Abstract
Bovine vaginal cytobrush specimens were analyzed for the presence of Chlamydia spp. by a high-sensitivity, high-specificity quantitative PCR. The 53% prevalence of low-level Chlamydia psittaci and C. pecorum genital infection detected in virgin heifers suggests predominantely extragenital transmission of Chlamydia in cattle and conforms to the high seroprevalence of anti-Chlamydia antibodies.
Over the last 40 years, evidence has accumulated to suggest the ubiquitous presence of infections with intracellular bacteria of the genus Chlamydia in cattle and other livestock species. Despite some improvement in diagnostic techniques, our understanding about the prevalence and pathogenetic significance of these infections, succinctly reviewed by Shewen (11) in 1980, has not substantially changed since that time. In cattle, enzyme-linked immunosorbent assay examinations of sera for the antibody against Chlamydia psittaci suggest a high level of exposure to C. psittaci (6, 8, 10). The application of nested PCR to bovine clinical specimens substantiated such widespread, but mostly clinically inapparent, presumably low-level infections (3, 7), similar to the findings for human C. pneumoniae infections (1). However, due to high technical demands, these PCR methods were rarely transferred from research settings to systematic epidemiological investigations and diagnostic use. A simple, highly specific, fluorescent-probe-based single-tube LightCycler quantitative PCR (qPCR) platform was recently developed for the detection of Chlamydia DNA. This platform optimizes sample nucleic acid preservation, extraction, and recovery, as well as qPCR methodology, for maximum sensitivity in the detection of Chlamydia (2). This has opened the possibility for the sensitive routine diagnosis of chlamydial infection as well as for systematic epidemiological studies. Such investigations would benefit from both the high sensitivity and the ability to ascertain quantitative differences in chlamydial burdens between animals, which may be required to understand disease mechanisms. In this initial epidemiological application of the Chlamydia qPCR platform, we addressed the question of chlamydial infection of the bovine genital tract in animals that had not previously been exposed to the possibility of sexual transmission of chlamydiae. We report here a high prevalence of genital tract infection with C. psittaci and C. pecorum in clinically normal virgin cattle.
A herd of 51 virgin Holstein heifers, 14 to 16 months old, was sampled four times at weekly intervals. These animals were clinically normal, but low-grade vaginitis was common. Vaginal cytobrush specimens (Histobrush; Fisher Scientific, Suwanee, Ga.) were obtained by 10-s rotation in the vaginal vestibulum, brushes were immediately transferred to 400 μl of RNA-DNA stabilization reagent (Roche Applied Science, Indianapolis, Ind.) in a 1.5-ml screw-cap microcentrifuge tube, samples were stirred, and the brushes were cut off. Brushes were removed after a 5-min centrifugation at 3,000 × g at room temperature; nucleic acids were extracted by glass fiber binding (High Pure PCR template kit; Roche Molecular Biochemicals, Indianapolis, Ind.) and eluted two times at 72°C in 20 μl of 0.1 mM EDTA-10 mM Tris-HCl, pH 8.4. Extractions with the High Pure kit were performed after the direct addition of 10% proteinase K solution to stabilized specimens. Fluorescence resonance energy transfer (FRET) qPCRs for the detection and typing of Chlamydia 23S rRNA and of the C. psittaci B577 omp1 and C. pecorum omp1 genes were performed as described previously (2, 5) with the following modifications: the Chlamydia 23S rRNA qPCR used the genus-specific primers CHL23SUP (5′-GGGGTTGTAGGGTYGAGRAIAWRRGATC-3′) and CHL23SDN (5′-GAGAGTGGTCTCCCCAGATTCARACTA-3′), the Lightcycler Red 640 probe CHL23LCR (5′-LCRed640-CCTGAGTAGRRCTAGACACGTGAAAC-Phosphate-3′), and the C. psittaci-, C. pecorum-, and C. pneumoniae-specific carboxyfluorescein probe CP23FLU(5′-ACGAAARAACAARAGACKCTAWTCGAT-FAM-3′).All Lightcycler Red 640 probes were used at a concentration of 0.2 μM, the carboxyfluorescein probes were used at 0.1 μM, and Platinum Taq polymerase (Invitrogen, Carlsbad, Calif.) was used at 1.5 U per 20-μl qPCR (2). Thermal cycling for the 23S rRNA or omp1 qPCRs, respectively, was performed in a step-down protocol of 6 cycles for 12 s at 64 or 60°C, for 11 s at 72°C, and for 0 s at 95°C; 9 cycles for 12 s at 62 or 58°C, for 11 s at 72°C, and for 0 s at 95°C; 3 cycles for 12 s at 60 or 56°C, for 11 s at 72°C, and for 0 s at 95°C; and 40 cycles for 8 s at 54 or 50°C. Fluorescence acquisition was performed for 11 s at 72°C and for 0 s at 95°C (2).
