Abstract
Vimentin intermediate filaments undergo spatial reorganization in cultured smooth muscle cells in response to contractile activation; however, the role of vimentin in physiologic properties of smooth muscle has not been well elucidated. In this report, tracheal smooth muscle strips were loaded with antisense oligonucleotides against vimentin, and these muscle strips were then cultured for 2 days to allow for protein degradation. The treatment with vimentin antisense but not sense oligonucleotides suppressed vimentin protein expression; neither antisense nor sense oligonucleotides affected protein levels of desmin and actin. Force development in response to stimulation with acetylcholine (ACh) or KCl depolarization was lower in vimentin-deficient tissues than in muscle tissues treated with sense oligonucleotides or in muscle strips not treated with oligonucleotides. Passive tension was also depressed in vimentin-depleted muscle tissues. Vimentin downregulation did not attenuate increases in myosin light chain phosphorylation in response to contractile stimulation or basal myosin light chain phosphorylation. In smooth muscle strips that had been treated with vimentin sense or without oligonucleotides, the desmosomal protein plakoglobin was primarily localized in cell periphery. The membrane-associated localization of plakoglobin was reduced in vimentin-depleted muscle tissues. These studies suggest that vimentin filaments play an important role in mediating active force development and passive tension, which is not regulated by myosin light chain phosphorylation. Vimentin downregulation impairs the structural organization of desmosomes, which may be associated with the decrease in force development.
Keywords: Intermediate filaments, cytoskeleton, contraction, smooth muscle, vimentin, desmin
INTRODUCTION
The intermediate filament network is one of the three cytoskeletal systems in smooth muscle cells. Intermediate filaments link to the membrane at the desmosome, an intercellular junction. At the desmosome, the cytoplasmic domains of transmembrane desmocollin and desmoglein connect with intermediate filaments via desmosomal proteins such as plakoglobin. The extracellular domains of desmocollin and desmoglein engage with their counterparts of adjacent cells to form the intercellular connection. In addition, in the myoplasm, intermediate filaments connect to dense bodies to which actin filaments also attach (1, 8, 15, 25).
Five types of structurally-related intermediate filament proteins have been identified; their expression is cell-type specific (13). Vimentin is a major intermediate filament protein in airway and vascular smooth muscle, whereas desmin is expressed in airway smooth muscle and microarteries (12, 14, 28, 39).
We have previously shown that contractile stimulation leads to vimentin phosphorylation at ser-56 and spatial reorientation of the vimentin network in cultured smooth muscle cells (28). In addition, acetylcholine stimulation of canine tracheal smooth muscle strips results in the increase in the amount of soluble vimentin (33), indicating that vimentin may undergo remodeling in differentiated smooth muscle tissues. The spatial reorganization of vimentin intermediate filaments also occurs in endothelial cells and fibroblasts in response to stimulation with platelet-derived growth factor (PDGF) and epidermal growth factor (EGF). The remodeling of vimentin filaments has been implicated in regulating the division of endothelial cells and fibroblasts (19, 38).
Plakoglobin plays a pivotal role in mediating desmosomal formation in certain cell types (3, 6). In human fibrosarcoma cells and COS-7 cells, the overexpression of plakoglobin promotes clustering of desmosomal plaque complexes at cell-cell borders (3). Biochemical and morphological analysis indicates that junctional incorporation of desmosomal component is impaired in plakoglobin-deficient murine keratinocytes. Re-expression of plakoglobin in these plakoglobin-deficient cells largely reverses the effects (40).
This study was undertaken to assess the role of vimentin in smooth muscle by evaluating the effects of vimentin downregulation with antisense oligonucleotides on physiological properties of smooth muscle. Our results suggest that vimentin is involved in passive tension and active force development and membrane-associated plakoglobin distribution but not myosin light chain phosphorylation in smooth muscle, indicating that vimentin intermediate filaments are involved in force development in smooth muscle tissues.
MATERIALS AND METHODS
Preparation of smooth muscle tissues
Mongrel dogs (20–25 kg) were anesthetized with pentobarbital sodium (30 mg/kg, i.v.) and quickly exsanguinated. All experimental protocols were approved by the Institutional Animal Care and Usage Committee. A 15 cm segment of extra-thoracic trachea was immediately removed and placed at room temperature in physiological saline solution (PSS) containing 110 mM NaCl, 3.4 mM KCl, 2.4 mM CaCl2, 0.8 mM MgSO4, 25.8 mM NaHCO3, 1.2 mM KH2PO4, and 5.6 mM glucose. The solution was aerated with 95%O2-5%CO2 to maintain a pH of 7.4. Rectangular strips of tracheal muscle 0.6–0.8 mm in width, 0.2–0.3 mm in thickness and 9–10 mm in length were dissected from the trachea after removal of the epithelium and connective tissue layer. For canine tracheal muscle strips, the use of muscle strips with aforementioned width, thickness, and length was critical for maintaining muscle contractility during the incubation period and for the successful introduction of oligonucleotides throughout the muscle strip.
Measurement of tension development in smooth muscle
Each muscle strip was placed in PSS at 37°C in a 25-ml organ bath and attached to a Grass force transducer that had been connected to a Gould recorder or a computer with A/D converter (Grass). At the beginning of each experiment, muscle strips were stretched to the reference muscle length (9–10 mm). After 30 min equilibrium they were stimulated with 10−5M acetylcholine (ACh) repeatedly until contractile responses and passive tension reached stable. In untreated muscle tissues, an average passive tension was 0.2 g while an average active force was 4 g.
Oligodeoxynucleotides (ODNs) dissolved in Tris-EDTA buffer were introduced into muscle strips according to experimental procedures described previously (29, 36). Muscle strips were then incubated for 2 days with ODNs in Dulbecco’s Modified Eagle Medium (DMEM). The strips were returned to PSS at 37°C in 25-ml organ baths and stretched to the corresponding reference muscle length. Following repeated stimulation with ACh, each contractile response and passive tension was compared with corresponding preincubation value. For biochemical analysis, muscle strips were frozen using liquid N2-cooled tongs, and then pulverized under liquid N2 using a mortar and pestle.
Loading of oligodeoxynucleotides and organ culture
Antisense ODNs with the following sequence were designed to selectively suppress vimentin expression in canine tracheal smooth muscle: 5′-AGGACACGGACCTGGTGG-3′ (based on human vimentin cDNA sequence, accession number XM 167414). The vimentin sense sequence (5′-CCACCAGGTCCGTGTCCT-3′) and scrambled sequence (5′-CGAAATCGGTAGCGGGTA-3′) are used as a control. According to sequence matching results obtained from The National Center for Biotechnology Information, these sequences are not homologous to sequences of any other contractile proteins and cytoskeletal proteins. The antisense molecule targets to a region of mRNA that is unique to vimentin. The phosphorothioate ODNs were synthesized and purified by Invitrogen Corporation, Carlsbad, CA, USA. The ODNs were introduced into the smooth muscle strips by chemical loading (also referred to as reversible permeabilization) using methods we have previously described (29, 35, 36).
