Abstract
Surveillance of Norwalk-like virus (NLV) infections in cases of pediatric gastroenteritis between April 1996 and March 2000 showed that NLVs were an important causative agent in viral gastroenteritis cases among children between November and January in those years. The predominant type of NLV was closely related to Lordsdale virus in genogroup 2. During the 1999-2000 season, Arg320-like strains, which may be genetic recombinants, suddenly appeared and spread.
Norwalk-like viruses (NLVs) are single-stranded, positive-sense RNA viruses in the family Caliciviridae (6). NLV is a major cause of both sporadic cases and outbreaks of acute nonbacterial gastroenteritis in all age groups (14). In children, group A rotavirus has been recognized as the most important cause of viral gastroenteritis, but recent work has demonstrated that NLVs are also important causative agents. Seroprevalence studies of NLV infections in pediatric cases of gastroenteritis suggested high exposure to NLVs among children (5, 20). Since the development of the reverse transcription (RT)-PCR (2, 12) method for detection of NLVs, NLV infections among children have been reported, and NLVs have been identified as a causative agent of gastroenteritis (3, 9, 18, 21). In this study, we addressed the causative role of NLV infections in cases of pediatric gastroenteritis and the molecular epidemiology of NLVs in Osaka City, Japan.
A total of 669 fecal specimens were collected from an equal number of patients with acute nonbacterial gastroenteritis attending sentinel pediatric clinics in Osaka City, Japan, between April 1996 and March 2000. The study included only fecal specimens from sporadic cases, excluding outbreaks, as assessed by the pediatrician's interview with the patient. All patients were <12 years of age, and 512 of the 669 (76.5%) were <3 years of age. Initially, all fecal specimens were examined for group A rotavirus and adenovirus serotypes 40 and 41 using antigen and enzyme-linked immunosorbent assay kits (ROTACLONE and ADENOCLONE-E; Meridian Bioscience, Inc., Cincinnati, Ohio) and were used for adenovirus and enterovirus tissue culture isolation with Vero and RD-18S cells. Detection of NLVs was performed using RT-PCR with G1 and G2 primer sets (2) amplifying a 123-bp RNA polymerase region as previously described (10, 11). The virus-negative samples were tested using an electron microscopy method with negative staining (8, 16). All NLV strains detected by RT-PCR were classified into six probe types (P1-A, P1-B, P2-A, P2-B [2], SOV, and 96065 [10]) by Southern hybridization and sequencing as previously described (10). The alignment of the nucleotide and amino acid sequences was analyzed using Clustal X (version 1.63b) with initial fixed parameter values and the matrix Clustal W (1.6) in the DNA weight matrix option (23). Further molecular analysis of NLV strains was carried out by amplifying a 322-bp sequence of the capsid region using two additional primers, mon381 and mon383 (19).
The viruses detected in the pediatric gastroenteritis cases were group A rotavirus (189 strains), NLVs (105 strains), adenovirus (17 strains), enteroviruses (6 strains), and astrovirus (1 strain). Four specimens contained mixed viral infections: rotavirus and enteric adenovirus, rotavirus and NLV, adenovirus type 2 and NLV, and coxsackie B virus type 5 and NLV. Group A rotaviruses were the most common among the pediatric cases (28.6%), followed by NLVs (15.7%). Among the NLV-positive patients, 91.4% were <3 years old, 7.6% were 4 to 12 years old, and the ages of the others were unknown. The major symptoms in NLV-positive patients were nausea, vomiting, diarrhea, and abdominal pain. There were no differences in symptoms among the NLV strains. Seven of the 105 NLV-associated gastroenteritis cases involved benign afebrile convulsions associated with diarrhea; all seven were in patients <2 years old. The relationship between NLV infections and benign afebrile convulsions is unclear. Benign afebrile convulsions are not observed in adults infected with NLV (10, 11). There have been some studies of rotavirus gastroenteritis causing afebrile convulsions (4, 17; Y. Minamiura, H. Hattori, S. Kadotani, M. Nishimoto, T. Nakajima, A. Nishimura, M. Murakami, T. Kato, and K. Murakami, abstract from the Proceedings of the 30th Congress of the Japan Epilepsy Society, Tokyo, Japan, 4 to 5 October 1996, Epilepsia 38(Suppl. 6):78, 1997). Abe et al. (1), and Seto et al. (22) suggested that afebrile convulsions with mild diarrhea may have similar pathogeneses for both rotavirus and NLVs.