In an enzyme-linked immunosorbent assay for a peptide of the C. psittaci major outer membrane protein (6), the sera of all 51 heifers had high antibody titers, indicating recent or active infection with Chlamydia. In an initial screen, we analyzed all cytobrush specimens by single 23S rRNA, C. psittaci B577 omp1, and C. pecorum omp1 qPCRs. Cumulative results for the 23S rRNA qPCR, shown in Table 1, revealed a surprisingly high prevalence of Chlamydia in vaginal infections, with 51% of virgin heifers positive at least once during the 4-week sample collection period. C. pecorum accounted for 67% of these infections, and C. psittaci accounted for 33%. Two heifers were positive for both chlamydial species on different days, and 10 heifers were positive for the same Chlamydia species on multiple days. Compared to the 23S rRNA qPCR assay, the omp1 qPCR assays were less sensitive, with 22% of the heifers positive at least once during the 4-week sample collection period (Table 1). Repeated detection of the same or different chlamydial species was similar in proportion to the 23S rRNA qPCR. The number of chlamydiae per specimen detected by all qPCRS was very low, approximately one genome per qPCR (Table 1).
TABLE 1.
Prevalence of Chlamydia vaginal infection in heifers as determined by Chlamydia 23S rRNA and Chlamydia omp1 FRET-qPCRs
| qPCR methoda | No. C. psittaci positive (%) | No. C. pecorum positive (%) | Total no. Chlamydia positive (%) | Chlamydia genomes per PCR-positive specimen (mean ± SD) |
|---|---|---|---|---|
| 23S rRNA | 9 (18%) | 19 (37%) | 26 (51%) | 0.51 ± 1.9 |
| omp1 | 4 (8%) | 8 (16%) | 11 (22%) | 0.80 ± 1.0 |
| Totalb | 12 (24%) | 20 (39%) | 27 (53%) | 0.59 ± 1.3 |
Vaginal cytobrush swabs were collected four times at weekly intervals from a herd of 51 clinically normal virgin Holstein heifers. DNA from swab specimens was extracted, and each sample was examined by a single Chlamydia 23S rRNA, C. psittaci B577 omp1, and C. pecorum omp1 qPCRs. Heifers were scored as positive if any of the four specimens was positive. In the Chlamydia 23S rRNA qPCR, 10 heifers were repeatedly positive for the same Chlamydia species and 2 heifers were positive at different times for C. psittaci and C. pecorum. In the omp1 qPCRs, three heifers were repeatedly positive for the same Chlamydia species and one heifer was positive at different times for C. psittaci and C. pecorum. All negative controls extracted along with the specimens were negative.
Number of heifers scored as positive at least once by 23S rRNA and/or omp1 qPCR.
To ascertain the specificity of the assays, we selected, for additional evaluation, all discordant vaginal cytobrush specimens (n = 39) that were Chlamydia positive in a single qPCR but not in both the 23S rRNA and omp1 qPCRs or those that were positive for different chlamydial species in the 23S rRNA and omp1 qPCRs (Table 2). These specimens were assayed multiple times by the 23S rRNA or omp1 qPCRs. Eighteen of the 39 specimens were found to be Chlamydia positive by both the 23S rRNA and omp1 qPCRs; 16 of these specimens were concordant for Chlamydia species, and 2 were discordant for Chlamydia species.
