Abstract
Rho GTPases are key regulators of the actin cytoskeleton in membrane trafficking events. We previously reported that Cdc42 facilitates exocytosis in neuroendocrine cells by stimulating actin assembly at docking sites for secretory granules. These findings raise the question of the mechanism activating Cdc42 in exocytosis. The neuronal guanine nucleotide exchange factor, intersectin-1L, which specifically activates Cdc42 and is at an interface between membrane trafficking and actin dynamics, appears as an ideal candidate to fulfill this function. Using PC12 and chromaffin cells, we now show the presence of intersectin-1 at exocytotic sites. Moreover, through an RNA interference strategy coupled with expression of various constructs encoding the guanine nucleotide exchange domain, we demonstrate that intersectin-1L is an essential component of the exocytotic machinery. Silencing of intersectin-1 prevents secretagogue-induced activation of Cdc42 revealing intersectin-1L as the factor integrating Cdc42 activation to the exocytotic pathway. Our results extend the current role of intersectin-1L in endocytosis to a function in exocytosis and support the idea that intersectin-1L is an adaptor that coordinates exo–endocytotic membrane trafficking in secretory cells.
Keywords: Cdc42, endocrine cells, exocytosis, intersectin, secretion
Introduction
In most secretory cells, actin forms a dense network of filaments beneath the plasma membrane. Activation of secretion triggers a fine reorganization of these peripheral actin filaments in order to allow access of the granules to the exocytotic sites (Sontag et al, 1988; Vitale et al, 1995). Rho GTPases are key regulators of the actin cytoskeletal organization in a wide range of membrane trafficking aspects (Ridley, 2001). In neuroendocrine chromaffin and PC12 cells, we previously described that reorganization of actin at the site of exocytosis is under the combined control of two members of the Rho family, RhoA bound to secretory granules (Gasman et al, 1997, 1998) and Cdc42 present at the plasma membrane (Gasman et al, 2004). Experiments based on the expression of constitutively active GTP-loaded mutants led to the observation that RhoA and Cdc42 have negative and positive influences, respectively, on the exocytotic machinery (Bader et al, 2004; Gasman et al, 2004). We investigated further the molecular pathway integrating Cdc42 to exocytosis and found that in secretagogue-stimulated PC12 cells, activated Cdc42 increased the secretory response through a mechanism involving the neural Wiskott–Aldrich syndrome protein (N-WASP) and the formation of actin filaments under the plasma membrane (Gasman et al, 2004). These findings raised the question of the upstream events leading to the activation of Cdc42 in the course of exocytosis. Hence, by integrating various cellular signals to the activation of Cdc42, candidates for the regulation of Cdc42 guanine nucleotide exchange activity are ideally suited to spatially and temporally coordinate the actin cytoskeletal rearrangements to the membrane trafficking events underlying exocytosis.
Rho family guanine nucleotide exchange factors (GEFs) are characterized by a conserved catalytic domain, the Dbl homology (DH) domain, catalyzing the release of GDP from Rho GTPases and their subsequent activation by GTP binding (Rossman et al, 2005). In addition to the DH domain, the vast majority of Dbl family proteins possesses an adjacent pleckstrin homology (PH) domain that is thought to influence their catalytic activity (Rossman et al, 2003). Intersectin-1L is a Rho family GEF that is mainly expressed in neurons and contains, in addition to the DH–PH domains, two N-terminal Eps15 homology domains (EH1 and EH2), a central coiled-coil region, five Src homology 3 (SH3) domains and a carboxy-terminal C2 domain (Guipponi et al, 1998). In vertebrates, there is a shorter splice variant of the protein, designated intersectin-1S, which is ubiquitously expressed and which lacks the tandem DH/PH and the C2 domains (Guipponi et al, 1998; Roos and Kelly, 1998; Yamabhai et al, 1998; Okamoto et al, 1999). Because of its interactions with dynamin (Hussain et al, 1999; Okamoto et al, 1999; Predescu et al, 2003; Sengar et al, 1999) and other endocytic proteins like Eps15 (Sengar et al, 1999), synaptojanin (Yamabhai et al, 1998), SCAMP1 (Fernandez-Chacon et al, 2000) and stonin/stoned-B (Martina et al, 2001; Kelly and Phillips, 2005), intersectin-1 has been mainly proposed to function as a scaffolding protein regulating the assembly of multi-protein complexes at the sites of clathrin-mediated endocytosis or caveolae-induced internalization (Predescu et al, 2003). Such a role has been confirmed using in vitro endocytosis assays (Simpson et al, 1999) and more recently through analysis of Drosophila null mutants (Koh et al, 2004; Marie et al, 2004).
We reasoned that intersectin-1L would be an interesting candidate as the activator of Cdc42 responsible for the release of secretory granules for several reasons. First, intersectin-1L promotes GDP/GTP exchange on Cdc42, but not on Rac1 or RhoA (Hussain et al, 2001; Karnoub et al, 2001). Second, intersectin-1 has been reported to interact not only with endocytotic proteins, but also with SNAP-25, a protein of the exocytotic machinery that is critical for the docking of vesicles to the plasma membrane (Okamoto et al, 1999). This led to the idea that intersectin-1 may function as a scaffolding protein that participates in coupling exo- and endocytosis. Finally, N-WASP, the effector through which Cdc42 stimulates exocytosis in neuroendocrine cells (Gasman et al, 2004), binds intersectin-1L and upregulates its GEF activity (Hussain et al, 2001).
The present study provides the first direct evidence of a role for intersectin-1L in exocytosis. Intersectin-1L is present at the site of exocytosis of dense-core secretory granules in chromaffin and PC12 cells. Using a variety of direct means, we demonstrate that Cdc42 activation is an essential step in the exocytotic process and we provide evidence that intersectin-1L is the GEF promoting nucleotide exchange and activation of Cdc42 in response to secretagogues. We propose that intersectin-1L is a key component of the pathway that activates Cdc42-dependent reactions and thereby links extracellular signals to the actin rearrangements required for exocytosis.
Results
Calcium-regulated exocytosis requires Cdc42
We recently demonstrated that the constitutively active Cdc42Q61L mutant was able to stimulate secretion from PC12 cells by recruiting N-WASP to the plasma membrane and providing actin filaments to the exocytotic sites (Gasman et al, 2004). These findings suggested but did not prove that Cdc42 represents a true component of the basic exocytotic machinery. To address this question, we studied the functional importance of endogenous Cdc42 in PC12 cell exocytosis using a short interference RNA (siRNA) strategy (McManus and Sharp, 2002). SiRNAs can be generated in mammalian cells with plasmids that direct the transcription of short hairpin RNAs (Brummelkamp et al, 2002). In the present experiments, we employed a plasmid (pGHsuper) that expresses full-length human growth hormone (GH) protein and allows transcription of the siRNA targeted against the sequence of Cdc42. GH specifically stored into secretory granules can be used as a reporter for exocytosis in the subpopulation of cells that transiently express the siRNAs (Vitale et al, 2005).
