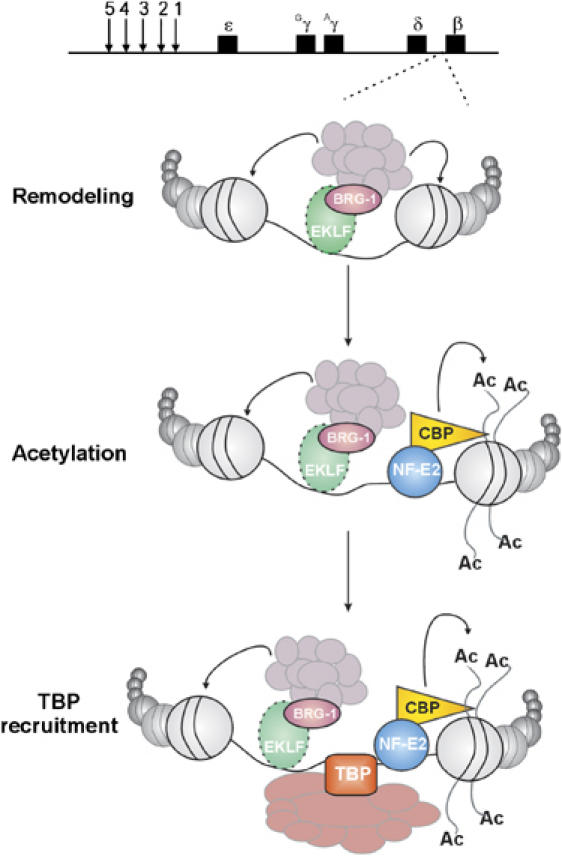
Model of huβ-globin gene potentiation in ln2 HPC. Schematic representation of huβ-gene potentiation in multipotent ln2 HPC (see Discussion for more details). Nucleosomes are represented by beads and transcriptional activators are indicated as colored objects.
TBP and Pol II association to the huβ-globin locus in HPC
We demonstrate for the first time that the absence of EKLF in HPC affects basal levels of huβ-gene expression and, to some extent, benefits huγ- over huβ-gene expression. This is also supported by the preferential recruitment of TBP at huβ-promoter in ln2 HPC, and at huγ-promoters in ln2 EKLF−/− HPC. Huβ- and huγ-promoters should not compete for βLCR interaction in hematopoietic progenitors (Palstra et al, 2003). However, EKLF might contribute to huγ-gene silencing in adult HPC. Indeed, EKLF can interact with Sin3A and histone deacetylase 1 (HDAC1) corepressors, and it can induce repression of a reporter gene in huγ-expressing cell lines (Chen and Bieker, 2004). A similar repression mechanism involving EKLF could take place in HPC.
Since Pol II is not detected at huβ- or huγ-promoters in HPC, it is likely that basal levels of huβ-gene expression result from a limiting number of Pol II molecules (difficult to detect by ChIP) engaged at the promoter at any given time, and also from stochastic Pol II–huβ-promoter interactions. Indeed, only a subset of ln2 HPC express huβ- or huγ-genes (Bottardi et al, 2003), a circumstance that might further affect ChIP sensitivity. We also demonstrate that Pol II is recruited at βLCR HS2 in multipotent HPC. This observation is reminiscent of what has been observed at the mouse globin βLCR in EryC and cell lines (Johnson et al, 2001). The fact that Pol II binding to βLCR HS2 in ln2 EKLF−/− HPC is not significantly affected suggests that, as in the case of MEL cells (Johnson et al, 2003), Pol II–HS2 interaction occurs independent of histone acetylation/methylation patterns or transcriptional status.