Analysis of protein expression
Pulverized muscle strips were mixed with 50 μl of extraction buffer containing 20mM Tris-HCl at pH 7.4, 2% Triton X-100, 0.2% SDS, 2 mM EDTA, phosphatase inhibitors (2 mM sodium orthovanadate, 2 mM molybdate, and 2 mM sodium pyrophosphate) and protease inhibitors (2 mM benzamidine, 0.5 mM aprotinin and 1 mM phenylmethylsulfonyl fluoride). Each sample was kept on ice for 1 h and then centrifuged for the collection of supernatant. Muscle extracts were boiled in sample buffer (1.5% dithiothreitol, 2% SDS, 80 mM Tris-HCl (pH 6.8), 10% glycerol and 0.01% bromophenol blue) for 5 min and then separated by SDS polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to nitrocellulose, after which the membrane was cut into two parts for immunoblotting of different proteins. The upper part of the membrane was blocked with 5% milk for 1 h and probed with monoclonal antibody against vimentin (BD Pharmingen, San Diego, CA) followed by horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Amersham Pharmacia Biotech, Piscataway, NJ). The nitrocellulose membranes were then stripped of bound antibodies and reprobed with desmin monoclonal antibody (BD Pharmingen, San Diego, CA). The lower part of the membrane was reacted with a monoclonal antibody against actin (clone 1A4, Sigma) followed by HRP-conjugated anti-mouse IgG. Proteins were visualized by enhanced chemiluminescence (ECL) and quantitated by scanning densitometry. Densitometric values of vimentin, desmin and actin were determined for sense-treated and antisense-treated strips and normalized to those of no ODN-treated strips. The ratios of these proteins were calculated to verify that changes in protein expression were selective for vimentin.
Electron microscopy of smooth muscle tissues
Tracheal smooth muscle strips were placed in organ baths containing PSS, and fixed at the reference length at 37°C for 15 min in the solution containing 2% glutaraldehyde, 2% paraformaldehyde and 2% tannic acid in 0.1 M sodium cacodylate buffer, pH 7.2. The samples were removed from the organ baths, cut into small blocks (1 x 0.5 x 0.1 mm in dimension), and placed in the same fixing solution for 2 h at 4°C followed by three 10-min washes in 0.1 M sodium cacodylate buffer. The tissues were then fixed with 1% OsO4 in 0.1 M sodium cacodylate buffer for 2 h. After washing three times with distilled water, the samples were stained en bloc in 1% uranyl acetate for 1 h, and then washed and dehydrated through a graded series of ethanol of 50, 70, 80, 90, and 95% for 10 min each, followed by 100% ethanol. They were embedded in Spurr’s resin. Ultrathin sections of ~ 90 nm were cut with a diamond knife and picked upon on Formvar coated grids. The sections were stained in 1% uranyl acetate followed by lead citrate and examined on a JEOL 100CX transmission electron microscope.
Assessment of myosin light chain phosphorylation
Myosin light chain phosphorylation in tracheal smooth muscle strips was assessed by immunoblot analysis as described previously (29–32, 37).
Cell dissociation and immunofluorescence analysis
Tracheal smooth muscle strips were minced and transferred to 5 ml of dissociation solution containing 130 mM NaCl, 5 mM KCl, 1.0 mM CaCl2, 1.0 mM MgCl2, 10 mM Hepes, 0.25 mM EDTA, 10 mM D-glucose, 10 mM taurine, pH 7, collagenase (400 U/ml, type I), papain (30 U/m, type IV), bovine serum albumin (1mg/ml), and DTT (1 mM). All enzymes were obtained from Sigma. The strips were then placed in a 37ºC shaking water bath, at 80 oscillations per min for 10 minutes. The strips were then washed three times with a Hepes-buffered saline solution (composition in mM: 130 NaCl, 5 KCl, 1.0 CaCl2, 1.0 MgCl2, 20 Hepes, 10 D-glucose, pH 7.4) and triturated with a pipette to liberate individual smooth muscle cells from the tissue. The solution containing the dissociated cells was poured over slides, and the cells were allowed to adhere to the slides for 2–3 hrs at room temperature (20).
The smooth muscle cells were fixed for 10 min in 4% paraformaldehyde, and were then washed three times in Tris-buffered saline (TBS) containing 50 mM Tris, 150 mM NaCl, and 0.1% NaN3 followed by permeabilization with 0.2% Triton X-100 dissolved in TBS for 5 min. Cells were then incubated with vimentin monoclonal antibody (BD Pharmingen, 1:100 dilution) or desmin goat polyclonal antibody (Santa Cruz Biotechnology, CA, 1:20 dilution) for 45 min at 37ºC. Cells were then washed and incubated with a secondary antibody conjugated to either Alexa 488, or Alexa 546 fluoroprobe (Molecular Probes, Eugene, OR) for 30 min at 37ºC. Cells were washed and slides were covered by coverslips using fluoromount G. The cellular localization of fluorescently labeled proteins was viewed under laser scanning confocal microscopy (Zeiss LSM 510) using an Apo 63x oil immersion objective. Alexa 488-labeled proteins (green) were excited with a 488-nm argon laser light and fluorescent emissions were collected at 500–550 nm. The fluorescence of Alexa 546-labeled protein (red) was excited with a helium/neon laser at 543 nm and emissions were collected at 565–615 nm.
Immunohistochemistry
Tracheal smooth muscle tissues were embedded in frozen tissue embedding media (Neg 52, Richard-Allen Scientific) and cryosectioned using Cryostats (Richard-Allen Scientific). Sections were air-dried, incubated with plakoglobin monoclonal antibody (BD Pharmingen, 1:20 dilution) and vinculin rabbit polyclonal antibody (Santa Cruz Biotechnology, CA, 1:100 dilution) at room temperature for 1 hour. These sections were washed with PBS and incubated with a secondary antibody conjugated to either Alexa 488, or Alexa 546 fluoroprobe (Molecular Probes, Eugene, OR) for 30 min at 37ºC. The samples were covered and viewed under laser scanning confocal microscopy.
Image analysis for protein distribution was performed using the previously-described method with modification (20). Briefly, images of smooth muscle cells in the tissues were analyzed for distribution differences of stained proteins by quantifying the pixel intensity with a series of 3–4 line scans cross the boarder of cells in tissue cross-sections. The ratio of pixel intensities between the cell borders and interior was determined for each line scan by taking the ratio of the average maximal pixel intensities at the cell periphery to the minimal pixel intensities in the cell interior. The ratios of pixel intensities between the cell periphery and the cell interior for all of the line scans performed on a given cell were averaged to obtain a single value for the ratio of each cell.
Statistical analysis
All statistical analysis was performed using Prism 4 software (GraphPad Software, San Diego, CA). Comparison among multiple groups was performed by one-way analysis of variance followed by post test (Tukey’s multiple comparison test). Differences between pairs of groups were analyzed by Student-Newman-Keuls test or Dunn’s method. Values of n refer to the number of experiments used to obtain each value. P < 0.05 was considered to be significant.