TABLE 2.
Reexamination of 39 discordant specimens with multiple qPCRs
| qPCR resulta | omp1 qPCR positive | omp1 qPCR negative | Total |
|---|---|---|---|
| 23S rRNA qPCR positive | 18 | 20 | 38 |
| 23S rRNA qPCR negative | 1 | 0 | 1 |
| Total | 19 | 20 | 39 |
DNA from vaginal cytobrush specimens had been initially examined by a single Chlamydia 23S rRNA, C. psittaci B577 omp1, and C. pecorum omp1 qPCRs. Thirty-nine discordant specimens identified as Chlamydia positive by one qPCR but as negative by the others or positive in both 23S rRNA and omp1 qPCRs but discordant in Chlamydia species identification were reexamined by reamplification by the respective negative qPCR four or five times. Specimens were assayed multiple times to compensate for the random distribution of single target genes in 5-μl-sample DNA aliquots at the observed low target concentrations (12). Specimens were scored as positive if any single of the multiple qPCRs was positive.
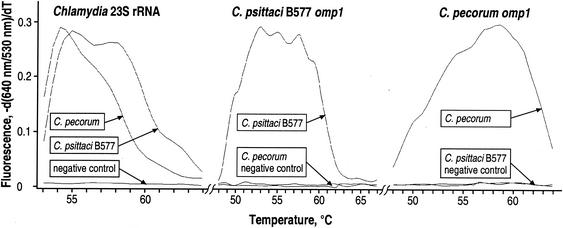
In the absence of an accepted, highly sensitive gold standard for the detection of chlamydiae and, consequently, in the absence of data on the true prevalence of chlamydiae in cattle, the specificity, sensitivity, and predictive values of the Chlamydia qPCR assays must be inferred from the probabilistic comparative data of this study (4). The question of specificity can be tentatively answered by (i) considering the digital nature of qPCR-positive versus -negative results, (ii) evaluation of the concordance of species identification between the positive 23S rRNA and omp1 qPCRs, and (iii) considering the results of the parallel analysis of the control specimens included in each batch of cytobrush specimens. First, an important strength of the qPCR method is that detection specificity is confirmed not only by the appearance of a fluorescent signal during amplification but also by melting point analysis of the saturation amplification products (Fig. 1). These data indicated exquisite, virtually 100% specificity without unspecific background. For instance, both omp1 qPCRs used the same Chlamydia genus-specific primers and Lightcycler Red 640 probe but the exchange of the single C. psittaci B577 probe for the C. pecorum probe and vice versa completely abolished the fluorescent signal for the respective noncognate species (Fig. 1). Similarly, three polymorphic nucleotides in the 54-bp probe binding region allowed for species differentiation in the Chlamydia 23S rRNA qPCR (Fig. 1). This fluorescent signal was much more specific and easier to differentiate from background binding than the fluorescent antibody binding used for the detection of low-level Chlamydia-infected cell cultures. Second, chlamydial species identification in the 23S rRNA and omp1 qPCR assays was 89% concordant. This suggests virtually 100% concordant species identification, considering the random detection of targets in 5-μl-sample DNA aliquots given the Poisson distribution of single target genes at the observed low target concentrations (12) and given the high probability of the presence of both chlamydial species in cytobrush specimens from heifers with mixed infection. Third, throughout the course of this study, we did not observe positive amplication in any negative control specimen, presumably because the closed, single-tube format eliminated product carryover. Collectively, these data suggest 100% specificity of the Chlamydia qPCRs.
FIG. 1.
Specificity of the Chlamydia 23S rRNA, C. psittaci B577 omp1, and C. pecorum omp1 FRET-qPCRs. Melting curves of amplification products from C. psittaci strain B577, C. pecorum, and Escherichia coli-negative control DNAs were acquired in the Chlamydia 23S rRNA, C. psittaci B577 omp1, and C. pecorum omp1 FRET-qPCRs. The omp1 qPCRs yielded product melting curves only for the cognate species. Amplification products of C. psittaci B577 and C. pecorum in the Chlamydia 23S rRNA qPCR can be clearly distinguished by their different melting curves and melting temperature (Tm) peaks of ∼55 and ∼54°C, respectively. The negative control template of irrelevant E. coli DNA did not yield any hybridizing amplification products. These data indicate 100% specificity of the observed fluorescent signal.