Throughout this study, very similar observations were made in ln2 HPC versus human CD34+ cells. However, TBP and Pol II binding to huγ-promoters are, respectively, three- and four-fold higher in CD34+ cells than in ln2 HPC. This discrepancy is likely due to the fact that CD34+ cells were purified from apheresis samples of normal patients who underwent SCF and G-CSF treatment to increase CD34+ cell mobilization. SCF can induce γ-globin expression in adult human erythroblasts (Bhanu et al, 2005). Even though we did not detect a significant difference in huγ/huβ-gene basal levels of expression between CD34+ cells and ln2 HPC (data not shown), increased TBP and Pol II binding at huγ-promoters in CD34+ cells suggests that SCF might modify globin gene potentiation at early stages of hematopoiesis.
Chromatin organization of the huβ-globin locus in EryC
In 13.5 d.p.c. fetal liver EryC, globin expression is linked to GATA-1, p45, CBP, BRG1 and TBP recruitment at the locus, and to efficient Pol II CTD phosphorylation at the 3′ end of huβ-gene. Analyses of EKLF−/− EryC allowed us to highlight some of the molecular defects associated with EKLF ablation. Specifically, H3 acetylation/methylation, GATA-1, p45, CBP, BRG1 and TBP recruitment at HS2 and huβ-promoter are significantly affected. These results support and extend previous studies (Wijgerde et al, 1996; Armstrong et al, 1998) by suggesting that in EryC EKLF influences chromatin organization at the huβ-promoter and, to a lesser extent, at the βLCR. In addition, the delayed switching from huγ- to huβ-gene expression in the absence of EKLF (Perkins et al, 1996; Wijgerde et al, 1996) correlates well with increased TBP and Pol II binding to the huγ-promoters in ln2 EKLF−/− EryC. However, the fact that in ln2 EKLF−/− EryC hyperphosphorylated Pol II is absent at the 3′ end of the huβ-gene strongly suggests that transcriptional elongation is also impaired in these cells. Accordingly, it has been reported that EKLF is important for βLCR-βmaj promoter interactions (Drissen et al, 2004), which favor transcriptional elongation (Sawado et al, 2001).
In summary, we provide the first evidence that the lineage-associated transcriptional activators EKLF and p45, which are expressed at basal levels in multipotent progenitor cells, can be functional and involved in gene potentiation in multipotent HPC, that is, before transcriptional activation. Finally, based on the results presented here, we propose a model that recapitulates the significant ‘steps' in the chain of events required to activate chromatin and to render the huβ-globin gene and promoter permissive for transcriptional machinery assembly during hematopoiesis.
Materials and methods
Mouse transgenic lines
Homozygous ln2 mice (Strouboulis et al, 1992) were bred with CD1 females and ln2+/− 13.5 d.p.c. fetal livers were isolated. Otherwise, ln2+/+:EKLF+/− (Nuez et al, 1995) or ln2+/+:p45+/− (Shivdasani and Orkin, 1995) mice were crossed with EKLF+/− or p45+/− mice respectively and 13.5 d.p.c. ln2+/−:EKLF−/− or ln2+/−:p45−/− fetal livers were isolated.
HPC purification and cell culture
Human CD34+ cells were purified as previously described (Bottardi et al, 2003). Five up to 10 13.5 d.p.c. fetal livers were first incubated with rat anti-Ter119, -B220 and -Gr-1 antibodies (Ab) followed by anti-rat FITC-conjugated Ab. After a brief wash in PBS 5% heat inactivated rat serum, cells were stained with biotynilated anti-CD31 Ab followed by SAV-TC Ab. For in vitro clonal assays, HPC were plated onto MethoCult M3434 medium (StemCell Technology) and colony types were scored at days 3 and 14.
ChIP and duplex PCR analyses
ChIP assays were carried out using at least 3 × 105 HPC or 106 CD34+ cells or EryC, as per the manufacturer's instruction (Upstate Biotechnology) and chromatin was reduced in size by sonication in order to obtain fragments of 400-bp average size. Antibodies were raised against acetylated histone H4, H3 or dimethylated (K4) histone H3 (Upstate Biotechnology); TBP (SI-1), NF-E2 (C-19), GATA-1 (N6), Pol II (N-20), CBP (A-22) and BRG1 (H-88) (Santa Cruz); or Ser2 phosphorylayed Pol II (H5) (Covance). For the latter, chromatin preclearing was carried out with anti-mouse IgM antibodies coupled to agarose beads (Sigma). On average, 1/30 of each ChIP sample was used in quantitative duplex PCR, as previously described (Bottardi et al, 2003). Primer sequences are available on request.