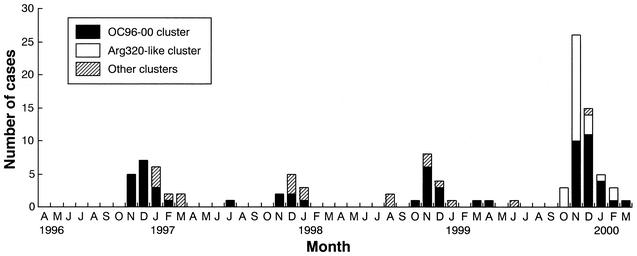
The monthly distribution of NLVs in pediatric gastroenteritis cases is shown in Fig. 1. NLVs were detected in pediatric gastroenteritis cases throughout the year, but 82.9% of the NLV cases occurred from November to January (i.e., late autumn to winter) in those 4 years. In this period, NLVs were an important causative agent in cases of viral gastroenteritis among children.
FIG. 1.
Monthly distribution of NLV-associated pediatric gastroenteritis cases in Osaka City, Japan, between April 1996 and March 2000. The months are represented by their initial letters.
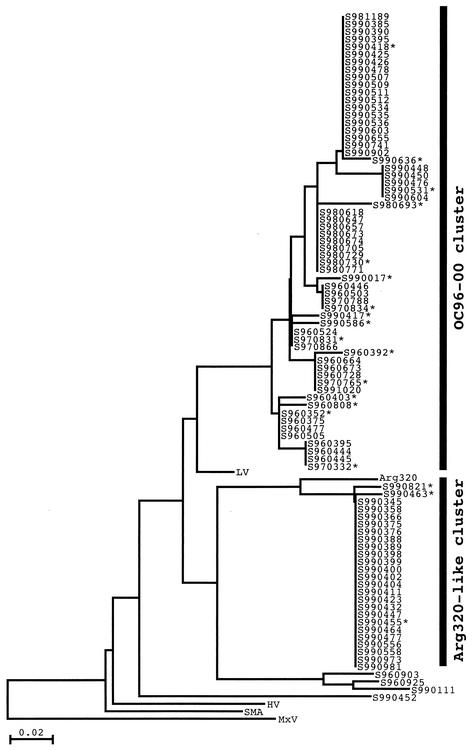
Each of the 105 NLV strains could be classified as a single probe type. These were 1 P1-A strain (0.9%), 8 P1-B strains (7.5%), 4 P2-A strains (3.8%), 90 P2-B strains (84.9%), and 2 96065 strains (1.9%). The P2-B strains were predominant in pediatric gastroenteritis cases in each season. Phylogenetic analysis showed that 86 of the 90 P2-B strains formed two major clusters, one of 61 strains and another of 25 strains (Fig. 2). The first of these clusters, called the OC96-00 cluster, consisted of strains with 96.3 to 100% amino acid sequence identity and 92.6 to 100% nucleotide sequence identity to each other. These strains showed similarity to the Lordsdale virus (LV), with 96.3 to 100% amino acid sequence identity in the region sequenced. Strains of the OC96-00 cluster were detected during all 4 years (Fig. 1). Strains of the second cluster, called the Arg320-like cluster, had 100% amino acid sequence identity and 97.5 to 100% nucleotide sequence identity to each other. These strains showed 96.3% amino acid sequence identity to the Arg320 strain in the region sequenced (13). Arg320-like strains were detected only during the 1999-2000 season (Fig. 1). The Arg320-like strains had 96.3% amino acid sequence identity and 80.2 to 87.7% nucleotide sequence identity to the strains of the OC96-00 cluster.
FIG. 2.
Phylogenetic tree of the 90 P2-B strains and 5 reference strains from GenBank, based on 81-nucleotide sequences from the RNA polymerase region. The distances were calculated by Kimura's two-parameter method with Clustal X. The tree was drawn by the neighbor-joining method with Clustal X. The asterisks indicate the 19 P2-B strains subjected to sequence analysis of the capsid region. The GenBank accession numbers for reference strains used in this analysis are as follows: Arg320, AF190817; Hawaii virus, U07611; LV, X86557; MxV, U22498; and Snow Mountain Agent, L23831.
To further compare these clusters, 19 strains with different nucleotide sequences in the polymerase region were chosen for further sequence analysis. A 277-bp portion (excluding the primer regions) of the capsid region was sequenced. The Arg320-like strains had a sequence related to that of the Mexico virus (MxV) (97.8% amino acid sequence identity), with little intracluster variation. Among the 16 OC96-00 strains, there was also little variation (97.8 to 100% amino acid sequence identity), but the sequence was closely related to that of LV (95.7 to 96.7% amino acid identity) and not to that of MxV (75.0 to 76.1% amino acid identity). Pairwise comparison of the OC96-00 strains and the Arg320-like strains showed amino acid sequence identities of 77.2 to 78.3% in the capsid region.