RESULTS
Spatial distribution of vimentin and desmin in freshly-dissociated smooth muscle cells
We evaluated the spatial distribution of the type III intermediate filament proteins vimentin and desmin in freshly-dissociated tracheal smooth muscle cells. Smooth muscle cells were freshly dissociated from canine tracheal smooth muscle cells and immunostained for vimentin and desmin. Protein distribution in these cells was viewed under confocal fluorescence microscope. Vimentin was mainly localized in the myoplasm, whereas desmin was primarily detected on cell periphery. Both vimentin and desmin were not found in the nucleus of the freshly-dissociated smooth muscle cells (Fig. 1).
Figure 1. Subcellular distribution of vimentin and desmin in freshly-dissociated smooth muscle cells.
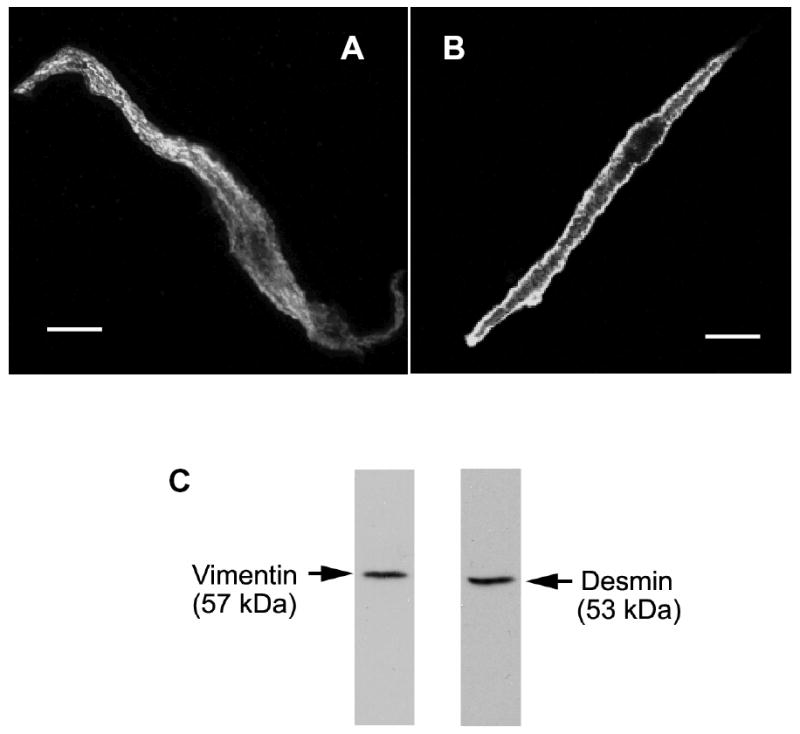
Smooth muscle cells were freshly dissociated from canine tracheal smooth muscle tissues. These cells were then immunofluorescently labeled for vimentin (A) or for desmin (B). Vimentin is distributed throughout the myoplasm, whereas desmin is more concentrated at the membrane. The nucleus appeared as a dark area. Bar, 5 μm. C, immunoblots of muscle extracts were obtained with antibodies against vimentin or desmin.
Vimentin downregulation attenuates force development in response to stimulation with acetylcholine (ACh)
To evaluate the role of vimentin in smooth muscle, vimentin antisense, sense oligodeoxynucleotides or scrambled oligonucleotides were introduced into canine tracheal smooth muscle strips by chemical loading (29, 31, 32, 36). These muscle strips were incubated for 2 days to allow for protein depletion. Protein expression from smooth muscle tissues was assessed by immunoblot analysis. The expression of vimentin protein was lower in muscle tissues treated with antisense ODNs than in tissues treated with sense or scrambled ODNs, or no ODNs. However, the expression of desmin and α-actin was not affected by antisense treatment (Fig. 2, A and B, n = 4 – 6).
Figure 2. Downregulation of vimentin protein by vimentin antisense oligodeoxynucleotides (ODNs).
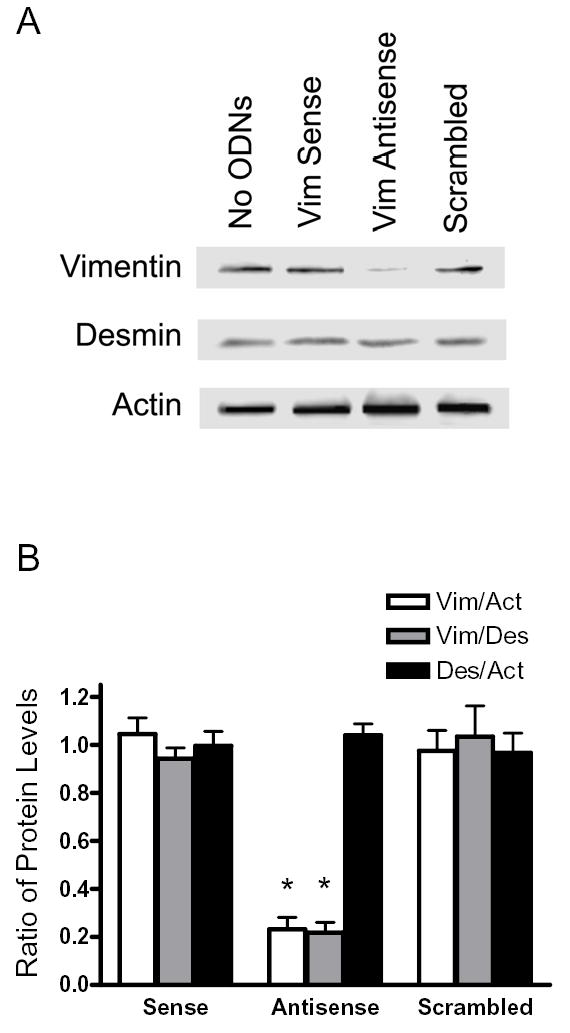
A, blots of protein extracts from muscle strips that had been cultured for 2 days without ODNs (No ODNs), with vimentin (Vim) sense ODNs or vimentin antisense ODNs, or scrambled ODNs were probed with antibodies against vimentin, desmin, and actin. The level of vimentin was lower in antisense-treated muscle strips than in strips not treated with ODNs, or sense-treated or scrambled sequence-treated muscle strips. Similar amounts of desmin and actin were detected in all four strips. Molecular mass markers are indicated on left. B, ratios of protein expression obtained from muscle strips treated with vimentin sense or antisense, or scrambled ODNs. Ratios in sense-treated, antisense-treated or scrambled sequence-treated muscle strips are normalized to ratios obtained in muscle strips not treated with ODNs. Vim, vimentin; Act, actin; Des, desmin. Values represent mean ± SE (n = 4–6). Asterisk indicates significantly lower ratios in antisense-treated strips as compared to no-ODN-treated, sense-treated and scrambled sequence-treated muscle strips (P < 0.05). C & D, representative electron micrographs of the longitudinal sections of smooth muscle tissues treated with vimentin sense (C) and tissues treated with antisense ODNs (D). Black arrows indicate intermediate filaments; white arrows, thin filaments; black arrow heads, thick filaments. Bar, 200 nm.
