Abstract
Background
Lemierre's syndrome presents a classic clinical picture, the pathophysiology of which remains obscure. Attempts have been made to trace genetic predispositions that modify the host detection of pathogen or the resultant systemic reaction.
Case presentation
A 17-year old female, with no previous medical history, was admitted to the intensive care unit for septic shock, acute respiratory distress syndrome and Lemierre's syndrome. Her DNA was assayed for single nucleotide polymorphisms previously incriminated in the detection of the pathogen, the inflammatory response and the coagulation cascade. We observed functional variations in her Toll like 5 receptor (TLR 5) gene and two coagulation variations (Tissue Factor (TF) 603 and Plasminogen-Activator-Inhibitor-1 (PAI-1) 4G-4G homozygosity) associated with thrombotic events.
Conclusion
The innate immune response and the prothrombogenic mutations could explain, at least in part, the symptoms of Lemierre's syndrome. Genomic study of several patients with Lemierre's syndrome may reveal its pathophysiology.
Background
Lemierre's syndrome presents a classic [1] but exceptional clinical picture (0.8 per million people per year) [2], the physiopathology of which remains obscure. Over the last few years, there has been a noticeable increase in efforts to identify genetic predispositions that modify the detection of the pathogen by the host or the resultant systemic reaction, efforts that may enhance our understanding of this syndrome. This article describes a young woman with Lemierre's syndrome who was found to carry several genetic polymorphisms: a single nucleotide polymorphism (SNP) in the TLR 5, and SNPs on the gene promoters coding for TF and PAI-1.
Case presentation
A 17-year old female with no previous medical history was admitted to the emergency room of a general hospital due to a deterioration of her general condition, with fever, dyspnea and a paroxysmal severe cough. Two weeks previously, she had presented with an acute pharyngitis, which was treated with amoxicillin for 8 days. At admission, she presented with persistent lateral cervical pain, hyperleukocytosis (leukocyte count of 13,300/m-3, including 90% neutrophils) and a CRP at 450 mg/L, combined with an X-ray shadow on the bottom left lung. This led to a diagnosis of community acquired pneumonia. Blood samples were collected for culture and the patient benefited from treatment with amoxicillin/clavulanic acid and erythromycin.
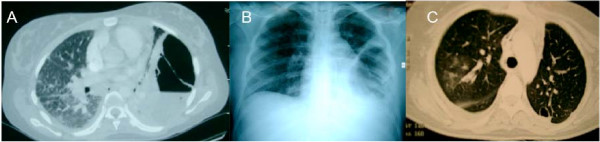
After 48 hours in hospital, the blood cultures were positive for anaerobic Gram negative bacilli. Her antibiotic therapy was modified, to a combination of cefotaxime, gentamicin and metronizadole. Following the appearance of pain in the two hypochondria, a thoraco-abdominal CT scan was performed, which revealed a voluminous build-up of air and fluid in the left thorax (Fig 1A) and a heterogeneous hepato-splenomegaly. Within a few hours, the patient presented with a body temperature of 39°7, respiratory failure with hypotension and awareness disorders, requiring the use of mechanical ventilation and vascular filling with crystalloids followed by the administration of norepinephrine (1 μg/kg/min). She was diagnosed with Lemierre's syndrome and transferred to the intensive care unit of a university hospital center.
Figure 1.
Initial thoracic CT-scan (A) and chest X-ray (B), thoracic CT-scan after chest tube and under mechanical ventilation (C).
At admission to the intensive care unit, the patient presented with severe ARDS, with a PaO2/FiO2 ratio at 65 mmHg, PEEP14, bilateral alveolo-interstitial syndrome, left pyopneumothorax on chest x-ray (Fig 1B) and septic shock. Biologically, her CRP was 475 mg/L and her procalcitonine was 92 mg/L. A left thoracic drain was installed, which removed 350 ml of the purulent liquid. Mechanical ventilation was implemented according to the recommendations of the National Institutes of Health. Her antibiotic regimen was modified to a combination of imipenem/cilastatin (3000 mg/day) and metronidazole (1500 mg/day). An ACTH test was performed, which showed 840 nmol/Lat baseline and 895 nmol/L 1 hour after injection, and she was started on treatment with 300 mg/day hydrocortisone.