RT–PCR and quantitative real-time PCR
Total RNA was extracted by Trizol (Invitrogen), treated with DNaseI-RNase free (Invitrogen) and used for cDNA synthesis with oligo(dT)15 primers and SuperScript Reverse Transcriptase (Invitrogen). Semiquantitative PCR was carried out with primers specific for murine SCL, GATA-1, p45, GATA-2, EKLF, GATA-3, C/EBPα, NPM and fetal human (γ) and mouse embryonic (βH1) globin transcripts (Epner et al, 1998) or adult human (β and δ) and mouse (βmin and βmaj) globin transcripts (Reik et al, 1998). Primer sequences are available on request.
Quantitative real-time RT–PCR analysis was carried out as in (Bottardi et al, 2005) with minor changes. Total RNA was treated with DNaseI-RNase free (Invitrogen) and used for cDNA synthesis with oligo(dT)15 primers and SuperScript Reverse Transcriptase (Invitrogen). Real-time PCR was performed with Qiagen QuantiTect probes specific for huβ- or huγ-globin cDNA. Mouse actin, GATA-1, p45, EKLF or βmin/βmaj globin cDNA were detected by Sybr Green (Bio-Rad). Primer sequences are available on request.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7
Supplementary Figure 8
Acknowledgments
We thank E Drobetsky, M Trudel and W de Laat for useful discussions and critical reading of the manuscript, G D'Angelo and J Hebert for Wright–Giemsa staining analysis, DC Roy for human apheresis samples, RA Shivdasani for kindly providing p45 knock-out mice and V Bourgoin and A Orimoto for technical assistance. VB is supported by the CIHR. EM is a scholar of the CIHR and this work was supported by a grant from the CIHR to EM.
References
- Agalioti T, Chen G, Thanos D (2002) Deciphering the transcriptional histone acetylation code for a human gene. Cell 111: 381–392 [DOI] [PubMed] [Google Scholar]
- Akashi K, Traver D, Miyamoto T, Weissman IL (2000) A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404: 193–197 [DOI] [PubMed] [Google Scholar]
- Amrolia PJ, Ramamurthy L, Saluja D, Tanese N, Jane SM, Cunningham JM (1997) The activation domain of the enhancer binding protein p45NF-E2 interacts with TAFII130 and mediates long-range activation of the alpha- and beta-globin gene loci in an erythroid cell line. Proc Natl Acad Sci USA 94: 10051–10056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews NC, Erdjument-Bromage H, Davidson MB, Tempst P, Orkin SH (1993) Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature 362: 722–728 [DOI] [PubMed] [Google Scholar]
- Anguita E, Hughes J, Heyworth C, Blobel GA, Wood WG, Higgs DR (2004) Globin gene activation during haemopoiesis is driven by protein complexes nucleated by GATA-1 and GATA-2. EMBO J 23: 2841–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JA, Bieker JJ, Emerson BM (1998) A SWI/SNF-related chromatin remodeling complex, E-RC1, is required for tissue-specific transcriptional regulation by EKLF in vitro. Cell 95: 93–104 [DOI] [PubMed] [Google Scholar]
- Bender MA, Bulger M, Close J, Groudine M (2000) Beta-globin gene switching and DNase I sensitivity of the endogenous beta-globin locus in mice do not require the locus control region. Mol Cell 5: 387–393 [DOI] [PubMed] [Google Scholar]
- Bhanu NV, Trice TA, Lee YT, Gantt NM, Oneal P, Schwartz JD, Noel P, Miller JL (2005) A sustained and pancellular reversal of gamma-globin gene silencing in adult human erythroid precursor cells. Blood 105: 387–393 [DOI] [PubMed] [Google Scholar]
- Bieker JJ (2001) Kruppel-like factors: three fingers in many pies. J Biol Chem 276: 34355–34358 [DOI] [PubMed] [Google Scholar]
- Blank V, Andrews NC (1997) The Maf transcription factors: regulators of differentiation. Trends Biochem Sci 22: 437–441 [DOI] [PubMed] [Google Scholar]