We also assessed the effects of vimentin antisense oligonucleotides on the ultrastucture of smooth muscle tissues. Electron microscopy analysis revealed that the amount of intermediate filaments was reduced in antisense-treated tissues compared to control tissues. However, the organization of thin and thick filaments in smooth muscle tissues treated with vimentin antisense was not markedly different from tissues treated with sense ODNs (Fig. 2, C and D).
We evaluated isometric force development in response to ACh in smooth muscle strips not treated with ODNs or in strips treated with vimentin sense or antisense ODNs. Active stress of the tracheal muscle strips in response to ACh stimulation (10−5 M, 5 min) is 95–100 mN/mm2 before incubation (20, 29, 30, 33, 34, 36, 42). Force development in response to 10−5 M ACh was compared before and after 2-day incubation period. In muscle strips not treated with ODNs, contractile force in response to stimulation with ACh for 5 min was similar to preincubation force (n = 10, P > 0.05). Contractile response in vimentin sense-treated tissues was 97% of preincubation force (n = 10, P > 0.05). However, ACh-stimulated force in antisense-treated tissues was significantly reduced to 24% of corresponding preincubation force (n = 10, P < 0.01) (Fig 3).
Figure 3. Effects of vimentin depletion on contractile force in response to stimulation with acetylcholine.
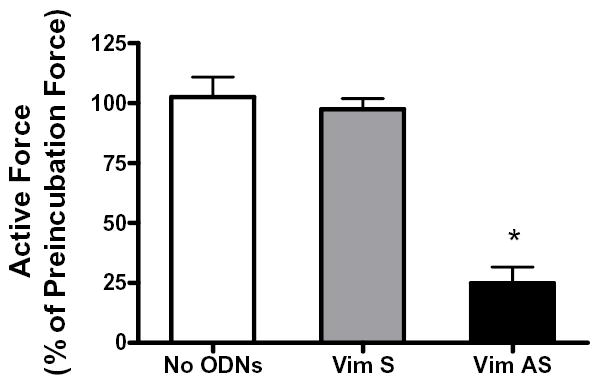
The contractile responses of canine tracheal smooth muscle strips were assessed, after which vimentin sense or vimentin antisense was loaded into these muscle strips, and these strips were incubated for 2 days to allow for protein depletion. The contractile responses of these muscle strips were then determined. Vimentin antisense inhibited ACh-induced contraction in smooth muscle strips after 2-day incubation. The contractile responses of no-ODN-treated or sense-treated muscle strips were similar to preincubation force after 2 days of culture. Mean active force in response to 10−5M ACh was quantitated as percent of ACh-induced force in each strip before incubation. Values are mean ± SE. Asterisk indicates significantly lower response as compared to muscles treated with sense ODNs or not treated with ODNs (n = 10, P < 0.05).
Antisense inhibition of vimentin depresses passive tension in smooth muscle
We assessed passive tension in smooth muscle strips treated with vimentin antisense or sense ODNs, or not treated with ODNs. Without ODN treatment, passive tension was similar before and after incubation (n = 10, P > 0.05). The passive strain in vimentin sense-treated tissues was also not distinguishable prior and after incubation (n = 10, P > 0.05). In contrast, passive tension in antisense-treated tissues was reduced after the incubation period as compared to preincubation strain (n = 10, P < 0.01) (Fig 4).
Figure 4. Passive tension in smooth muscle strips treated with vimentin antisense.
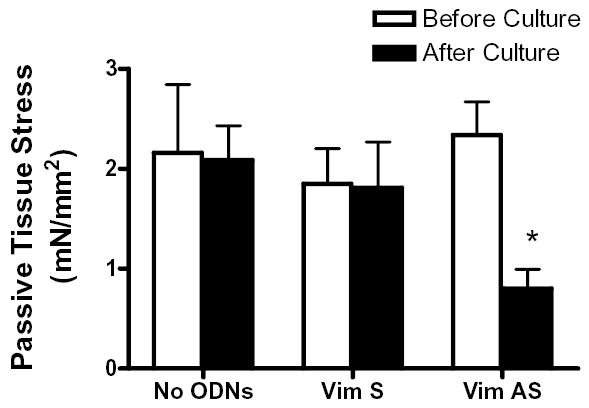
The passive tension of tracheal smooth muscle strips that had been treated with vimentin sense or antisense, or not treated with ODNs was determined before and after the organ culture. The passive tension in muscle strips treated with sense or in strips not treated with ODNs was comparable to preincubation tension after 2-day culture. The tension in muscle strips exposed to vimentin antisense was decreased after the incubation. Values are mean ± SE. Asterisk indicates significantly lower tension as compared to the value before incubation (n = 10, P < 0.05).
Myosin light chain phosphorylation is not attenuated in vimentin-deficient smooth muscle
Myosin light chain phosphorylation has been thought to mediate tension development in smooth muscle (26, 29). To determine whether vimentin depletion affects myosin light chain phosphorylation, a subset of tracheal smooth muscle strips that had been incubated with vimentin antisense or sense ODNs, or without ODNs were stimulated with 10−5 M ACh for 5 min, or they were not stimulated. These muscle strips were then frozen for the analysis of myosin light chain phosphorylation.
Although passive tension was significantly depressed, basal myosin light chain phosphorylation in antisense-treated tissues was not significantly distinguishable compared with muscle strips not treated with ODNs or with tissues treated with sense ODNs (Fig. 5, n = 4, P > 0.05). In addition, active force was suppressed in vimentin-deficient tissues. However, ACh stimulation was able to lead to a significant increase in myosin light chain phosphorylation in tissues treated with vimentin antisense ODNs. The average increases in myosin light chain phosphorylation in strips not treated with ODNs, or sense-treated and antisense-treated tissues were not significantly different 5 min after ACh stimulation (Fig. 5, n = 4, P > 0.05).
Figure 5. Effects of vimentin downregulation on myosin light chain phosphorylation.
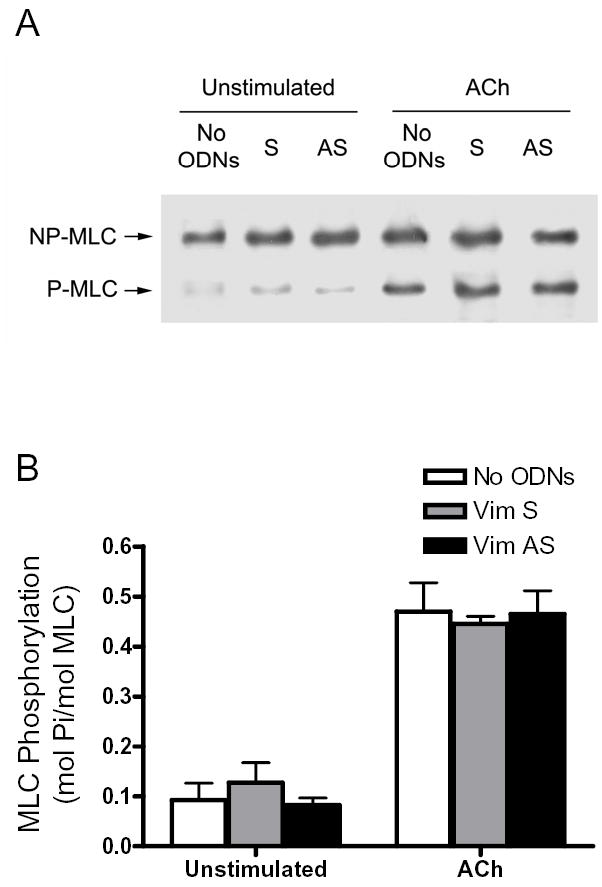
A, representative immunoblot showing unstimulated or ACh (10−5M, 5 min) stimulated myosin light chain (MLC) phosphorylation in tracheal smooth muscle strips that had been incubated with no ODNs, or with vimentin sense ODNs (S) or vimentin antisense ODNs (AS). NP-MLC, non-phosphorylated MLC; P-MLC, phosphorylated MLC. B, Differences between antisense-treated, sense-treated, and no ODNs-treated strips were not statistically significant (P > 0.05). Values shown are mean ± SE (n = 4–5).