Transfected PC12 cells were first assessed by Western blot. Transient expression of the Cdc42 siRNA selectively reduced the level of Cdc42 but did not affect the level of Rac1, a related Rho GTPase (Figure 1A). Densitometry scans from five independent experiments revealed that the level of endogenous Cdc42 was reduced by ∼75%, a decrease that was consistent with the efficiency of transfection (60–80% of the cells were transfected using electroporation). Although the available Cdc42 antibodies work for Western blot, they are not effective for immunofluorescence. We thus probed the silencing of the siRNA on cells expressing GFP-Cdc42. Expression of GFP-Cdc42 was almost completely blocked in GH-positive PC12 cells containing the siRNAs (Figure 1B), a result that was also confirmed by Western blot (Supplementary Figure 1).
Figure 1.

Reduction of endogenous Cdc42 by RNA interference inhibits GH secretion from PC12 cells. PC12 cells were transfected with the pGHsuper vector or with the Cdc42-shRNA plasmid (siRNA) for 72 h. (A) Cells were lysed and aliquots (40 μg of proteins) were used for electrophoresis and Western blot analysis using monoclonal anti-Cdc42, anti-Rac1 and anti-actin antibodies. (B) PC12 cells coexpressing Cdc42-GFP and pGHsuper or Cdc42-siRNA (siRNA) were fixed and stained with anti-GH antibodies. GH antibodies were visualized with Alexa 555-conjugated secondary antibodies. Bar, 5 μm. (C) Transfected PC12 cells were washed and subsequently incubated for 10 min in calcium-free Locke's solution (Basal) or stimulated for 10 min with 59 mM K+ (K+-stimulated). Data are given as the mean values±s.e.m. obtained from three independent experiments performed on three different cell cultures (n=3). ***P<0.001 (Student's t-test).
We then examined the effect of Cdc42 silencing on GH secretion from PC12 cells. Expression of Cdc42 siRNAs did not modify the expression level of GH (not shown) nor did it affect the distribution of GH in secretory granules (Figure 1B). However, reduction of endogenous Cdc42 significantly inhibited the secretion of GH stimulated by a depolarizing concentration of K+ (Figure 1C). These findings are in line with the idea that Cdc42 plays an essential function in dense-core granule exocytosis and prompted us to investigate the upstream molecular event leading to Cdc42 activation in secretagogue-stimulated cells.
Expression and subcellular localization of intersectin-1 proteins in chromaffin and PC12 cells
Reverse transcription–PCR and immunoblot analysis revealed that both intersectin-1L and intersectin-1S are expressed in chromaffin and PC12 cells (Supplementary Figure 2). The subcellular localization of intersectin-1 in chromaffin cells was assessed by immunofluorescence and confocal microscopy, using a polyclonal antibody raised against the EH domain of frog intersectin-1, which has been successfully used to localize mammalian intersectins in various cell types (Hussain et al, 1999). As illustrated in Figure 2A (left panel), intersectin-1 immunoreactivity appeared as small patches 175–400 nm in diameter in the cell periphery, and to a lesser extent as a diffuse staining in the cytoplasm. Polar confocal sections in the plane of the plasma membrane (Figure 2A, middle and right panels) and three-dimensional reconstitution (Supplementary Movie 1) demonstrated that the majority of the patches/clusters of intersectin-1 were restricted to the cell periphery, suggesting an association with the plasma membrane, consistent with Hussain et al (1999). To confirm this hypothesis, we performed double labeling experiments with antibodies against the plasma membrane marker SNAP25. Most of the intersectin-1 patches in periphery colocalized with SNAP25, in agreement with the presence of intersectin-1 at the plasma membrane in chromaffin cells (Figure 2B). Endogenous intersectin-1 was also detected at the plasma membrane in PC12 cells (not shown). In both cell types, the distribution of intersectin-1 in resting versus secretagogue-stimulated cells was not modified (Figure 3).
Figure 2.
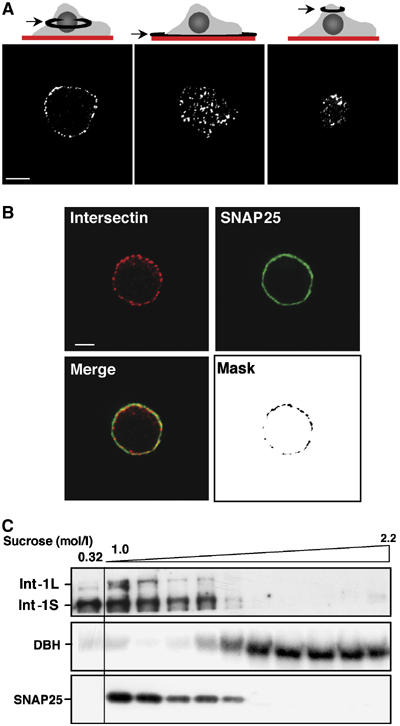
Endogenous intersectin-1 forms plasma membrane-associated patches in chromaffin cells. (A) Equatorial (left panel) and polar (middle and right panels) optical confocal sections of fixed chromaffin cells stained with anti-intersectin-1 antibodies and Alexa 555-conjugated secondary antibodies. Bar, 5 μm. (B) Confocal images obtained by labeling chromaffin cells with anti-intersectin-1 antibodies visualized with Alexa 555-conjugated secondary antibodies and monoclonal anti-SNAP25 antibodies visualized with Alexa 488-conjugated secondary antibodies. Mask representing the regions of intersectin-1/SNAP25 colocalization was generated by selecting the double-labeled pixels. Bar, 5 μm. (C) Distribution of intersectin-1 in subcellular fractions from bovine adrenal medulla. A total of 11 fractions (20 μg of protein/fraction) collected from a 10 ml continuous sucrose density gradient (1–2.2 M sucrose) layered with 1 ml crude chromaffin cell membranes (suspended in 0.32 M sucrose) were subjected to gel electrophoresis and immunodetection on nitrocellulose sheets using anti-SNAP25 antibodies to detect plasma membranes, anti-DBH to detect chromaffin granules and anti-intersectin-1 antibodies.
Figure 3.
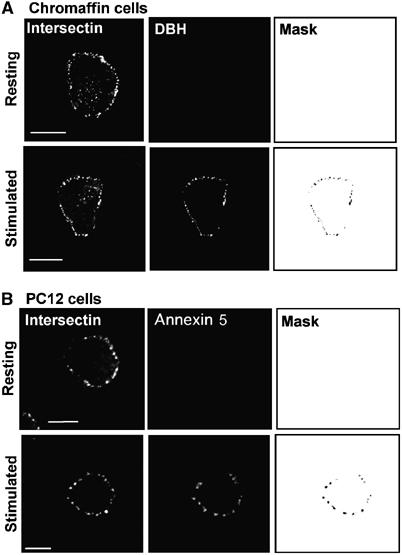
Intersectin-1 clusters at the plasma membrane colocalize with the exocytotic sites in stimulated chromaffin and PC12 cells. (A) Chromaffin cells maintained in resting conditions or stimulated for 10 min with 10 μM nicotine were incubated for 1 h at 4°C in the presence of rat anti-DBH antibodies to detect exocytotic sites. Cells were then fixed and processed for immunofluorescence with the anti-intersectin-1 antibodies and analyzed by confocal microscopy. DBH and intersectin-1 staining were revealed with Alexa 488-conjugated anti-rat antibodies and Alexa 555-conjugated anti-rabbit antibodies, respectively. (B) PC12 cells were maintained in resting conditions or stimulated for 10 min with 59 mM K+ in the presence of Alexa 568-conjugated annexin 5. Cells were then fixed and stained with the anti-intersectin-1 antibodies. Masks were generated by selecting the double-labeled pixels. Bar, 5 μm.