- Blobel GA, Nakajima T, Eckner R, Montminy M, Orkin SH (1998) CREB-binding protein cooperates with transcription factor GATA-1 and is required for erythroid differentiation. Proc Natl Acad Sci USA 95: 2061–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifer C (1999) Long-distance chromatin mechanisms controlling tissue-specific gene locus activation. Gene 238: 277–289 [DOI] [PubMed] [Google Scholar]
- Bottardi S, Aumont A, Grosveld F, Milot E (2003) Developmental stage-specific epigenetic control of human beta-globin gene expression is potentiated in hematopoietic progenitor cells prior to their transcriptional activation. Blood 102: 3989–3997 [DOI] [PubMed] [Google Scholar]
- Bottardi S, Bourgoin V, Pierre-Charles N, Milot E (2005) Onset and inheritance of abnormal epigenetic regulation in hematopoietic cells. Hum Mol Genet 14: 493–502 [DOI] [PubMed] [Google Scholar]
- Brand M, Ranish JA, Kummer NT, Hamilton J, Igarashi K, Francastel C, Chi TH, Crabtree GR, Aebersold R, Groudine M (2004) Dynamic changes in transcription factor complexes during erythroid differentiation revealed by quantitative proteomics. Nat Struct Mol Biol 11: 73–80 [DOI] [PubMed] [Google Scholar]
- Bultman SJ, Gebuhr TC, Magnuson T (2005) A Brg1 mutation that uncouples ATPase activity from chromatin remodeling reveals an essential role for SWI/SNF-related complexes in beta-globin expression and erythroid development. Genes Dev 19: 2849–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor AB, Orkin SH (2002) Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene 21: 3368–3376 [DOI] [PubMed] [Google Scholar]
- Carter D, Chakalova L, Osborne CS, Dai YF, Fraser P (2002) Long-range chromatin regulatory interactions in vivo. Nat Genet 32: 623–626 [DOI] [PubMed] [Google Scholar]
- Chen X, Bieker JJ (2004) Stage-specific repression by the EKLF transcriptional activator. Mol Cell Biol 24: 10416–10424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daftari P, Gavva NR, Shen CK (1999) Distinction between AP1 and NF-E2 factor-binding at specific chromatin regions in mammalian cells. Oncogene 18: 5482–5486 [DOI] [PubMed] [Google Scholar]
- de la Serna IL, Ohkawa Y, Berkes CA, Bergstrom DA, Dacwag CS, Tapscott SJ, Imbalzano AN (2005) MyoD targets chromatin remodeling complexes to the myogenin locus prior to forming a stable DNA-bound complex. Mol Cell Biol 25: 3997–4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drissen R, Palstra RJ, Gillemans N, Splinter E, Grosveld F, Philipsen S, de Laat W (2004) The active spatial organization of the beta-globin locus requires the transcription factor EKLF. Genes Dev 18: 2485–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drissen R, von Lindern M, Kolbus A, Driegen S, Steinlein P, Beug H, Grosveld F, Philipsen S (2005) The erythroid phenotype of EKLF-null mice: defects in hemoglobin metabolism and membrane stability. Mol Cell Biol 25: 5205–5214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epner E, Reik A, Cimbora D, Telling A, Bender MA, Fiering S, Enver T, Martin DI, Kennedy M, Keller G, Groudine M (1998) The beta-globin LCR is not necessary for an open chromatin structure or developmentally regulated transcription of the native mouse beta-globin locus. Mol Cell 2: 447–455 [DOI] [PubMed] [Google Scholar]
- Feng D, Kan YW (2005) The binding of the ubiquitous transcription factor Sp1 at the locus control region represses the expression of beta-like globin genes. Proc Natl Acad Sci USA 102: 9896–9900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester WC, Epner E, Driscoll MC, Enver T, Brice M, Papayannopoulou T, Groudine M (1990) A deletion of the human beta-globin locus activation region causes a major alteration in chromatin structure and replication across the entire beta-globin locus. Genes Dev 4: 1637–1649 [DOI] [PubMed] [Google Scholar]