Vimentin depletion inhibits contractile force without attenuating increases in myosin light chain phosphorylation stimulated by KCl
We also assessed the effect of vimentin depletion on force development and myosin light chain phosphorylation in tracheal smooth muscle strips stimulated by KCl. Force and myosin light chain phosphorylation were measured 5 min after stimulation (22, 29, 36). Contractile force in response to KCl stimulation was lower in antisense-treated muscle strips than in muscle strips not treated with ODNs or strips treated with sense ODNs (Fig. 6A). Increases in myosin light chain phosphorylation in muscle tissues not treated with ODNs were similar to those in muscle strips treated with vimentin sense or antisense ODNs (Fig. 6B, P > 0.05).
Figure 6. Contractile force and myosin light chain phosphorylation in vimentin-deficient muscle strips stimulated by KCl.
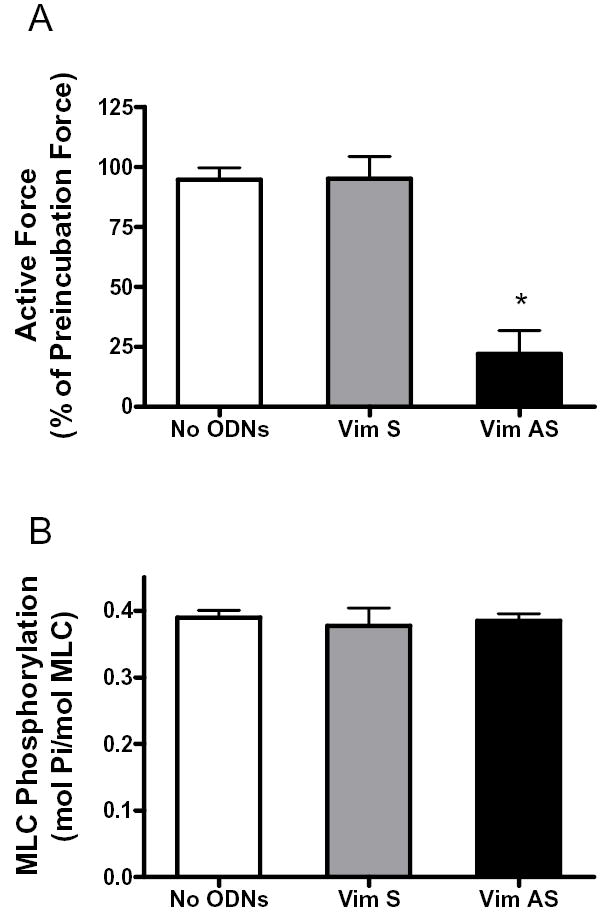
A, smooth muscle strips were contracted with 60 mM KCl before and after 2-day incubation without ODNs, or with vimentin sense or antisense. Mean active force in response to 60 mM KCl was quantitated as percent of KCl-induced force in each strip before incubation. Values are mean ± SE. Asterisk indicates significantly lower response as compared to muscles not treated with ODNs, or treated with sense ODNs (n = 6–7) (P < 0.05). B, myosin light chain phosphorylation in muscle strips incubated without ODNs, or with vimentin sense or antisense. Myosin light chain phosphorylation was measured in smooth muscle strips 5 min after stimulation with 60 mM KCl. Differences between antisense-treated, sense-treated and no ODNs-treated strips were not statistically significant. Values shown are mean ± SE (n = 4).
Downregulation of vimentin disrupts membrane-associated distribution of plakoglobin in smooth muscle tissues
We assessed the subcellular localization of plakoglobin in smooth muscle tissues exposed to vimentin antisense or sense ODNs. Smooth muscle strips that had been treated with vimentin antisense or sense oligonucleotides, or with no ODNs were cross-sectioned. These tissues were then immunofluorescently labeled for plakoglobin. As a control, these sections were also immunostained for vinculin, a cytoskeletal protein. Subcellular localization of these proteins was viewed under laser-scanning fluorescence microscope and quantitatively analyzed (See Materials and methods).
Plakoglobin staining in cells from tissues not treated with ODNs was primarily detected in cell periphery (Fig. 7A, panel a). In vimentin sense-treated tissues, plakoglobin was also localized on the membrane (Fig. 7A, panel b). However, the membrane distribution of plakoglobin in muscles treated with vimentin antisense ODNs was reduced dramatically (Fig. 7A, panel c). The subcellular localization of vinculin was comparable in tissues not treated with ODNs or in tissue treated with vimentin sense or antisense ODNs (Fig. 7A, panel a’, b’, and c’).
Figure 7. Subcellular distribution of plakoglobin in vimentin-depleted smooth muscle tissues.