Finally, we analyzed the distribution of intersectin-1 by subcellular fractionation of bovine adrenal medulla (Figure 2C). Both intersectin-1S and intersectin-1L were predominantly localized in the upper fractions of a sucrose density gradient layered with a crude membrane preparation. These fractions were enriched in SNAP25 immunoreactivity, consistent with the plasma membrane localization of the intersectin proteins. Note that the intersectins were not detected in gradient fractions containing secretory granules identified by the presence of dopamine β-hydroxylase (DBH). Taken together, these findings suggest that intersectin-1 is predominantly associated to the plasma membrane in chromaffin and PC12 cells.
Intersectin-1 is a functional element of the exocytotic machinery in chromaffin and PC12 cells
As intersectin-1 is not localized homogeneously throughout the plasma membrane but rather at specific region of the membrane, it was tempting to speculate that these areas could represent hot spots of exocytosis in which Cdc42 needs to be activated. To test this hypothesis, we compared the distribution of intersectin-1 with the exocytotic spots that appear in cells activated with a secretagogue. Exocytosis in living cells can be visualized by fluorescence in chromaffin and PC12 cells with either anti-DBH antibodies (Gasman et al, 1997) or fluorescent annexin 5 (Vitale et al, 2001), present in the cell incubation medium before fixation. In chromaffin cells, the granule-associated DBH becomes accessible to the antibody only at sites of exocytosis, thus leading to the appearance of fluorescent patches at the cell surface. Similarly, the phosphatidylserine-binding protein annexin 5 reveals the exocytotic sites where granules are transiently inserted into the plasma membrane, as phosphatidylserine is concentrated on the inner face of the granule membrane. As illustrated in Figure 3, resting chromaffin (Figure 3A) and PC12 cells (Figure 3B) displayed intersectin-1 clusters at the plasma membrane but no DBH or annexin 5 patches, indicating the low level of baseline exocytotic activity in the absence of secretagogues. Stimulation of the cells triggered the appearance of a patchy pattern of DBH or annexin 5 surface staining corresponding to the exocytotic sites (Figure 3A and B). Interestingly, a large proportion of the exocytotic hot spots revealed in stimulated cells (see mask in Figure 3A and B) contained intersectin-1, in line with the idea that intersectin-1 plays a role in the exocytotic process. Quantification of the proportion of DBH or annexin 5 signals that colocalized with intersectin-1 indicated that 92±3.2% of the total DBH immunoreactivity and 95±2.6% of the total annexin 5 signal were associated with intersectin-1 in stimulated cells (n=25 cells).
To directly establish the implication of intersectin-1 in exocytosis, we took advantage of the short hairpin RNA approach. PC12 cells were transfected with a plasmid that expresses an siRNA targeted against the sequence of the EH domain of intersectin-1 and GH. The intersectin silencing efficiency was verified by immunofluorescence, as transfected cells can be identified by the expression of GH. Cells expressing the intersectin siRNAs displayed significantly lower levels of intersectin-1 immunoreactivity in the cell periphery (Figure 4A). Fluorescence quantification (Figure 4B) and Western blot analysis of total cell extracts (Figure 4C) revealed that the level of endogenous intersectin-1 in cells expressing siRNAs was decreased by ∼80%. The role of intersectin-1 in exocytosis was first estimated by measuring GH secretion in response to high K+. Expression of intersectin siRNAs did not reduce the overall level of GH protein (see Figure 6A), nor did it affect the cellular distribution of GH-containing secretory granules (Figure 4A). However, selective knockdown of intersectin-1 by siRNAs inhibited the amount of GH secreted in response to stimulation by 59 mM K+ (Figure 4D), in line with a possible role for intersectin-1 in granule exocytosis.
Figure 4.

Intersectin-1 is involved in regulated exocytosis in PC12 cells. PC12 cells were transfected with pGHsuper or pGHsuper encoding intersectin shRNA (siRNA) for 72 h. (A) Cells were fixed and stained with anti-GH antibodies revealed by Alexa 488-labeled anti-rabbit antibodies. Cells were then post-fixed, stained with anti-intersectin-1 antibodies, visualized with Alexa 555-conjugated anti-rabbit antibodies and analyzed by confocal microscopy. Transfected cells are indicated with an asterisk. Bar, 5 μm. (B) Histogram representing a semiquantitative analysis of intersectin-1 immunoreactivity detected in non-transfected cells (NT), in pGHsuper-transfected cells and in cells expressing intersectin siRNAs. n=25 cells. (C) Transfected cells were lysed and aliquots (40 μg of proteins) were used for electrophoresis and Western blot analysis using anti-intersectin-1 and anti-actin antibodies. (D) Assay for GH release activity. Cells were washed and subsequently incubated for 10 min in Locke's solution (Basal) or stimulated for 10 min with 59 mM K+ (K+-stimulated). Data are given as the mean values±s.e.m. obtained in three experiments performed on three different cell cultures (n=3). #P>0.05, ***P<0.001 (Student's t-test). (E) Assay for exocytosis using fluorescent annexin 5. PC12 cells transfected with pEGFP-RNAi encoding intersectin shRNA (siRNA) were maintained in resting conditions or stimulated for 10 min with 59 mM K+ in the presence of Alexa 568-conjugated annexin 5. Non-transfected cells (GFP negative) are indicated with an asterisk. Bar, 5 μm. (F) Semiquantitative analysis of annexin 5 binding detected in resting and K+-stimulated conditions in non-transfected cells (NT), in pEGFP-RNAi transfected cells and in cells expressing intersectin siRNAs. n=25 cells for each experimental condition.
Figure 6.
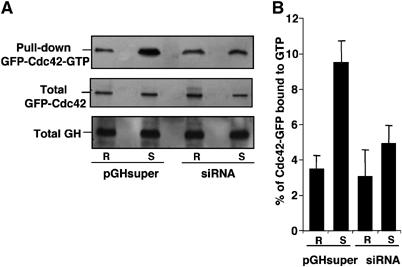
Intersectin-1L mediates secretagogue-induced Cdc42 activation in PC12 cells. (A) GTP-loaded Cdc42 pull-down assay in resting and stimulated PC12 cells coexpressing GFP-Cdc42 with either pGHsuper or pGHsuper encoding intersectin siRNA (siRNA). Cells were maintained in Locke's solution (R) or stimulated with 59 mM K+ (S) for 10 min. Cells were then immediately lysed by addition of ice-cold lysis buffer, and the lysates (700 μg) were used for affinity precipitation of GTP-loaded Cdc42. Pulled down GFP-Cdc42-GTP, and total GFP-Cdc42 and GH found in lysates (1/50 of the total) were detected by immunoblotting using anti-GFP and anti-GH antibodies. (B) Semiquantitative analysis of GFP-Cdc42 activation in response to K+-evoked stimulation in control cells or cells expressing intersectin-1 siRNA. Values obtained by scanning densitometry analysis are given as the mean values±s.e.m. obtained from three independent experiments (n=3) and expressed as the percentage of GTP-loaded GFP-Cdc42 relative to the total GFP-Cdc42.