Four days later, a second set of thoracic, cerebral and abdominal CT scans was performed. In addition to the abscess of thelingula and the drained pyopneumothorax, these scans revealed a large number of small abscesses of the upper and median lobes of the right lung (Fig 1C), a stable appearance of the hepato-splenomegaly and a partial thrombosis of the left internal jugular vein, without any appearance of stroke.
The anaerobic Gram negative bacillus was identified as a Fusobacterium necrophorum sensitive to penicillin, and she was treated with penicillin G for 15 days. The patient improved, becoming apyretic and with normal blood pressure. Withdrawal of ventilation was started and the patient was weaned from the the respiratory asssitance after 3 days. She was discharged from the intensive care unit 12 days after admission.
Her DNA was assayed for SNPs previously incriminated in the detection of the pathogen, the inflammatory response and the coagulation cascade (Table 1). We observed functional variations in her TLR 5 gene and two coagulation variations (heterozygous TF603 and PAI-1 4G-4G homozygosity) associated with thrombotic events.
Table 1.
Genotype findings at all loci tested. Functional variations were observed in the TLR 5 gene, the flagellin receptor, TLR5-F616L, a heterozygous mutation TLR5-R392 and two coagulation variations resulting in thrombotic events (HT TF603 and PAI-1 4G-4G homozygous). Genes encoding proteins involved in inflammation genes were normal.
| Ref | WT/WT | WT/M | M/M | WT/WT | WT/M | M/M | |||
| TLR2 R753Q | rs5743708 | X | IL 10-1082 | rs1800872 | X | ||||
| TLR5-R392 | rs5744168 | X | TNF b1/b2 | rs2229094 | X | ||||
| TLR5-N592S | rs2072494 | X | TNF 238 | rs361525 | X | ||||
| TLR5-F616L | rs5744174 | X | TNF 308 | rs1800629 | X | ||||
| TLR4 D299G | rs4986790 | X | TNF 376 | rs1800750 | X | ||||
| Fc-GrIIα | rs1801274 | X | PAI-1 | rs1799768 | X | ||||
| CD 14 | rs2569190 | X | Fibrinogen | rs6050 | X | ||||
| SPD 11 | rs721917 | X | TF p603 | [14] | X | ||||
| SPD 160 | rs2243639 | X | EPCR | rs867186 | X | ||||
| MIF | rs755622 | X | Factor II | rs1799963 | X | ||||
| IL 6 | rs1800795 | X | Factor V | Rs6025 | X | ||||
| IL 10-592 | rs1800872 | X | Factor VII | Rs6046 | X |
Abreviations : Toll like receptor (TLR); Fc receptor for IgG (Fc-gamma RII); macrophage migration inhibitory factor (MIF); Endothelial protein C receptor (EPCR); Tissue factor (TF); Tumor Necrosis Factor (TNF); Surfactant Protein D(SPD); WT: Wild type concerns the frequent allele; M: mutation concerns the rare allele. Ref: reference number for the studied SNP.
Discussion
Lemierre's syndrome is frequently due to infection with a strictly anaerobic Gram negative bacillus, Fusobacterium necrophorum, a saprophyte of the oropharynx, digestive tract and genital pathways. Lemierre's syndrome, which mainly affects young patients without any previous medical history, consists of a combination of fever, shivering, deterioration of the general condition, and pain and/or cervical tumefaction along the sterno-cleido-mastoid muscle, resulting from a suppurative thrombophlebitis of the tonsillar and peri-tonsillar veins that can extend to the internal jugular vein. This rare syndrome usually occurs following a banal pharyngitis. The appearance of pulmonary symptomatology (cough, dyspnea, thoracic pain) and/or abdominal symptomatology (hepato and/or spleno-megaly, hepatalgia, cholestasis and/or biological cytolysis) suggesting septic metastases, points to Lemierre's syndrome and justifies the implementation of a suitable antibiotic regimen. Other septic phenomena, such as arthritis, mediastinitis, meningitis and/or endocarditis, are found more rarely.
The physiopathology of Lemierre's syndrome remains controversial. Fusobacterium necrophorum [3] is a commensal of the normal flora of the human oropharynx, digestive tract, genital and urinary pathways and normally does not invade those mucosae. It is not known why Fusobacterium necrophorum becomes pathogenic in certain individuals, although a lowering of local pharyngeal defenses following a viral or bacterial infection may encourage this invasion by creating an anaerobic micro environment [4]. Another explanation is based on the capacity of these bacteria to secrete hemolysins, hemagglutinins and leukocidins, resulting in the formation of micro-abscesses by fibrin and platelet aggregation.