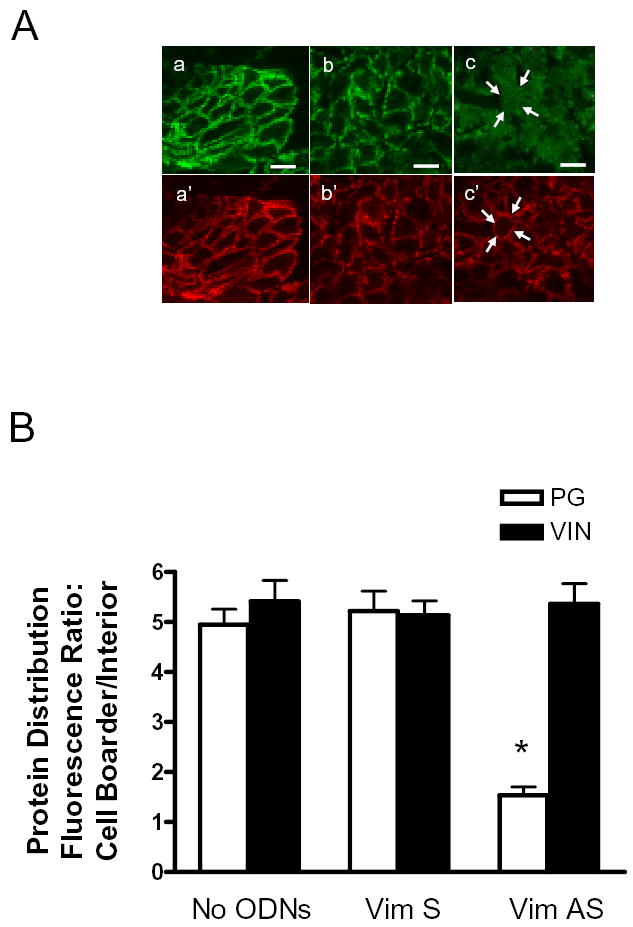
Tracheal smooth muscle strips that had been treated with vimentin antisense or sense oligonucleotides, or without oligonucleotides were cryosectioned. These sections were then immunofluorescently labeled for plakoglobin and vinculin. A, plakoglobin is mainly localized on the membrane in cells not treated with oligonucelotides (a) and in cells treated with vimentin sense oligonucleotides (b). The membrane distribution of plakoglobin is reduced in tissues treated with vimentin antisense oligonucleotides (c). However, vinculin localization is similar in tissues not treated with oligonucleotides (a’) and in muscle strips treated with vimentin sense (b’) or antisense (c’) oligonuceleotides. Bar, 5 μm. Arrows indicate the same cross section of the cell in two different images (c and c’). B, mean ratios of fluorescence intensity for plakoglobin (PG) and vinculin (VIN) in tracheal smooth muscle tissues not treated with ODNs (no ODNs), or treated with vimentin sense (Vim S) or vimentin antisense (Vim AS) oligonucleotides. Protein distribution in cells of the tissue cross-sections was expressed as the ratio of pixel intensity of the cell boarder to the cell interior (See Materials and methods). Each mean value was obtained from an average of 3–4 line scans in each of 20 no-ODN-treated cells, 20 sense-treated cells, and 20 antisense-treated cells from 3 experiments. * The fluorescence intensity ratio for plakoglobin in the antisense-treated muscles was significantly lower as compared to muscle tissues not treated with ODNs or tissues treated with sense ODNs (P < 0.05).
A total of 20 no-ODN-treated cells, 20 sense-treated cells, and 20 antisense-treated cells from 3 experiments were quantitatively analyzed for protein distribution. Plakoglobin fluorescence intensity ratio of cell periphery to interior in tissues treated with vimentin sense oligonucleotides was not significantly different from that in tissues not treated with ODNs (P > 0.05, n = 20). However, the fluorescence intensity ratio in tissues treated with vimentin antisense was significantly lower than tissues treated with no ODNs or vimentin sense ODNs (P < 0.05, n = 20). Pixel ratios of cell boarder to interior for the focal adhesion associated protein vinculin were not statistically different among smooth muscle tissues treated with no ODNs, or treated with vimentin sense or antisense ODNs (P > 0.05, n = 20).
DISCUSSION
Our present results demonstrate that vimentin downregulation in smooth muscle affects passive tension, active force, and plakoglobin distribution, but not myosin light chain phosphorylation. We propose that vimentin may play an essential role in tension development in smooth muscle, which is not mediated by myosin light chain phosphorylation.
Although it has long been shown that vimentin and desmin are major intermediate filament proteins in smooth muscle, their spatial distribution in differentiated smooth muscle cells is not fully understood. In this report, we have observed that vimentin is mainly localized in the myoplasm whereas desmin is primarily distributed in cell cortex. These results lead us to suggest that vimentin filaments in smooth muscle cells may link to dense bodies and to desmosomes while desmin filaments localized in cell periphery may reinforce the linkage of vimentin filaments to the desmosomes.
Primary function of the intermediate filament network has been thought to maintain cell shape and tissue integrity (1, 8, 25). To evaluate the role of the intermediate filament protein vimentin, smooth muscle strips were treated with antisense oligonucleotides against vimentin. Treatment with antisense oligonucleotides selectively downregulated vimentin protein expression. Moreover, electron microscopy analysis revealed that the ultrastructural organization of contractile filaments was not disrupted in smooth muscle tissues treated with vimentin antisense oligonucleotides. Microfilaments of astrocytes from vimentin knockout mice have also been shown to be similar to those from wild type mice (16). Vimentin downregulation inhibited passive tension and active force in response to stimulation with the muscarinic agonist or KCl depolarization. These results suggest that the depression of basal tension and active force cannot be attributed to general decline in protein expression or the disruption of contractile filaments in smooth muscle tissues treated with vimentin antisense oligonucleotides.
Phosphorylation of the 20-kDa regulatory light chain of myosin by Ca2+/calmodulin-regulated myosin light chain kinase initiates cross-bridge cycling and smooth muscle contraction (26, 27). The state of myosin light chain phosphorylation can also be regulated by Rho kinase (26). Furthermore, the subcellular localization of vimentin filaments affects the distribution of Rho kinase in Hela cells (24). Thus we considered the possibility that the depression of active force development in vimentin-deficient tissues in response to activation with contractile stimuli could be due to the inhibition of contractile protein activation. However, myosin light chain phosphorylation in response to contractile stimulation is normal while active force in the vimentin-depleted strips is dramatically depressed. Additionally, basal myosin light chain phosphorylation has also been thought to be associated with passive tension. In this report, passive tension, but not basal myosin light chain phosphorylation, was attenuated in vimentin-depleted tissues. Therefore, the inhibition of active force and passive tension in the vimentin-deficient tissues does not stem from the inhibition of myosin light chain phosphorylation.
Desmosomes are complex intercellular junctions specialized to provide strong but dynamic cell-cell adhesion in a variety of cell types and tissues including smooth muscle cells (15, 18, 25). As a major component of the desmosomes, plakoglobin links intermediate filaments to desmosomal cadherins (desmoglein and desmocollin) on the membrane, and has been used as a desmosomal marker (15, 25). Lack of plakoglobin in murine keratinocytes impairs the junctional incorporation of desmosomal components (40). Overexpression of plakoglobin in human fibrosarcoma cell and COS-7 cells promotes clustering of desmosomal plaque at intercellular borders (3). In this report, the downregulation of vimentin in smooth muscle impaired the membrane-associated localization of plakoglobin, indicating that the structure of desmosomes may be disrupted in vimentin-deficient smooth muscle cells. The disruption of desmosomal assembly may affect intercellular mechanical transduction and thus force development in these smooth muscle tissues.
Contractile stimulation induces the spatial reorganization of vimentin intermediate filaments in cultured smooth muscle cells. The spatial rearrangement of vimentin filaments may be part of cytoskeletal remodeling that affects force development during activation with contractile agonists (28). In addition, Ca2+/calmodulin-dependent kinase IIγ (CamKIIγ) has been shown to regulate arterial smooth muscle contraction (23). CamKIIγ G-2 (a novel form of CamKII) is associated with vimentin filaments in unstimulated differentiated arterial smooth muscle cells. Upon contractile stimulation, CamKIIγ G-2 is released into the cytosol and subsequently targeted to cortical dense plaques, which has been proposed to be an important signaling process in smooth muscle cells (17). Thus, it is possible that the structural reorganization of the vimentin network may facilitate the redistribution of signaling molecules such as CamKIIγ G-2 during contractile activation of smooth muscle. Deficiency of vimentin protein may impair the translocation of certain signaling molecules in smooth muscle in response to contractile activation.