To further probe this idea, we decided to use another assay for calcium-regulated exocytosis. Therefore, we examined the effect of intersectin siRNAs on the exocytotic activity visualized and quantified by the appearance of fluorescent annexin 5 patches at the cell surface in stimulated PC12 cells. As illustrated in Figure 4E and F (and Supplementary Figure 3), expression of intersectin siRNAs did not trigger the appearance of fluorescent annexin 5 patches in resting cells but it efficiently inhibited annexin 5 binding in cells stimulated with elevated K+. Thus, reduction of endogenous intersectin-1 level did not trigger spontaneous exocytotic activity but it clearly impaired secretagogue-evoked exocytosis, in line with the idea that intersectin-1 plays a positive role in the exocytotic machinery.
Given the important role of Cdc42 and intersectin in endocytosis, we examined whether the inhibition of secretagogue-evoked GH secretion in cells expressing intersectin siRNAs could be the indirect consequence of a blockage of granule recycling. Therefore, K+-evoked GH release was measured in PC12 cells expressing Eps15 mutants (ED95/295 and DIII domain) or dynamin mutants (Dynamin 1 and 2-K44A) that have been demonstrated to block clathrin- and dynamin-mediated endocytosis, respectively (Damke et al, 1994; Benmerah et al, 1999). Expression of these different constructs in PC12 cells efficiently blocked transferrin uptake (not shown) and transferrin receptor internalization (Jarousse et al, 2003), but it did not affect K+-induced GH release (Supplementary Figure 4). Thus, the inhibition of GH secretion observed in cells with reduced endogenous intersectin-1 is most likely due to a blockage of the exocytotic machinery per se rather than to an inhibition of the endocytotic process. Altogether, these findings are consistent with the idea that intersectin-1 plays an essential function in the exocytotic pathway of large dense-core secretory granules. However, as the siRNA prevents the expression of both variant of intersectin-1, that is, intersectin-1S and intersectin-1L, the question of the isoform implicated in the exocytotic machinery remains to be answered.
Intersectin-1L functions as a Cdc42 activator in the exocytotic pathway
To determine whether intersectin-1S, intersectin-1L (Figure 5A) or both function in exocytosis, we first expressed the two isoforms as Flag-tagged proteins and examined their localization in PC12 cells. We found that Flag-intersectin-1L was exclusively localized at the cell periphery, whereas Flag-intersectin-1S localized both at the periphery and throughout the cytoplasm (Figure 5B). As for the endogenous intersectins, there was no difference in the distribution of Flag-tagged proteins between resting and K+-stimulated cells. We then examined the effect of the expression of these Flag-tagged intersectins on K+-evoked GH secretion. Both Flag-tagged intersectin-1S and intersectin-1L produced an ∼10% inhibition of GH release (not shown). This effect, possibly due to the sequestration of endogenous SNAP25 or N-WASP, known to bind intersectin-1 and to be important for exocytosis, did not allow for a conclusion regarding the participation of the intersectin isoforms in the exocytotic machinery.
Figure 5.
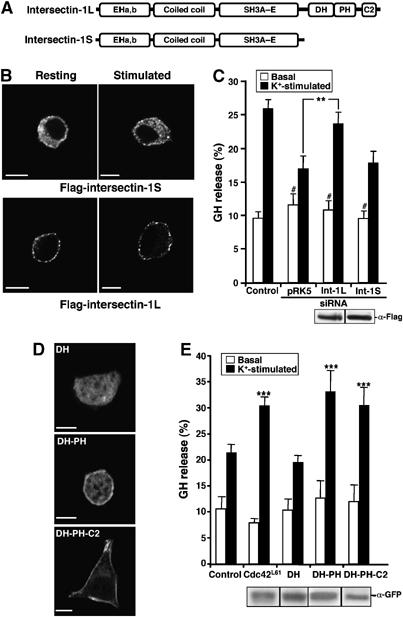
Human intersectin-1L restores exocytosis in PC12 cells depleted of endogenous intersectin-1. (A) Schematic representation of the intersectin-1 isoforms depicting the position of the various functional domains. EH, epsin homology domains; DH, Dbl homology domain; PH, pleckstrin homology domain. (B) Transfected PC12 cells expressing the indicated Flag-tagged human intersectin-1 variants were maintained under resting conditions or stimulated with 59 mM K+ and then processed for immunofluorescence using anti-Flag antibodies and Alexa 488-conjugated secondary antibodies. Bar, 5 μm. (C) Cells were cotransfected with pRK5 encoding the indicated Flag-tagged human intersectin-1 variants along with the pGHsuper encoding the shRNA for intersectin (siRNA). Control cells were cotransfected with pGHsuper and the empty pRK5 vector. At 72 h after transfection, cells were incubated for 10 min in Locke's solution (Basal) or stimulated for 10 min with 59 mM K+ (K+-stimulated) and processed for GH release assay. Data are given as the mean values±s.e.m. obtained from three independent experiments performed on three different cell cultures (n=3). **P<0.01, #P>0.05 compared to control cells (Student's t-test). The level of Flag-tagged construct expression is assessed by Western blot. (D, E) Intersectin-1L constructs displaying nucleotide exchange activity and targeted to the cell periphery stimulate exocytosis in PC12 cells. Cells were transfected with plasmids encoding the indicated GFP-tagged intersectin-1 constructs alone (D) or along with the plasmid encoding GH (E). At 48 h post-transfection, cells were processed for immunocytochemistry and confocal microscopy (bar, 5 μm) or GH release assay. ***P<0.001 compared to control cells (Student's t-test). The level of GFP-tagged construct expression is assessed by Western blot.
To further assess the importance of intersectin-1L versus intersectin-1S, we attempted to rescue the inhibition of GH release produced by the reduction of endogenous intersectin-1. Therefore, PC12 cells were cotransfected with the plasmid that expresses the intersectin-1 siRNAs and GH, and a plasmid coding for either the human Flag-tagged intersectin-1S or human Flag-tagged intersectin-1L. We took advantage of the human intersectins, which differ from the rat by two nucleotides in the sequence recognized by the siRNA and are insensitive to the siRNA as confirmed by immunofluorescence (not shown). We found that human intersectin-1L partially restored the secretory activity of PC12 cells depleted of endogenous intersectin-1, whereas human intersectin-1S had no significant effect (Figure 5C). Thus, intersectin-1L is most likely the form that plays a role in exocytosis. As the two intersectin variants differ only by their C-terminal region containing the GEF domain for Cdc42 (Figure 5A), these findings suggested that the function of intersectin-1 in the exocytotic machinery is likely related to the activation of Cdc42.
To probe directly the importance of the intersectin-1 GEF domain in the exocytotic response, we transfected PC12 cells with cDNAs encoding either the DH domain alone, or the DH–PH domains or the tail DH–PH–C2 and examined the effect of these constructs on K+-evoked GH secretion. The confocal images presented in Figure 5D show that the overexpressed DH domain displayed a predominant cytosolic distribution, whereas the DH–PH or the DH–PH–C2 associated to the cell periphery in K+-stimulated cells. Both DH–PH and DH–PH–C2 domains enhanced K+-evoked GH release whereas the DH domain alone failed to stimulate exocytosis (Figure 5E), most likely because DH was not properly targeted to the cell periphery and the exocytotic sites. Together, these results are consistent with a role for the guanine nucleotide exchange activity of intersectin-1L in its ability to regulate exocytosis.