The question of the relative sensitivity of the qPCRs has been addressed earlier (5). Compared to the optimized 23S rRNA qPCR method, the standard diagnosis of chlamydial infection by cell culture isolation is approximately 300 times less sensitive (5). At the low concentration of chlamydiae found in the vaginal cytobrush specimens, cell culture methods would have been uniformly negative. Nested PCR methods are also capable of detecting single copies of target genes (1, 7, 12). However, the overall sensitivity of investigations by nested PCR methods might have been lower due to the suboptimal preservation and concentration of DNA. The approximately twofold-increased detection frequency of chlamydiae in this study by the 23S rRNA qPCR, compared to that by the omp1 qPCR, is consistent with the shorter target sequence of the 23S rRNA qPCR, which increases amplification efficiency (7). Earlier Chlamydia omp1 qPCR methods that were not optimized for low target concentrations are approximately 20-fold less sensitive than the 23S rRNA qPCR and probably would not have detected Chlamydia in these heifers either (5).
Given our estimate of 100% specificity of the qPCR assays, we do not assume false-positive results in our study and calculated therefore a 100% predictive value for positive results (4). The absolute sensitivity and predictive value of negative results require data on the true prevalence of chlamydial shedding in cattle. We observed an apparent 4-week period prevalence of 53% and thus assumed a minimum 53% true 4-week period prevalence. Serological data in this and earlier studies indicate that all animals have been infected with Chlamydia (6), suggesting that a maximum 100% true prevalence of chlamydial shedding can be approached, given a high sampling density over an extended time period. Our unpublished preliminary data in cattle indicate similar chlamydial detection rates for other samples, such as milk or nasal and conjunctival cytobrush specimens. This suggests that sampling frequency and duration, more than the anatomical sampling site, are critical in establishing the true prevalence of chlamydial infection. Assuming 53, 75, and 98% true 4-week period prevalence of chlamydial shedding in the heifers in this study, the absolute sensitivity values were 96.4, 76, and 67.6%, respectively, for the 23S rRNA qPCRs and 62.8, 58.5, and 56.2% for the omp1 qPCRs. The predictive values of negative results under these assumptions were 96, 52, and 4%, respectively, for the 23S rRNA qPCRs and 60, 32.5, and 2.5% for the omp1 qPCRs.
Our application of the Chlamydia 23S rRNA and omp1 qPCR detection platforms yielded valuable information on the epidemiology of bovine chlamydial infection. Both the high sensitivity and quantitative aspects of the method proved valuable. The high sensitivity of the qPCR enabled us to detect low levels of chlamydiae in cattle with subclinical or low-grade vaginitis. These data conform to the high seroprevalence of chlamydial infection in cattle. The high frequency of chlamydial genital infection in virgin heifers suggests that extragenital transmission, most likely by social interaction such as mutual licking and possibly by inhalation, is the predominant mode of spread of chlamydial infection in cattle. These results have a direct impact on our understanding of the epidemiology of Chlamydia in cattle and confirm the ubiquitous nature of bovine chlamydial infection.