The intermediate network has been proposed to interact with the actin cytoskeleton in certain cell types including macrophages, epithelial cells, and fibroblasts (7, 10, 11). Treatment of epithelial cells with cytochalasin D, which is known to inhibit actin polymerization, disrupts the organization of keratin intermediate filament network (10). Recent studies have also shown that contractile activation of smooth muscle induces actin polymerization, which may be associated with tension development in smooth muscle (2, 9, 20, 30, 34, 37, 42). Although disruption of actin stress fibers does not affect the reorganization of vimentin filaments in cultured smooth muscle cells and Hela cells (4, 28), we do not rule out the possibility that lack of vimentin protein may interfere with the reorganization of actin cytoskeleton in the vimentin-deficient smooth muscle strips in response to contractile stimulation.
In fibroblasts, intermediate filaments and microtubules form closely associated parallel arrays that are distributed throughout the cytoplasm. Treatment with the microtubule disruption agents nocodazole or colchicines induces vimentin filament reorganization (5). Thus we speculate that vimentin deficiency could reciprocally influence structural array of microtubules in these smooth muscle strips, which might be associated with the decrease in force development. However, reports on the role of microtubules in affecting smooth muscle contraction are controversial (21, 41). Treatment with nocodazole slightly enhanced KCl-induced vascular muscle contraction by increasing intracellular calcium, not by affecting mechanical stiffness.
In summary, the downregulation of vimentin in smooth muscle inhibits passive tension and active force development, but not myosin light chain phosphorylation. The lack of vimentin protein decreases the membrane-associated distribution of plakoglobin, a desmosomal protein. We conclude that vimentin is necessary for force development in smooth muscle, which is not regulated by the state of myosin light chain phosphorylation. Vimentin deficiency impairs the structural organization of desmosomes, which may be associated with the decrease in force development.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grants HL-75388 and an American Heart Association Scientist Development Grant (to D.D.T.). The authors thank Susan J. Gunst, Amy M. Spinelli, and Ping Tao for their assistance.
References
- 1.Ashton FT, Somlyo AV, Somlyo AP. The contractile apparatus of vascular smooth muscle: intermediate high voltage stereo electron microscopy. J Mol Biol. 1975;98:17–29. doi: 10.1016/s0022-2836(75)80098-2. [DOI] [PubMed] [Google Scholar]
- 2.Barany M, Barron JT, Gu L, Barany K. Exchange of the actin-bound nucleotide in intact arterial smooth muscle. J Biol Chem. 2001;276:48398–48403. doi: 10.1074/jbc.M106227200. [DOI] [PubMed] [Google Scholar]
- 3.Bornslaeger EA, Godsel LM, Corcoran CM, Park JK, Hatzfeld M, Kowalczyk AP, Green KJ. Plakophilin 1 interferes with plakoglobin binding to desmoplakin, yet together with plakoglobin promotes clustering of desmosomal plaque complexes at cell-cell borders. J Cell Sci. 2001;114:727–738. doi: 10.1242/jcs.114.4.727. [DOI] [PubMed] [Google Scholar]
- 4.Chan W, Kozma R, Yasui Y, Inagaki M, Leung T, Manser E, Lim L. Vimentin intermediate filament reorganization by Cdc42: involvement of PAK and p70 S6 kinase. Eur J Cell Biol. 2002;81:692–701. doi: 10.1078/0171-9335-00281. [DOI] [PubMed] [Google Scholar]
- 5.Chang L, Goldman RD. Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol. 2004;5:601–613. doi: 10.1038/nrm1438. [DOI] [PubMed] [Google Scholar]
- 6.Chidgey M. Desmosomes and disease: an update. Histol Histopathol. 2002;17:1179–1192. doi: 10.14670/HH-17.1179. [DOI] [PubMed] [Google Scholar]
- 7.Correia I, Chu D, Chou YH, Goldman RD, Matsudaira P. Integrating the actin and vimentin cytoskeletons. adhesion-dependent formation of fimbrin-vimentin complexes in macrophages. J Cell Biol. 1999;146:831–842. doi: 10.1083/jcb.146.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrod DR, Merritt AJ, Nie Z. Desmosomal adhesion: structural basis, molecular mechanism and regulation. Mol Membr Biol. 2002;19:81–94. doi: 10.1080/09687680210132476. [DOI] [PubMed] [Google Scholar]
- 9.Gerthoffer WT, Gunst SJ. Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol. 2001;91:963–972. doi: 10.1152/jappl.2001.91.2.963. [DOI] [PubMed] [Google Scholar]
- 10.Green KJ, Geiger B, Jones JC, Talian JC, Goldman RD. The relationship between intermediate filaments and microfilaments before and during the formation of desmosomes and adherens-type junctions in mouse epidermal keratinocytes. J Cell Biol. 1987;104:1389–1402. doi: 10.1083/jcb.104.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green KJ, Goldman RD. Evidence for an interaction between the cell surface and intermediate filaments in cultured fibroblasts. Cell Motil Cytoskeleton. 1986;6:389–405. doi: 10.1002/cm.970060405. [DOI] [PubMed] [Google Scholar]
- 12.Halayko AJ, Salari H, MA X, Stephens NL. Markers of airway smooth muscle cell phenotype. Am J Physiol. 1996;270:L1040–51. doi: 10.1152/ajplung.1996.270.6.L1040. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann H, Aebi U. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol. 2000;12:79–90. doi: 10.1016/s0955-0674(99)00060-5. [DOI] [PubMed] [Google Scholar]
- 14.Johansson B, Eriksson A, Virtanen I, Thornell LE. Intermediate filament proteins in adult human arteries. Anat Rec. 1997;247:439–448. doi: 10.1002/(SICI)1097-0185(199704)247:4<439::AID-AR1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 15.Ko KS, McCulloch CA. Intercellular mechanotransduction: cellular circuits that coordinate tissue responses to mechanical loading. Biochem Biophys Res Commun. 2001;285:1077–1083. doi: 10.1006/bbrc.2001.5177. [DOI] [PubMed] [Google Scholar]
- 16.Lepekhin EA, Eliasson C, Berthold CH, Berezin V, Bock E, Pekny M. Intermediate filaments regulate astrocyte motility. J Neurochem. 2001;79:617–625. doi: 10.1046/j.1471-4159.2001.00595.x. [DOI] [PubMed] [Google Scholar]
- 17.Marganski WA, Gangopadhyay SS, Je HD, Gallant C, Morgan KG. Targeting of a novel Ca+2/calmodulin-dependent protein kinase II is essential for extracellular signal-regulated kinase-mediated signaling in differentiated smooth muscle cells. Circ Res. 2005;97:541–549. doi: 10.1161/01.RES.0000182630.29093.0d. [DOI] [PubMed] [Google Scholar]