We recently described that secretagogue-induced stimulation triggers the nucleotide exchange and activation of Cdc42 in PC12 cells (Gasman et al, 2004). In view of the present results, we decided to examine whether intersectin-1L is the GEF mediating the activation of Cdc42 in response to secretagogues. PC12 cells were cotransfected with a plasmid coding for GFP-Cdc42 and with the pGHsuper with or without siRNA for intersectin-1. Cells were maintained under resting conditions or were stimulated for 10 min with 59 mM K+, and GFP-Cdc42 activation was measured by a pull-down assay using the PAK1-binding domain as a bait to trap the GTPase in its GTP-bound form (Figure 6). Consistent with our previous observation (Gasman et al, 2004), stimulation with high K+ rapidly increased the level of GTP-loaded Cdc42 in control cells expressing only GH but not siRNA. In contrast, reduction of endogenous intersectin-1 by siRNA expression almost completely abolished the secretagogue-induced activation of Cdc42 (Figure 6A and B). These results strongly support the idea that intersectin-1L is the nucleotide exchange factor that catalyzes the activation of Cdc42 and turns on the Cdc42-dependent reactions required for exocytosis.
Discussion
Over the past years, the small GTPase Cdc42 has emerged as a key player in various membrane trafficking events that require dynamic regulation of the actin cytoskeleton (Cerione, 2004). In neuroendocrine cells, calcium-induced exocytosis is a tightly regulated process that requires coordinated interactions between membranes and the actin cytoskeleton (Bader et al, 2004). Actin forms a dense network of filaments just beneath the plasma membrane in these cells. Thus, a transient depolymerization of the subplasmalemmal actin cytoskeleton accompanies granule transport and fusion events (Sontag et al, 1988; Vitale et al, 1995) and actin was first described as a physical barrier that controls the access of secretory vesicles to their release sites at the plasma membrane (Aunis and Bader, 1988). More recently, evidence has emerged that actin is also an active player responsible for late stages in the exocytotic process (Lang et al, 2000; Gasman et al, 2004). Using chromaffin and PC12 cells, we previously described that activation of Cdc42 stimulates secretion by a pathway involving N-WASP, and the formation of actin structures at the interface between granules and the plasma membrane. This actin reorganization seems to render secretion more efficient most likely by facilitating a post-docking step of the exocytotic machinery (Gasman et al, 2004). Our results indicated that Cdc42 is able to maintain a sustained exocytotic activity, but they did not prove that the participation of Cdc42 is required for the basic exocytotic machinery. Indeed, expression of the dominant-negative Cdc42N17 mutant had only a modest inhibitory effect on secretion (Gasman et al, 2004). We show here that the secretory response is drastically inhibited in cells having reduced levels of endogenous Cdc42. Thus, activation of Cdc42 is a prerequisite for exocytosis in neuroendocrine cells. In line with these results, the active participation of Cdc42 in exocytosis has been described in mast cells and pancreatic β cells (Hong-Geller and Cerione, 2000; Nevins and Thurmond, 2003). These findings raise the question of the mechanisms of Cdc42 activation. Hence, if Cdc42 is an essential component of the exocytotic machinery and is activated in response to secretagogue-induced stimulation (Gasman et al, 2004), then there should be an exchange factor that specifically integrates Cdc42 to the molecular cascade coupling stimulus to secretion.
Intersectin-1L is a neuronal specific GEF for Cdc42 displaying various properties that make it an ideal candidate to functionally link Cdc42 activation to the exocytotic pathway. To investigate the functional importance of intersectin-1 in regulated exocytosis, we used a loss-of-function approach. We found that reduction of total intersectin-1 expression (S and L isoforms) using siRNA inhibited the exocytotic activity of PC12 cells. To discriminate between the two isoforms, we performed rescue experiments with cDNAs encoding human intersectin variants, which are insensitive to the siRNAs used. Only intersectin-1L was able to significantly restore the exocytotic activity. These results led us to conclude that intersectin-1L is the isoform that participates in large dense-core granule exocytosis. We further demonstrated a causal relationship between the intersectin-1L-mediated activation of Cdc42 and the exocytotic process by establishing that the GEF domain of intersectin-1L stimulates exocytosis and that reduction of endogenous intersectin-1 prevents secretagogue-evoked activation of Cdc42. From these results, we propose that intersectin-1L is the exchange factor responsible for the activation of Cdc42 in the course of neuroendocrine secretion. So far, the participation of intersectin-1L has been described in dendritic spine development (Irie and Yamaguchi, 2002), cellular transformation (Wang et al, 2005) and endocytosis (Hussain et al, 1999). To our knowledge, this is the first report demonstrating a functional role for intersectin-1L in a secretory process.
We observed that endogenous intersectin-1 localizes to specific regions of the plasma membrane, which most likely represent the active sites of exocytosis where secretory granules dock and fuse in stimulated cells. The mechanisms targeting intersectin-1 to these exocytotic sites remain to be investigated. As both intersectin-1S and intersectin-1L are found at the plasma membrane, the specific C-terminal region of intersectin-1L including the PH domain is probably not the major determinant for membrane localization. Indeed, it has been shown that EH domains are important for plasma membrane targeting (Hussain et al, 1999). Moreover, in endothelial cells, a large proportion of intersectin-1 is concentrated in caveolae (Predescu et al, 2003), which have been described as stably cholesterol-dependent assembled membrane domains (Tagawa et al, 2005). Interestingly, we have recently demonstrated that exocytosis in chromaffin cells occurs preferentially at cholesterol-enriched lipid rafts formed in the plasma membrane of stimulated cells (Chasserot-Golaz et al, 2005). Thus, intersectin-1L may be targeted to the exocytotic sites through its recruitment to the secretagogue-evoked lipid rafts required for regulated exocytosis.
PH domain may also to some extent play an important role in localizing intersectin-1L at the sites of exocytosis. Indeed, the sole intersectin-1L C-terminal constructs that stimulate exocytosis are those displaying the PH domain and found at the plasma membrane. Interestingly, phosphatidylinositol 4,5 bisphosphate (PI4,5P2) located at the plasma membrane is important for exocytosis in many cell types including chromaffin and PC12 cells and it has been proposed that the docking and fusion sites for exocytosis are defined as PI4,5P2-containing microdomains in the plasma membrane that allow structural and spatial organization of the secretory machinery (Holz et al, 2000; Cremona and De Camilli, 2001). Thus, intersectin-1L may also follow this route to enter into the exocytotic sites.