The Arg320-like strains, which had suddenly appeared and spread during the 1999-2000 season, may have belonged to genogroup 2, like LV and MxV. Jiang et al. reported that the Arg320 strain might be a recombinant between strains in the MxV and LV genetic clusters of NLVs (13). The Arg320-like strains also appear to be recombinants, as the capsid region is very similar to that of MxV while the polymerase region is more similar to that of LV. Vinje and Koopmans reported that the NLV strains in the Rotterdam cluster were clearly distinct from MxV in the polymerase region and closely related to the capsid region of MxV (24). Hardy et al. (7), Jiang et al. (13), and Katayama et al. (15) reported the naturally occurring recombinants of NLV strains. However, the mechanism of recombination among different NLV strains is unclear. The definitive classification of Arg320-like strains will hinge on the analysis of the other genomic regions.
The surveillance that we conducted for NLV infections included both sporadic and outbreak cases. This study shows that the NLV P2-B probe type in genogroup 2 was consistently the one found in sporadic pediatric cases and that these NLV strains were closely related to the LV prototype. In contrast, the predominant probe type of NLV strains detected from outbreaks, which mainly occurred in adults, changed every season in this community (10, 11), and its genetic type differed from that of the predominant strain causing sporadic pediatric cases (data not shown). As the NLV-associated outbreaks tended to occur more frequently from January to March (10, 11), the pediatric NLV infections tended to precede the NLV-associated outbreaks. Further epidemiological investigations are required to clarify the differences between NLV infections in children and adults.
Nucleotide sequence accession numbers.
The nucleotide sequences of the 19 P2-B NLV strains subjected to sequence analysis of the capsid region have been deposited in DDBJ/EMBL/GenBank under the accession numbers AB089860 to AB089863, AB089866 to AB089872, and AB089874 to AB089881.
Acknowledgments
We thank Teruo Kimura for helpful advice and Kaoru Takino and Shouji Minoshiro for technical assistance.
This work was supported by a grant from the Daido Seimei Social Welfare Foundation.
REFERENCES
- 1.Abe, T., M. Kobayashi, K. Araki, H. Kodama, Y. Fujita, T. Shinozaki, and H. Ushijima. 2000. Infantile convulsions with mild gastroenteritis. Brain Dev. 22:301-306. [DOI] [PubMed] [Google Scholar]
- 2.Ando, T., S. S. Monroe, J. R. Gentsch, Q. Jin, D. C. Lewis, and R. I. Glass. 1995. Detection and differentiation of antigenically distinct small round-structured viruses (Norwalk-like viruses) by reverse transcription-PCR and southern hybridization. J. Clin. Microbiol. 33:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bon, F., P. Fascia, M. Dauvergne, D. Tenenbaum, H. Planson, A. M. Peiton, P. Pothier, and E. Kohli. 1999. Prevalence of group A rotavirus, human calicivirus, astrovirus, and adenovirus type 40 and 41 infections among children with acute gastroenteritis in Dijon, France. J. Clin. Microbiol. 37:3055-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Contino, M. F., T. Lebby, and E. L. Arcinue. 1994. Rotaviral gastrointestinal infection causing afebrile seizures in infancy and childhood. Am. J. Emerg. Med. 12:94-95. [DOI] [PubMed] [Google Scholar]
- 5.Cukor, G., N. R. Blacklow, P. Echeverria, M. K. Bedigian, H. Puruggan, and V. Basaca-Sevilla. 1980. Comparative study of the acquisition of antibody to Norwalk virus in pediatric populations. Infect. Immun. 29:822-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green, K. Y., T. Ando, M. S. Balayan, T. Berke, I. N. Clarke, M. K. Estes, D. O. Matson, S. Nakata, J. D. Neil, and M. J. Studdert. 2000. Taxonomy of the caliciviruses. J. Infect. Dis. 181(Suppl. 2):322-330. [DOI] [PubMed] [Google Scholar]
- 7.Hardy, M. E., S. F. Kramer, J. J. Treanor, and M. K. Estes. 1997. Human caliciviruses genogroup II capsid sequence diversity revealed by analyses of the prototype Snow Mountain agent. Arch. Virol. 142:1469-1479. [DOI] [PubMed] [Google Scholar]