The ubiquitous presence of two chlamydial species in cattle begs a number of questions. (i) Are individual animals long-term persistently infected, or do we observe mainly short-lived reinfections? (ii) What is the impact on herd health and production? (iii) What is the significance for public health? It is clear that we need to understand chlamydial infection as a continuum, ranging from severe overt disease to low-grade inflammatory change, with little, if any, difference between clinical signs of low-level infections with different chlamydial species. The question of persistence versus reinfection is difficult to answer in the current epidemiological situation. Most likely, some animals will harbor and shed the organisms for an extended period of time and continuously reinfect their herd mates while the majority of the herd will eliminate chlamydiae more rapidly. Understanding the balance between these response patterns and the impact of low-level infection on herd health and production will require investigations into the interaction of chlamydial infection with herd nutrition, population density, and host genetics. The significance of high-dose infection with animal chlamydiae has long been recognized for human ornithosis and for human abortion caused by infection with C. psittaci contracted from aborting ewes (9, 11). We do not know the public health significance of human exposure to the pervasive low-level infections of cattle. A preponderance of evidence suggests that specific chlamydial strains are associated with the long-term infection of specific mammalian hosts, such as C. pneumoniae with humans. Given the close association of humans with low-level C. psittaci- and C. pecorum-infected livestock, and the frequent identification of human infection with C. pneumoniae but not with C. psittaci or C. pecorum, it appears likely that low-level chlamydial infection of cattle only transiently crosses over into human hosts, if at all, and does not cause overt disease.
REFERENCES
- 1.Boman, J., C. A. Gaydos, and T. C. Quinn. 1999. Molecular diagnosis of Chlamydia pneumoniae infection. J. Clin. Microbiol. 37:3791-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeGraves, F. J., D. Gao, and B. Kaltenboeck. 2003. A high-sensitivity quantitative PCR platform. BioTechniques 34:106-115. [DOI] [PubMed]
- 3.Domeika, M., A. Ganusauskas, M. Bassiri, G. Fröman, and P.-A. Mårdh. 1994. Comparison of polymerase chain reaction, direct immunofluorescence, cell culture and enzyme immunoassay for the detection of Chlamydia psittaci in bull semen. Vet. Microbiol. 42:273-280. [DOI] [PubMed] [Google Scholar]
- 4.Galen, R. S. 1986. Use of predictive value theory in clinical immunology, p 966-970. In N. R. Rose, H. Friedman, and J. L. Fahey (ed.), Manual of clinical laboratory immunology, 3rd ed. American Society for Microbiology, Washington, D.C.
- 5.Huang, J., F. J. DeGraves, D. Gao, P. Feng, T. Schlapp, and B. Kaltenboeck. 2001. Quantitative detection of Chlamydia spp. by fluorescent PCRs in the LightCycler. BioTechniques 30:151-157. [DOI] [PubMed] [Google Scholar]
- 6.Kaltenboeck, B., D. Heard, F. J. DeGraves, and N. Schmeer. 1997. Use of synthetic antigens improves detection by enzyme-linked immunosorbent assay of antibodies against abortigenic Chlamydia psittaci in ruminants. J. Clin. Microbiol. 35:2293-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaltenboeck, B., N. Schmeer, and R. Schneider. 1997. Evidence for numerous omp1 alleles of porcine Chlamydia trachomatis and novel chlamydial species obtained by PCR. J. Clin. Microbiol. 35:1835-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Martinez, J. A., N. Schmeer, and J. Storz. 1986. Bovine chlamydial abortion: serodiagnosis by modified complement-fixation and indirect inclusion fluorescence tests and enzyme-linked immunosorbent assay. Am. J. Vet. Res. 47:1501-1506. [PubMed] [Google Scholar]
- 9.Schachter, J. 1999. Infection and disease epidemiology, p. 139-169. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, D.C.
- 10.Schmeer, N., K. L. Schnorr, J. A. Perez-Martinez, and J. Storz. 1987. Dominance of Chlamydia psittaci-specific IgG2 subclass in the humoral immune response of naturally and experimentally infected cattle. Vet. Immunol. Immunopathol. 15:311-322. [DOI] [PubMed] [Google Scholar]
- 11.Shewen, P. E. 1980. Chlamydial infections in animals: a review. Can. Vet. J. 21:2-11. [PMC free article] [PubMed] [Google Scholar]
- 12.Smieja, M., J. B. Mahoney, C. H. Goldsmith, S. Chong, A. Petrich, and M. Chernesky. 2001. Replicate PCR testing and probit analysis for detection and quantitation of Chlamydia pneumoniae in clinical specimens. J. Clin. Microbiol. 39:1796-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]