One particular feature of intersectin-1L is the regulation of its nucleotide exchange activity by N-WASP (Hussain et al, 2001), the effector by which Cdc42 controls actin organization in neuroendocrine cells (Gasman et al, 2004). In fact, the SH3 domain of intersectin-1L inhibits exchange activity through direct interaction with the DH domain and blockage of Cdc42 binding (Hussain et al, 2001; Zamanian and Kelly, 2003). It has been proposed that N-WASP binding to intersectin-1L enhances the ability of the DH domain to interact with GDP-bound Cdc42 and to catalyze its conversion to GTP-bound Cdc42 (Hussain et al, 2001). Thus, by regulating the enzymatic activity of intersectin-1L, N-WASP might participate in its own activation. Yet, the incorporation of GEFs and effectors within a protein complex has the advantage to couple the GTPase activation to its downstream effector. Although we did not study the functional importance of N-WASP in regulating intersectin-1L-mediated activation of Cdc42 during exocytosis, one possible speculation is that intersectin-1L positions a first subset of N-WASP molecules at the exocytotic sites of the plasma membrane. The complex intersectin-1L/N-WASP then creates clusters of activated Cdc42 specifically at granule docking sites, which subsequently recruit additional N-WASP molecules to form the actin filaments necessary for exocytosis (Gasman et al, 2004). Similar functional synergies involving GEF/effector complexes have been previously described, for instance between the Rab5 effector Rabaptin and the nucleotide exchange factor Rabex-5 (Lippe et al, 2001).
Finally, one of the most fascinating aspects of intersectin-1L is its multi-modular aspect allowing for interaction with various proteins, including SNAP25, which is essential for the interaction of secretory vesicles with the plasma membrane in exocytosis (Okamoto et al, 1999), Cdc42 and N-WASP regulating actin dynamics, and proteins of the endocytotic machinery like dynamin, Eps15 and synaptojanin. To keep a constant cell surface area, regulated exocytosis is followed by compensatory endocytosis (Smith and Neher, 1997). In neuroendocrine cells, the molecular mechanism by which this membrane balance is maintained and regulated is poorly understood. However, patch-clamp and imaging studies suggest that there is a spatial and temporal coupling of exocytosis with endocytosis (Artalejo et al, 1995; Smith and Neher, 1997; Tsuboi et al, 2000), which involves dynamin (Artalejo et al, 2002). Intersectin-1L promotes actin polymerization (Hussain et al, 2001; McGavin et al, 2001), is found in presynaptic peri-active zones in neurons (Hussain et al, 1999; Roos and Kelly, 1999) and, as shown here, is associated with the exocytotic sites in neuroendocrine cells. Therefore, it is tempting to speculate that intersectin-1L couples the activation of Cdc42 and the subsequent actin remodeling necessary for hormone release to the recruitment of the endocytotic machinery for granule membrane retrieval.
Materials and methods
Plasmids and short interference RNA
Flag-tagged human intersectin-1S and -1L and constructs encoding various domains of intersectin-1L tail region fused to GFP (Hussain et al, 2001), Cdc42 constructs (Gasman et al, 2004) as well as pGHsuper vector (Vitale et al, 2005) were as described previously. The sequences of siRNA, derived from the target transcript, were obtained from Ambion. Rat DNA fragments encoding the siRNA sequence of Cdc42 (GGGCAAGAGGATTATGACATT) and intersectin (GGCACAATCATTTGATGTATT) separated from its reverse complement by a short spacer were annealed and cloned in front of the H1-RNA promoter from the pGHsuper vector as previously described (Vitale et al, 2005). For annexin 5-binding experiments, the siRNA sequence of intersectin was cloned in the pEGFP-RNAi plasmid (kindly provided by Dr JP Borg, INSERM U-599, Marseille). Intersectin siRNA is targeted against both isoforms (-S and -L) of rat intersectin-1 and Cdc42 siRNA is targeted against both G25 K and Hs forms.
Culture and transfection
Chromaffin and PC12 cells culture conditions were as described (Gasman et al, 1997, 2004). Mammalian expression vectors were introduced into PC12 cells (24-well dishes, 1 × 105 cells, 0.5 μg/well of each plasmid) using GenePorter (Gene Therapy Systems) on adherent or suspension cells according to the manufacturer's instructions (5–25% of transfection efficiency). To check the efficiency of siRNA by immunoblotting, 3 μg of shRNA-plasmid was electroporated in 5 × 106 PC12 cells (Nucleofector, Amaxa). Under these conditions, the transfection efficiency reached 60–80%.
Growth hormone release from PC12 Cells
GH release experiments were performed 48 h/72 h after transfection. PC12 cells were washed twice with Locke's solution (140 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 11 mM glucose and 15 mM Hepes, pH 7.2) and then incubated for 10 min in calcium-free Locke's solution (Basal release) or in Locke's solution (Basal release in cells expressing siRNAs against intersectin) or stimulated with an elevated K+ solution (Locke's containing 59 mM KCl and 85 mM NaCl). The supernatant was collected, and the cells were harvested by scraping in 10 mM phosphate-buffered saline. The amounts of GH secreted into the medium and retained in the cells were measured using an ELISA assay (Roche). GH secretion is expressed as a percentage of total GH present in the cells before stimulation. In the figures, data are given as the mean values±s.e.m. obtained in three independent experiments performed on three different cell cultures.
Subcellular fractionation of bovine adrenal medulla
Plasma and chromaffin granule membranes were purified from bovine adrenal medulla as described previously (Gasman et al, 1998). Briefly, adrenal medullary glands were homogenized in 0.32 M sucrose (10 mM Tris–HCl, pH 7.4) and then centrifuged at 800 g for 15 min. After centrifugation at 20 000 g for 20 min, the pellet was resuspended in 0.32 M sucrose (10 mM Tris–HCl, pH 7.4), layered on a continuous sucrose density gradient (1–2.2 M sucrose, 10 mM Tris–HCl, pH 7.4) and centrifuged for 1 h at 100 000 g. Then, 1-ml fractions were collected from top (0.32 M sucrose) to bottom (2.2 M sucrose) and analyzed.
Antibodies, immunoblotting, immunofluorescence, confocal microscopy and image analysis
The following antibodies were used: rabbit polyclonal anti-intersectin-1 (Hussain et al, 1999); monoclonal anti-Cdc42 antibodies (BD, Transduction Laboratories); monoclonal anti-actin antibodies (Sigma); monoclonal anti-SNAP-25 antibodies (Sternberger Monoclonals Inc., Lutherville, MD); monoclonal anti-Flag antibodies (Sigma); monoclonal anti-Rac1 (Santa Cruz); polyclonal anti-GH antibodies (kindly provided by Dr AF Parlow, NIDDK, NIH, Bethesda, MD); monoclonal anti-GFP antibodies (Roche); rabbit and rat anti-DBH antibodies (Perrin and Aunis, 1985; Sontag et al, 1988). Alexa-labeled secondary antibodies and Alexa 568-conjugated annexin 5 were obtained from Molecular Probes. Blots were processed using the Super Signal detection system (Pierce). Immunoreactive bands from Western blot experiments were quantified using Image J 1.29x software (Wayne Rasband, National Institutes of Health, Bethesda, MD). For immunocytochemistry, chromaffin or PC12 cells grown on poly-D-lysine-coated glass coverslips were fixed and immunostained as described previously (Gasman et al, 1998). Stained cells were visualized using a confocal microscope LSM 510 (Carl Zeiss, Jena, Germany). Using the Zeiss CLSM instrument software 3.2, the amount of intersectin or annexin 5 labeling was measured and expressed as the average fluorescence intensity normalized to the corresponding surface area and divided by the total surface of each cell. This allows a quantitative cell-to-cell comparison of the intersectin-1/annexin 5 signal detected in cells. The proportion of DBH/annexin 5 colocalized with intersectin-1 was estimated from the double-labeled pixels, expressed as the average fluorescence intensity normalized to the corresponding surface area, and calculated as a percentage of the total DBH/annexin 5 fluorescence detected in each cell.