- 8.Haruki, K., Y. Seto, T. Murakami, and T. Kimura. 1991. Pattern of shedding of small, round-structured virus particles in stools of patients of outbreaks of food-poisoning from raw oysters. Microbiol. Immunol. 35:83-86. [DOI] [PubMed] [Google Scholar]
- 9.Inouye, S., K. Yamashita, S. Yamadera, M. Yoshikawa, N. Kato, and N. Okabe. 2000. Surveillance of viral gastroenteritis in Japan: pediatric cases and outbreak incidents. J. Infect. Dis. 181(Suppl. 2):S270-S274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iritani, N., Y. Seto, K. Haruki, M. Kimura, M. Ayata, and H. Ogura. 2000. Major change in the predominant type of Norwalk-like viruses in outbreaks of acute nonbacterial gastroenteritis in Osaka City, Japan, between April 1996 and March 1999. J. Clin. Microbiol. 38:2649-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iritani, N., Y. Seto, H. Kubo, K. Haruki, M. Ayata, and H. Ogura. 2002. The prevalence of “Norwalk-like virus” infections in outbreaks of acute nonbacterial gastroenteritis observed during the 1999-2000 season in Osaka City, Japan. J. Med. Virol. 66:131-138. [DOI] [PubMed] [Google Scholar]
- 12.Jiang, X., J. Wang, D. Y. Graham, and M. K. Estes. 1992. Detection of Norwalk virus in stool by polymerase chain reaction. J. Clin. Microbiol. 30:2529-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang, X., C. Espul, W. M. Zhong, H. Cuello, and D. O. Matson. 1999. Characterization of a novel human calicivirus that may be a naturally occurring recombinant. Arch. Virol. 144:2377-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapikian, A., M. Estes, and M. Chanock. 1996. Norwalk group viruses, p. 783-810. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Straus (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 15.Katayama, K., H. Shirato-Horikoshi, S. Kojima, T. Kageyama, T. Oka, F. B. Hoshino, S. Fukushi, M. Shinohara, K. Uchida, Y. Suzuki, T. Gojobori, and N. Takeda. 2002. Phylogenetic analysis of the complete genome of 18 Norwalk-like viruses. Virology 299:225-239. [DOI] [PubMed] [Google Scholar]
- 16.Kimura, T., and T. Murakami. 1977. Tubular structures associated with acute nonbacterial gastroenteritis in young children. Infect. Immun. 17:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komori, H., M. Wada, M. Eto, H. Oki, K. Aida, and T. Fujimoto. 1995. Benign convulsions with mild gastroenteritis: a report of 10 recent case detailing clinical varieties. Brain Dev. 17:334-337. [DOI] [PubMed] [Google Scholar]
- 18.Levett, P. N., M. Gu, B. Luan, M. Fearon, J. Stubberfield, F. Jamieson, and M. Petric. 1996. Longitudinal study of molecular epidemiology of small round-structured viruses in a pediatric population. J. Clin. Microbiol. 34:1497-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noel, J. S., T. Ando, J. P. Leite, K. Y. Green, K. E. Dingle, M. K. Estes, Y. Seto, S. S. Monroe, and R. I. Glass. 1997. Correlation of the patient immune responses with genetically characterized small round-structured viruses involved in outbreaks of nonbacterial acute gastroenteritis in the United States, 1990 to 1995. J. Med. Virol. 53:372-383. [DOI] [PubMed] [Google Scholar]
- 20.Numata, K., S. Nakata, X. Jiang, M. K. Estes, and S. Chiba. 1994. Epidemiological studies on Norwalk virus infection in Japan and Southeast Asia using enzyme-like immunosorbent assays with baculovirus-expressed Norwalk virus capsid protein. J. Clin. Microbiol. 32:121-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang, X. L., S. Honma, S. Nakata, and T. Vesikari. 2000. Human caliciviruses in acute gastroenteritis of young children in the community. J. Infect. Dis. 181(Suppl. 2):S288-S294. [DOI] [PubMed] [Google Scholar]
- 22.Seto, T., T. Yokoi, H. Hattori, K. Furukawa, K. Hayashi, O. Matsuoka, N. Iritani, Y. Seto, and H. Ogura. 1999. Three young children with afebrile seizures and a demonstration of SRSV in the diarrheal stool. Osaka J. Epilepsy Res. 10:45-50. (In Japanese.)
- 23.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinje, J., and M. P. G. Koopmans. 2000. Simultaneous detection and genotyping of “Norwalk-like viruses” by oligonucleotide array in a reverse line blot hybridization format. J. Clin. Microbiol. 38:2595-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]