- Forsberg EC, Downs KM, Bresnick EH (2000) Direct interaction of NF-E2 with hypersensitive site 2 of the beta-globin locus control region in living cells. Blood 96: 334–339 [PubMed] [Google Scholar]
- Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH (1996) Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc Natl Acad Sci USA 93: 12355–12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T (2002) Differentiation plasticity of hematopoietic cells. Blood 99: 3089–3101 [DOI] [PubMed] [Google Scholar]
- Grosveld F, van Assendelft GB, Greaves DR, Kollias G (1987) Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell 51: 975–985 [DOI] [PubMed] [Google Scholar]
- Hong W, Nakazawa M, Chen YY, Kori R, Vakoc CR, Rakowski C, Blobel GA (2005) FOG-1 recruits the NuRD repressor complex to mediate transcriptional repression by GATA-1. EMBO J 24: 2367–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Krause D, Greaves M, Sharkis S, Dexter M, Heyworth C, Enver T (1997) Multilineage gene expression precedes commitment in the hemopoietic system. Genes Dev 11: 774–785 [DOI] [PubMed] [Google Scholar]
- Hung HL, Kim AY, Hong W, Rakowski C, Blobel GA (2001) Stimulation of NF-E2 DNA binding by CREB-binding protein (CBP)-mediated acetylation. J Biol Chem 276: 10715–10721 [DOI] [PubMed] [Google Scholar]
- Jimenez G, Griffiths SD, Ford AM, Greaves MF, Enver T (1992) Activation of the beta-globin locus control region precedes commitment to the erythroid lineage. Proc Natl Acad Sci USA 89: 10618–10622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KD, Christensen HM, Zhao B, Bresnick EH (2001) Distinct mechanisms control RNA polymerase II recruitment to a tissue-specific locus control region and a downstream promoter. Mol Cell 8: 465–471 [DOI] [PubMed] [Google Scholar]
- Johnson KD, Grass JA, Park C, Im H, Choi K, Bresnick EH (2003) Highly restricted localization of RNA polymerase II within a locus control region of a tissue-specific chromatin domain. Mol Cell Biol 23: 6484–6493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S, McAlpine GS, Phelan ML, Kingston RE, Jones KA, Emerson BM (2000) Functional selectivity of recombinant mammalian SWI/SNF subunits. Genes Dev 14: 2441–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach KM, Vieira KF, Kang SH, Aslanian A, Teichmann M, Roeder RG, Bungert J (2003) Characterization of the human beta-globin downstream promoter region. Nucleic Acids Res 31: 1292–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milot E, Strouboulis J, Trimborn T, Wijgerde M, de Boer E, Langeveld A, Tan-Un K, Vergeer W, Yannoutsos N, Grosveld F, Fraser P (1996) Heterochromatin effects on the frequency and duration of LCR-mediated gene transcription. Cell 87: 105–114 [DOI] [PubMed] [Google Scholar]
- Morley BJ, Abbott CA, Sharpe JA, Lida J, Chan-Thomas PS, Wood WG (1992) A single beta-globin locus control region element (5′ hypersensitive site 2) is sufficient for developmental regulation of human globin genes in transgenic mice. Mol Cell Biol 12: 2057–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagl NG Jr, Zweitzig DR, Thimmapaya B, Beck GR Jr, Moran E (2006) The c-myc gene is a direct target of mammalian SWI/SNF-related complexes during differentiation-associated cell cycle arrest. Cancer Res 66: 1289–1293 [DOI] [PubMed] [Google Scholar]
- Nuez B, Michalovich D, Bygrave A, Ploemacher R, Grosveld F (1995) Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature 375: 316–318 [DOI] [PubMed] [Google Scholar]