Pull-down assay for Cdc42-GTP
The activation of cellular Cdc42 is based on a procedure originally developed for Ras and specifically adapted for Rac and Cdc42 (Bagrodia et al, 1998). After stimulation, PC12 cells expressing GFP-Cdc42 were immediately lysed in ice-cold lysis buffer (25 mM Hepes, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 5 mM EDTA, 1% NP-40, 10% glycerol, 0.1 mM Na3VO4, 4 mM NaF and mammalian protease inhibitor cocktail (Sigma)). GTP-bound GFP-Cdc42 was pulled down by incubating lysates containing equal amounts of proteins (700 μg) with the Cdc42-interacting domain (CRIB) domain of PAK1 (p21-activated kinase) for 2 h at 4°C. Lysates loaded with guanosine 5′-O-(3-thio)triphosphate and GDP served, respectively, as positive and negative controls. The precipitated GTP-bound Cdc42 was resolved on 12% polyacrylamide–SDS gels and immunoblotted with antibodies specific for GFP.
RNA isolation and reverse transcription-PCR
Total RNA was prepared from PC12 and chromaffin cell cultures using the GenElute kit (Sigma). Total RNA (5 μg) was transcribed into cDNA using oligo(dT) and SuperscriptII Reverse Transcriptase (Invitrogen). An aliquot of the cDNA produced was used as a template for amplification of the intersectin-1L fragments by PCR using Taq polymerase (Sigma) and specific primers (forward primer 5′-GTGACTGGTGGACGGCAGTG-3′ and reverse primer 5′-GGAGCCTCATCTGTCTTCTGCTG-3′). PCR reactions were run for 35 cycles and PCR products were resolved on 1% agarose gels.
Supplementary Material
Supplementary Figure 1
Legend to Supplementary Figure 1
Supplementary Figure 2
Legend to Supplementary Figure 2
Supplementary Figure 3
Legend Supplementary Figure 3
Supplementary Figure 4
Legend to Supplementary Figure 4
Supplementary Movie
Legend to Supplementary Movie
Acknowledgments
We acknowledge the generosity of Dr A Benmerah (Institut Cochin, Paris) and Dr C Lamaze (CNRS UMR 144, Paris) for kindly providing the Eps15 and dynamin mutants. We are grateful to Dr JP Borg (INSERM U-599, Marseille) for generously providing pEGFP-RNAi vector. We thank T Thahouly and F Gambino for technical assistance. This work was supported by a Human Frontier Science Program (HFSP) grant to SG (RGY40-2003C), by a French Ministry of Science ACI grant to MFB (ACI BCMS015) and by a Canadian Institutes of Health Research (CIHR) grant MOP-15396 to PSM. We acknowledge the confocal microscopy facilities of Plateforme Imagerie In Vitro of IFR 37.
References
- Artalejo CR, Elhamdani A, Palfrey HC (2002) Sustained stimulation shifts the mechanism of endocytosis from dynamin-1-dependent rapid endocytosis to clathrin- and dynamin-2-mediated slow endocytosis in chromaffin cells. Proc Natl Acad Sci USA 99: 6358–6363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo CR, Henley JR, McNiven MA, Palfrey HC (1995) Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc Natl Acad Sci USA 92: 8328–8332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aunis D, Bader MF (1988) The cytoskeleton as a barrier to exocytosis in secretory cells. J Exp Biol 139: 253–266 [DOI] [PubMed] [Google Scholar]
- Bader MF, Doussau F, Chasserot-Golaz S, Vitale N, Gasman S (2004) Coupling actin and membrane dynamics during calcium-regulated exocytosis: a role for Rho and ARF GTPases. Biochim Biophys Acta 1742: 37–49 [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Jordon KA, Van Aelst L, Cerione RA (1998) A novel regulator of p21-activated kinases. J Biol Chem 273: 23633–23636 [DOI] [PubMed] [Google Scholar]
- Benmerah A, Bayrou M, Cerf-Bensussan N, Dautry-Varsat A (1999) Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J Cell Sci 112 (Part 9): 1303–1311 [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553 [DOI] [PubMed] [Google Scholar]
- Cerione RA (2004) Cdc42: new roads to travel. Trends Cell Biol 14: 127–132 [DOI] [PubMed] [Google Scholar]
- Chasserot-Golaz S, Vitale N, Umbrecht-Jenck E, Knight D, Gerke V, Bader MF (2005) Annexin 2 promotes the formation of lipid microdomains required for calcium-regulated exocytosis of dense-core vesicles. Mol Biol Cell 16: 1108–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona O, De Camilli P (2001) Phosphoinositides in membrane traffic at the synapse. J Cell Sci 114: 1041–1052 [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL (1994) Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol 127: 915–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Achiriloaie M, Janz R, Albanesi JP, Sudhof TC (2000) SCAMP1 function in endocytosis. J Biol Chem 275: 12752–12756 [DOI] [PubMed] [Google Scholar]
- Gasman S, Chasserot-Golaz S, Hubert P, Aunis D, Bader MF (1998) Identification of a potential effector pathway for the trimeric Go protein associated with secretory granules. Go stimulates a granule-bound phosphatidylinositol 4-kinase by activating RhoA in chromaffin cells. J Biol Chem 273: 16913–16920 [DOI] [PubMed] [Google Scholar]
- Gasman S, Chasserot-Golaz S, Malacombe M, Way M, Bader MF (2004) Regulated exocytosis in neuroendocrine cells: a role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol Biol Cell 15: 520–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasman S, Chasserot-Golaz S, Popoff MR, Aunis D, Bader MF (1997) Trimeric G proteins control exocytosis in chromaffin cells. Go regulates the peripheral actin network and catecholamine secretion by a mechanism involving the small GTP-binding protein Rho. J Biol Chem 272: 20564–20571 [DOI] [PubMed] [Google Scholar]
- Guipponi M, Scott HS, Chen H, Schebesta A, Rossier C, Antonarakis SE (1998) Two isoforms of a human intersectin (ITSN) protein are produced by brain-specific alternative splicing in a stop codon. Genomics 53: 369–376 [DOI] [PubMed] [Google Scholar]
- Holz RW, Hlubek MD, Sorensen SD, Fisher SK, Balla T, Ozaki S, Prestwich GD, Stuenkel EL, Bittner MA (2000) A pleckstrin homology domain specific for phosphatidylinositol 4, 5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important in exocytosis. J Biol Chem 275: 17878–17885 [DOI] [PubMed] [Google Scholar]
- Hong-Geller E, Cerione RA (2000) Cdc42 and Rac stimulate exocytosis of secretory granules by activating the IP(3)/calcium pathway in RBL-2H3 mast cells. J Cell Biol 148: 481–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain NK, Jenna S, Glogauer M, Quinn CC, Wasiak S, Guipponi M, Antonarakis SE, Kay BK, Stossel TP, Lamarche-Vane N, McPherson PS (2001) Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nat Cell Biol 3: 927–932 [DOI] [PubMed] [Google Scholar]
- Hussain NK, Yamabhai M, Ramjaun AR, Guy AM, Baranes D, O'Bryan JP, Der CJ, Kay BK, McPherson PS (1999) Splice variants of intersectin are components of the endocytic machinery in neurons and nonneuronal cells. J Biol Chem 274: 15671–15677 [DOI] [PubMed] [Google Scholar]