The recent discovery of anti-infection defense mechanisms, in particular Toll like receptors, and the finding that these receptors have functional variations open up new avenues in the pathophysiology of the Lemierre's syndrome, as illustrated in this clinical case.
The innate immune response to Fusobacterium necrophorum is complex and involves both tissue immunity (cathelicidins and defensins) and cellular immunity (TLR receptors). Although Fusobacterium necrophorum is not a flagellate bacterium, it is capable of synthesizing Pilin [5]. Pilin and type IV pili (monomer), may be one of the TLR5 triggers. The TLR5-F616L and TLR5-R392 mutations, which are associated with infections by flagellate bacteria, such as Legionella pneumophila [6], may also be associated with Fusobacterium necrophorum infections. To date, there is no scientific proof with this assertion.
A second facet of the symptomatology of Lemierre's syndrome is the presence of hypercoagulability, resulting in thrombophilia. A procoagulating mutation of prothrombin was recently associated with this syndrome [7]. Interestingly, we observed a combination of one prothrombogenic and one anti-fibrinolytic variations in our patient. The last one was the 4G-4G homozygous gentotype of the PAI-1 gene which encodes for a primary anti-fibrinolytic molecule. This gentotype is responsible for an increase in plasma concentrations of PAI-1. Moreover, this mutation alone [8] or in combination with other prothrombotic genetic anomalies [9,10] is a risk factor for myocardial infarction and venous thrombo-embolic phenomena. The pathophysiology of sepsis is due to imbalances in the coagulation and fibrinolysis systems, and any factor that accentuates these imbalances can influence the host response. Patients suffering from meningococcemia and whose close relatives were carriers of the 4G-4G genotype were found to be 6 times more likely to develop septic shock rather than meningitis [11]. Moreover, this genotype was also found to be associated for an increase in the mortality of patients suffering from multiple injuries [12] and meningococcemia [13]. This SNP can therefore partly explain the physiopathology of Lemierre's syndrome, as well as the severity of the clinical picture in our patient. Similarly, the variation in tissue factor promoter results in a spontaneous and induced overexpression of this trigger of coagulation during sepsis. Of course we have assayed a selection of previously reported SNPs, but many other potentially important reported candidate SNPs have not been examined.
Conclusion
To our knowledge, this article is the first to identify SNPs in a patient suffering from Lemierre's syndrome. The innate immune response and the prothrombogenic mutations observed in this patient could explain, at least in part, the symptoms of Lemierre's syndrome. Genomic study of several patients with Lemierre's syndrome may help to reveal its complex pathophysiology.
Abbreviations
SNP: Single nucleotide polymorphism
TLR 5: Toll like 5 receptor gene
TF: Tissue Factor
PAI-1: Plasminogen-Activator-Inhibitor-1
PEEP: Positive end expiratory pressure
Fc-GRII: Fc receptor for IgG
MIF: macrophage migration inhibitory factor
EPCR: Endothelial protein C receptor
TNF: Tumor Necrosis Factor
SPD: Surfdactant Protein D
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
JMC, JPM and RG drafted the manuscript, and FG, OL, JPR, HL oversaw the sections on infectious disease. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The authors acknowledge the technicians of the Cochin Institute for Genetics Analysis, and Dr Scott Butler for his help in manuscript editing. Written consent was obtained from the patient for publication of study.
Contributor Information
Jean-Michel Constantin, Email: jmconstantin@chu-clermontferrand.fr.
Jean-Paul Mira, Email: jean-paul.mira@cch.ap-hop-paris.fr.
Renaud Guerin, Email: rguerin@chu-clermontferrand.fr.
Sophie Cayot-Constantin, Email: scayot@chu-clermontferrand.fr.
Olivier Lesens, Email: olesens@chu-clermontferrand.fr.
Florence Gourdon, Email: fgourdon@chu-clermontferrand.fr.
Jean-Pierre Romaszko, Email: jpromaszko@chu-clermontferrand.fr.
Philippe Linval, Email: philippe.linval@wanadoo.fr.
Henri Laurichesse, Email: hlaurichesse@chu-clermontferrand.fr.
Jean-Etienne Bazin, Email: jebazin@chu-clermontferrand.fr.