- Palstra RJ, Tolhuis B, Splinter E, Nijmeijer R, Grosveld F, de Laat W (2003) The beta-globin nuclear compartment in development and erythroid differentiation. Nat Genet 35: 190–194 [DOI] [PubMed] [Google Scholar]
- Perkins AC, Gaensler KM, Orkin SH (1996) Silencing of human fetal globin expression is impaired in the absence of the adult beta-globin gene activator protein EKLF. Proc Natl Acad Sci USA 93: 12267–12271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins AC, Sharpe AH, Orkin SH (1995) Lethal beta-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature 375: 318–322 [DOI] [PubMed] [Google Scholar]
- Pevny L, Simon MC, Robertson E, Klein WH, Tsai SF, D'Agati V, Orkin SH, Costantini F (1991) Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature 349: 257–260 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik A, Telling A, Zitnik G, Cimbora D, Epner E, Groudine M (1998) The locus control region is necessary for gene expression in the human beta-globin locus but not the maintenance of an open chromatin structure in erythroid cells. Mol Cell Biol 18: 5992–6000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez P, Bonte E, Krijgsveld J, Kolodziej KE, Guyot B, Heck AJ, Vyas P, de Boer E, Grosveld F, Strouboulis J (2005) GATA-1 forms distinct activating and repressive complexes in erythroid cells. EMBO J 24: 2354–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawado T, Igarashi K, Groudine M (2001) Activation of beta-major globin gene transcription is associated with recruitment of NF-E2 to the beta-globin LCR and gene promoter. Proc Natl Acad Sci USA 98: 10226–10231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani RA, Orkin SH (1995) Erythropoiesis and globin gene expression in mice lacking the transcription factor NF-E2. Proc Natl Acad Sci USA 92: 8690–8694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouboulis J, Dillon N, Grosveld F (1992) Developmental regulation of a complete 70-kb human beta-globin locus in transgenic mice. Genes Dev 6: 1857–1864 [DOI] [PubMed] [Google Scholar]
- Struhl K (2005) Transcriptional activation: mediator can act after preinitiation complex formation. Mol Cell 17: 752–754 [DOI] [PubMed] [Google Scholar]
- Szutorisz H, Canzonetta C, Georgiou A, Chow CM, Tora L, Dillon N (2005) Formation of an active tissue-specific chromatin domain initiated by epigenetic marking at the embryonic stem cell stage. Mol Cell Biol 25: 1804–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W (2002) Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol Cell 10: 1453–1465 [DOI] [PubMed] [Google Scholar]
- Vakoc CR, Letting DL, Gheldof N, Sawado T, Bender MA, Groudine M, Weiss MJ, Dekker J, Blobel GA (2005) Proximity among distant regulatory elements at the beta-globin locus requires GATA-1 and FOG-1. Mol Cell 17: 453–462 [DOI] [PubMed] [Google Scholar]
- Wijgerde M, Gribnau J, Trimborn T, Nuez B, Philipsen S, Grosveld F, Fraser P (1996) The role of EKLF in human beta-globin gene competition. Genes Dev 10: 2894–2902 [DOI] [PubMed] [Google Scholar]
- Xue L, Chen X, Chang Y, Bieker JJ (2004) Regulatory elements of the EKLF gene that direct erythroid cell-specific expression during mammalian development. Blood 103: 4078–4083 [DOI] [PubMed] [Google Scholar]
- Ye M, Iwasaki H, Laiosa CV, Stadtfeld M, Xie H, Heck S, Clausen B, Akashi K, Graf T (2003) Hematopoietic stem cells expressing the myeloid lysozyme gene retain long-term, multilineage repopulation potential. Immunity 19: 689–699 [DOI] [PubMed] [Google Scholar]
- Zhang W, Bieker JJ (1998) Acetylation and modulation of erythroid Kruppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc Natl Acad Sci USA 95: 9855–9860 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7
Supplementary Figure 8