- Irie F, Yamaguchi Y (2002) EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat Neurosci 5: 1117–1118 [DOI] [PubMed] [Google Scholar]
- Jarousse N, Wilson JD, Arac D, Rizo J, Kelly RB (2003) Endocytosis of synaptotagmin 1 is mediated by a novel, tryptophan-containing motif. Traffic 4: 468–478 [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Worthylake DK, Rossman KL, Pruitt WM, Campbell SL, Sondek J, Der CJ (2001) Molecular basis for Rac1 recognition by guanine nucleotide exchange factors. Nat Struct Biol 8: 1037–1041 [DOI] [PubMed] [Google Scholar]
- Kelly LE, Phillips AM (2005) Molecular and genetic characterization of the interactions between the Drosophila stoned-B protein and DAP-160 (intersectin). Biochem J 388: 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TW, Verstreken P, Bellen HJ (2004) Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron 43: 193–205 [DOI] [PubMed] [Google Scholar]
- Lang T, Wacker I, Wunderlich I, Rohrbach A, Giese G, Soldati T, Almers W (2000) Role of actin cortex in the subplasmalemmal transport of secretory granules in PC-12 cells. Biophys J 78: 2863–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe R, Miaczynska M, Rybin V, Runge A, Zerial M (2001) Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol Biol Cell 12: 2219–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie B, Sweeney ST, Poskanzer KE, Roos J, Kelly RB, Davis GW (2004) Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron 43: 207–219 [DOI] [PubMed] [Google Scholar]
- Martina JA, Bonangelino CJ, Aguilar RC, Bonifacino JS (2001) Stonin 2: an adaptor-like protein that interacts with components of the endocytic machinery. J Cell Biol 153: 1111–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin MK, Badour K, Hardy LA, Kubiseski TJ, Zhang J, Siminovitch KA (2001) The intersectin 2 adaptor links Wiskott Aldrich Syndrome protein (WASp)-mediated actin polymerization to T cell antigen receptor endocytosis. J Exp Med 194: 1777–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus MT, Sharp PA (2002) Gene silencing in mammals by small interfering RNAs. Nat Rev Genet 3: 737–747 [DOI] [PubMed] [Google Scholar]
- Nevins AK, Thurmond DC (2003) Glucose regulates the cortical actin network through modulation of Cdc42 cycling to stimulate insulin secretion. Am J Physiol Cell Physiol 285: C698–710 [DOI] [PubMed] [Google Scholar]
- Okamoto M, Schoch S, Sudhof TC (1999) EHSH1/intersectin, a protein that contains EH and SH3 domains and binds to dynamin and SNAP-25. A protein connection between exocytosis and endocytosis? J Biol Chem 274: 18446–18454 [DOI] [PubMed] [Google Scholar]
- Perrin D, Aunis D (1985) Reorganization of alpha-fodrin induced by stimulation in secretory cells. Nature 315: 589–592 [DOI] [PubMed] [Google Scholar]
- Predescu SA, Predescu DN, Timblin BK, Stan RV, Malik AB (2003) Intersectin regulates fission and internalization of caveolae in endothelial cells. Mol Biol Cell 14: 4997–5010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ (2001) Rho proteins: linking signaling with membrane trafficking. Traffic 2: 303–310 [DOI] [PubMed] [Google Scholar]
- Roos J, Kelly RB (1998) Dap160, a neural-specific Eps15 homology and multiple SH3 domain-containing protein that interacts with Drosophila dynamin. J Biol Chem 273: 19108–19119 [DOI] [PubMed] [Google Scholar]
- Roos J, Kelly RB (1999) The endocytic machinery in nerve terminals surrounds sites of exocytosis. Curr Biol 9: 1411–1414 [DOI] [PubMed] [Google Scholar]
- Rossman KL, Cheng L, Mahon GM, Rojas RJ, Snyder JT, Whitehead IP, Sondek J (2003) Multifunctional roles for the PH domain of Dbs in regulating Rho GTPase activation. J Biol Chem 278: 18393–18400 [DOI] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J (2005) GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6: 167–180 [DOI] [PubMed] [Google Scholar]
- Sengar AS, Wang W, Bishay J, Cohen S, Egan SE (1999) The EH and SH3 domain Ese proteins regulate endocytosis by linking to dynamin and Eps15. EMBO J 18: 1159–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson F, Hussain NK, Qualmann B, Kelly RB, Kay BK, McPherson PS, Schmid SL (1999) SH3-domain-containing proteins function at distinct steps in clathrin-coated vesicle formation. Nat Cell Biol 1: 119–124 [DOI] [PubMed] [Google Scholar]
- Smith C, Neher E (1997) Multiple forms of endocytosis in bovine adrenal chromaffin cells. J Cell Biol 139: 885–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag JM, Aunis D, Bader MF (1988) Peripheral actin filaments control calcium-mediated catecholamine release from streptolysin-O-permeabilized chromaffin cells. Eur J Cell Biol 46: 316–326 [PubMed] [Google Scholar]
- Tagawa A, Mezzacasa A, Hayer A, Longatti A, Pelkmans L, Helenius A (2005) Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J Cell Biol 170: 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, Zhao C, Terakawa S, Rutter GA (2000) Simultaneous evanescent wave imaging of insulin vesicle membrane and cargo during a single exocytotic event. Curr Biol 10: 1307–1310 [DOI] [PubMed] [Google Scholar]
- Vitale ML, Seward EP, Trifaro JM (1995) Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron 14: 353–363 [DOI] [PubMed] [Google Scholar]
- Vitale N, Caumont AS, Chasserot-Golaz S, Du G, Wu S, Sciorra VA, Morris AJ, Frohman MA, Bader MF (2001) Phospholipase D1: a key factor for the exocytotic machinery in neuroendocrine cells. EMBO J 20: 2424–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale N, Mawet J, Camonis J, Regazzi R, Bader MF, Chasserot-Golaz S (2005) The Small GTPase RalA controls exocytosis of large dense core secretory granules by interacting with ARF6-dependent phospholipase D1. J Biol Chem 280: 29921–29928 [DOI] [PubMed] [Google Scholar]
- Wang JB, Wu WJ, Cerione RA (2005) Cdc42 and Ras cooperate to mediate cellular transformation by intersectin-L. J Biol Chem 280: 22883–22891 [DOI] [PubMed] [Google Scholar]
- Yamabhai M, Hoffman NG, Hardison NL, McPherson PS, Castagnoli L, Cesareni G, Kay BK (1998) Intersectin, a novel adaptor protein with two Eps15 homology and five Src homology 3 domains. J Biol Chem 273: 31401–31407 [DOI] [PubMed] [Google Scholar]
- Zamanian JL, Kelly RB (2003) Intersectin 1L guanine nucleotide exchange activity is regulated by adjacent src homology 3 domains that are also involved in endocytosis. Mol Biol Cell 14: 1624–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Legend to Supplementary Figure 1
Supplementary Figure 2
Legend to Supplementary Figure 2
Supplementary Figure 3
Legend Supplementary Figure 3
Supplementary Figure 4
Legend to Supplementary Figure 4
Supplementary Movie
Legend to Supplementary Movie