- 18.Matsushita T, Oyamada M, Kurata H, Masuda S, Takahashi A, Emmoto T, Shiraishi I, Wada Y, Oka T, Takamatsu T. Formation of cell junctions between grafted and host cardiomyocytes at the border zone of rat myocardial infarction. Circulation. 1999;100:II262–II268. doi: 10.1161/01.cir.100.suppl_2.ii-262. [DOI] [PubMed] [Google Scholar]
- 19.Meriane M, Mary S, Comunale F, Vignal E, Fort P, Gauthier-Rouviere C. Cdc42Hs and Rac1 GTPases induce the collapse of the vimentin intermediate filament network. J Biol Chem. 2000;275:33046–33052. doi: 10.1074/jbc.M001566200. [DOI] [PubMed] [Google Scholar]
- 20.Opazo SA, Zhang W, Wu Y, Turner CE, Tang DD, Gunst SJ. Tension development during contractile stimulation of smooth muscle requires recruitment of paxillin and vinculin to the membrane. Am J Physiol Cell Physiol. 2004;286:C433–C447. doi: 10.1152/ajpcell.00030.2003. [DOI] [PubMed] [Google Scholar]
- 21.Paul RJ, Bowman PS, Kolodney MS. Effects of microtubule disruption on force, velocity, stiffness and [Ca(2+)](i) in porcine coronary arteries. Am J Physiol Heart Circ Physiol. 2000;279:H2493–H2501. doi: 10.1152/ajpheart.2000.279.5.H2493. [DOI] [PubMed] [Google Scholar]
- 22.Rembold CM, Murphy RA. Histamine concentration and Ca2+ mobilization in arterial smooth muscle. Am J Physiol. 1989;257:C122–C128. doi: 10.1152/ajpcell.1989.257.1.C122. [DOI] [PubMed] [Google Scholar]
- 23.Rokolya A, Singer HA. Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. Am J Physiol Cell Physiol. 2000;278:C537–C545. doi: 10.1152/ajpcell.2000.278.3.C537. [DOI] [PubMed] [Google Scholar]
- 24.Sin WC, Chen XQ, Leung T, Lim L. RhoA-binding kinase alpha translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol Cell Biol. 1998;18:6325–6339. doi: 10.1128/mcb.18.11.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Small JV. Structure-function relationships in smooth muscle: the missing links. Bioessays. 1995;17:785–792. doi: 10.1002/bies.950170908. [DOI] [PubMed] [Google Scholar]
- 26.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 522 Pt. 2000;2:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang DC, Stull JT, Kubota Y, Kamm KE. Regulation of the Ca2+ dependence of smooth muscle contraction. J Biol Chem. 1992;267:11839–11845. [PubMed] [Google Scholar]
- 28.Tang DD, Bai Y, Gunst SJ. Silencing of p21-activated kinase attenuates vimentin phosphorylation on Ser-56 and reorientation of the vimentin network during stimulation of smooth muscle cells by 5-hydroxytryptamine. Biochem J. 2005;388:773–783. doi: 10.1042/BJ20050065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang DD, Gunst SJ. Depletion of focal adhesion kinase by antisense depresses contractile activation of smooth muscle. Am J Physiol Cell Physiol. 2001;280:C874–C883. doi: 10.1152/ajpcell.2001.280.4.C874. [DOI] [PubMed] [Google Scholar]
- 30.Tang DD, Gunst SJ. The small GTPase Cdc42 regulates actin polymerization and tension development during contractile stimulation of smooth muscle. J Biol Chem. 2004;279:51722–51728. doi: 10.1074/jbc.M408351200. [DOI] [PubMed] [Google Scholar]
- 31.Tang DD, Tan J. Downregulation of profilin with antisense oligodeoxynucleotides inhibits force development during stimulation of smooth muscle. Am J Physiol Heart Circ Physiol. 2003;285:H1528–H1536. doi: 10.1152/ajpheart.00188.2003. [DOI] [PubMed] [Google Scholar]
- 32.Tang DD, Tan J. Role of Crk-Associated Substrate in the Regulation of Vascular Smooth Muscle Contraction. Hypertension. 2003;42:858–863. doi: 10.1161/01.HYP.0000085333.76141.33. [DOI] [PubMed] [Google Scholar]
- 33.Tang DD, Tao P, Bai Y. P21-activated protein kinase 1 (PAK1) is required for vimentin phosphorylation at ser-56 during stimulation of smooth muscle tissues. FASEB J. 2004;18:A1085. [Google Scholar]
- 34.Tang DD, Turner CE, Gunst SJ. Expression of non-phosphorylatable paxillin mutants in canine tracheal smooth muscle inhibits tension development. J Physiol. 2003;553:21–35. doi: 10.1113/jphysiol.2003.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang DD, Turner CE, Gunst SJ. Expression of non-phosphorylatable paxillin mutants in canine tracheal smooth muscle inhibits tension development. J Physiol. 2003;553:21–35. doi: 10.1113/jphysiol.2003.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang DD, Wu MF, Opazo Saez AM, Gunst SJ. The focal adhesion protein paxillin regulates contraction in canine tracheal smooth muscle. J Physiol. 2002;542:501–513. doi: 10.1113/jphysiol.2002.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang DD, Zhang W, Gunst SJ. The Adapter Protein CrkII Regulates Neuronal Wiskott-Aldrich Syndrome Protein, Actin Polymerization, and Tension Development during Contractile Stimulation of Smooth Muscle. J Biol Chem. 2005;280:23380–23389. doi: 10.1074/jbc.M413390200. [DOI] [PubMed] [Google Scholar]
- 38.Valgeirsdottir S, Claesson-Welsh L, Bongcam-Rudloff E, Hellman U, Westermark B, Heldin CH. PDGF induces reorganization of vimentin filaments. J Cell Sci. 1998;111 ( Pt 14):1973–1980. doi: 10.1242/jcs.111.14.1973. [DOI] [PubMed] [Google Scholar]
- 39.Wede OK, Lofgren M, Li Z, Paulin D, Arner A. Mechanical function of intermediate filaments in arteries of different size examined using desmin deficient mice. J Physiol. 2002;540:941–949. doi: 10.1113/jphysiol.2001.014910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin T, Getsios S, Caldelari R, Godsel LM, Kowalczyk AP, Muller EJ, Green KJ. Mechanisms of plakoglobin-dependent adhesion: desmosome-specific functions in assembly and regulation by epidermal growth factor receptor. J Biol Chem. 2005;280:40355–40363. doi: 10.1074/jbc.M506692200. [DOI] [PubMed] [Google Scholar]
- 41.Zhang D, Jin N, Rhoades RA, Yancey KW, Swartz DR. Influence of microtubules on vascular smooth muscle contraction. J Muscle Res Cell Motil. 2000;21:293–300. doi: 10.1023/a:1005600118157. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, Wu Y, Du L, Tang DD, Gunst SJ. Activation of the Arp2/3 complex by N-WASp is required for actin polymerization and contraction in smooth muscle. Am J Physiol Cell Physiol. 2005;288:C1145–C1160. doi: 10.1152/ajpcell.00387.2004. [DOI] [PubMed] [Google Scholar]