References
- Lemierre A. On certain septicaemias due to anaerobic organisms . Lancet. 1936;1:701–703. doi: 10.1016/S0140-6736(00)57035-4. [DOI] [Google Scholar]
- Hagelskjaer LH, Prag J, Malczynski J, Kristensen JH. Incidence and clinical epidemiology of necrobacillosis, including Lemierre's syndrome, in Denmark 1990-1995. Eur J Clin Microbiol Infect Dis. 1998;17:561–565. doi: 10.1007/BF01708619. [DOI] [PubMed] [Google Scholar]
- Citron DM. Update on the taxonomy and clinical aspects of the genus fusobacterium. Clin Infect Dis. 2002;35:S22–7. doi: 10.1086/341916. [DOI] [PubMed] [Google Scholar]
- Tan ZL, Nagaraja TG, Chengappa MM. Fusobacterium necrophorum infections: virulence factors, pathogenic mechanism and control measures. Vet Res Commun. 1996;20:113–140. doi: 10.1007/BF00385634. [DOI] [PubMed] [Google Scholar]
- Desvaux M, Khan A, Beatson SA, Scott-Tucker A, Henderson IR. Protein secretion systems in Fusobacterium nucleatum: genomic identification of Type 4 piliation and complete Type V pathways brings new insight into mechanisms of pathogenesis. Biochim Biophys Acta. 2005;1713:92–112. doi: 10.1016/j.bbamem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Hawn TR, Verbon A, Lettinga KD, Zhao LP, Li SS, Laws RJ, Skerrett SJ, Beutler B, Schroeder L, Nachman A, Ozinsky A, Smith KD, Aderem A. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. J Exp Med. 2003;198:1563–1572. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid T, Miskin H, Schlesinger Y, Argaman Z, Kleid D. Respiratory failure and hypercoagulability in a toddler With Lemierre's syndrome. Pediatrics. 2005;115:e620–2. doi: 10.1542/peds.2004-2505. [DOI] [PubMed] [Google Scholar]
- Balta G, Altay C, Gurgey A. PAI-1 gene 4G/5G genotype: A risk factor for thrombosis in vessels of internal organs. Am J Hematol. 2002;71:89–93. doi: 10.1002/ajh.10192. [DOI] [PubMed] [Google Scholar]
- Texereau J, Pene F, Chiche JD, Rousseau C, Mira JP. Importance of hemostatic gene polymorphisms for susceptibility to and outcome of severe sepsis. Crit Care Med. 2004;32:S313–9. doi: 10.1097/01.CCM.0000126363.46191.DC. [DOI] [PubMed] [Google Scholar]
- Segui R, Estelles A, Mira Y, Espana F, Villa P, Falco C, Vaya A, Grancha S, Ferrando F, Aznar J. PAI-1 promoter 4G/5G genotype as an additional risk factor for venous thrombosis in subjects with genetic thrombophilic defects. Br J Haematol. 2000;111:122–128. doi: 10.1046/j.1365-2141.2000.02321.x. [DOI] [PubMed] [Google Scholar]
- Westendorp RG, Hottenga JJ, Slagboom PE. Variation in plasminogen-activator-inhibitor-1 gene and risk of meningococcal septic shock. Lancet. 1999;354:561–563. doi: 10.1016/S0140-6736(98)09376-3. [DOI] [PubMed] [Google Scholar]
- Menges T, Hermans PW, Little SG, Langefeld T, Boning O, Engel J, Sluijter M, de Groot R, Hempelmann G. Plasminogen-activator-inhibitor-1 4G/5G promoter polymorphism and prognosis of severely injured patients. Lancet. 2001;357:1096–1097. doi: 10.1016/S0140-6736(00)04311-7. [DOI] [PubMed] [Google Scholar]
- Haralambous E, Hibberd ML, Hermans PW, Ninis N, Nadel S, Levin M. Role of functional plasminogen-activator-inhibitor-1 4G/5G promoter polymorphism in susceptibility, severity, and outcome of meningococcal disease in Caucasian children. Crit Care Med. 2003;31:2788–2793. doi: 10.1097/01.CCM.0000100122.57249.5D. [DOI] [PubMed] [Google Scholar]
- Ott I, Koch W, von Beckerath N, de Waha R, Malawaniec A, Mehilli J, Schomig A, Kastrati A. Tissue factor promotor polymorphism -603 A/G is associated with myocardial infarction. Atherosclerosis. 2004;177:189–191. doi: 10.1016/j.atherosclerosis.2004.07.006. [DOI] [PubMed] [Google Scholar]