Abstract
A family of histidine-rich peptides, histatins, is secreted by the parotid gland in mammals and exhibits marked inhibitory activity against a number of Candida species. We were particularly interested in the mechanism by which histidine-rich peptides inhibit fungal growth, because our laboratory has synthesized a variety of such peptides for drug and nucleic acid delivery. In contrast to naturally occurring peptides that are linear, peptides made on synthesizers can be varied with respect to their degrees of branching. Using this technology, we explored whether histidine-lysine (HK) polymers of different complexities and degrees of branching affect the growth of several species of Candida. Polymers with higher degrees of branching were progressively more effective against Candida albicans, with the four-branched polymer, H2K4b, most effective. Furthermore, H2K4b accumulated efficiently in C. albicans, which may indicate its ability to transport other antifungal agents intracellularly. Although H2K4b had greater antifungal activity than histatin 5, their mechanisms were similar. Toxicity in C. albicans induced by histatin 5 or branched HK peptides was markedly reduced by 4,4′-diisothiocyanato-stilbene-2,2′-disulfonate, an inhibitor of anion channels. We also determined that bafilomycin A1, an inhibitor of endosomal acidification, significantly decreased the antifungal activity of H2K4b. This suggests that the pH-buffering and subsequent endosomal-disrupting properties of histidine-rich peptides have a role in their antifungal activity. Moreover, the ability of the histidine component of these peptides to disrupt endosomes, which allows their escape from the lysosomal pathway, may explain why these peptides are both effective antifungal agents and nucleic acid delivery carriers.
The increased frequency of fungal infections in immunocompromised hosts and the emergence of strains resistant to conventional treatments indicate the need for new therapeutic approaches to combat these infections (11, 37). Within the Candida species, approximately half of the bloodstream infections are caused by C. albicans, with an increasing proportion due to C. glabrata, C. krusei, and C. parapsilosis. Candida is now the fourth most common cause of bloodstream infections in the United States, with an overall mortality from candidemia of 40% (39).
A promising group of agents that have activity against fungi resistant to amphotericin or azole agents is a class of cationic histidine-rich peptides called histatins (42). Histatins belong to a group of low-molecular-weight antimicrobial cationic peptides that have diverse amino acid sequences and structure and that are ubiquitous in the animal and plant kingdoms (12, 13, 17, 23, 32, 40). However, histatins are found exclusively in mammalian saliva (32). Of the 12 members in the histatin family, histatin 5, a proteolytic fragment of histatin 3, is the most potent and has fungistatic and/or fungicidal activity against several fungi, including Candida albicans, Candida glabrata, Candida krusei, Saccharomyces cerevisiae, Cryptococcus neoformans, and Aspergillus fumigatus.
Not surprisingly, the antifungal mechanism of histatins is different than the antimicrobial mechanism of other cationic peptides. Most cationic peptides are thought to solubilize bacterial membranes by interacting with the negative charges on the surface of the microbe. In contrast, histidine-rich peptides do not act by this mechanism; instead, these peptides act through a multistep process. After binding to the Ssa1/2 surface proteins (26), histatins appear to be internalized (16), and their primary intracellular target in fungi is the Trk1 potassium transporter (3). Interestingly, the Trk1p transporter has a dual function in mediating not only the uptake of potassium but also the efflux of chloride. Consequently, the fungistatic and fungicidal properties of histatin 5, manifested by loss of cytoplasmic small molecules and ions, including ATP and K+, may be reversed by anion channel inhibitors (e.g., 4,4′-diisothiocyanato-stilbene-2,2′-disulfonate [DIDS]) (3). In addition to its effects on ion channels, histatin 5 may chelate transitional metals, including zinc and copper, which have an important role in activating essential enzymes (27).
Since our laboratory has synthesized more than a hundred histidine-rich peptides for drug and nucleic acid delivery (9, 10, 24, 25), we were interested in examining their antifungal properties. In gene therapy studies, we found that branched HK polymers are significantly more effective than linear HK peptides at transporting genes (9, 24). The increased efficacy of branched HK polymers with their higher histidine content may be due in part to their ability to buffer and lyse endosomal vesicles more effectively than the linear HK polymers. In this study, we compared naturally occurring histidine-rich peptides with our synthetic HK polymers. Here we report that highly branched peptides are more fungistatic and fungicidal than those with fewer branches or naturally occurring linear peptides. Furthermore, our data indicate that by inducing endosomal lysis, the histidine constituent of these peptides promotes their antifungal activity.
MATERIALS AND METHODS
Cells.
Human microvascular endothelial cells (Cascade Biologics, Portland, OR), bovine aortic endothelial cells (Coriell Cell Repository, Camden, NJ), and human umbilical vein endothelial cells (pooled; Cascade Biologics) were used in this study. Bovine aortic endothelial cells were cultured in Dulbecco's modified essential medium (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum. Human microvascular endothelial cells and human umbilical vein endothelial cells were cultured in medium 131 with microvascular growth supplement (Cascade Biologics) and medium 200 with low serum growth supplement (Cascade Biologics), respectively. All mammalian cell lines were incubated at 37°C with 5% CO2 and 90% humidity.
Fungi.
The following fungi were obtained from the American Type Culture Collection (ATCC; Manassas, VA): Candida albicans, ATCC 10231, ATCC 76615, ATCC 90259, ATCC MYA-1237, and ATCC MYA-576 (fluconazole resistant); C. glabrata, ATCC 66032; C. tropicalis, ATCC 66029; C. kefyr, ATCC 66028; C. krusei, ATCC 14243; C. parapsilosis, ATCC 22019; Trichosporon cutaneum, ATCC 11115. These fungi were grown in yeast-maltose (YM) medium (Becton Dickinson, Sparks, MD) as detailed below in fungistatic studies. YM medium contains 0.3% yeast extract, 0.3% malt extract, 0.5% peptone, and 1.0% glucose.
Synthesis of polymers.
The biopolymer core facility at the University of Maryland synthesized the following nine HK polymers (see Table 1 for formulas and physical properties of HK polymers) on a Ranin Voyager solid-phase synthesizer (PTI, Tucson, AZ): H2K, H2K2b, H2K3b, H2K4b, H3K4b, H2K(L4)4b, H2K(L5)4b, H2L4b, and histatin 5. The linear and branched polymers were synthesized as previously described (45). If polymer purity was less than 95%, then polymers were further purified on a high-performance liquid chromatography column with System Gold operating software by using a Dynamax 21 4- by 250-mm C18 reversed-phase preparative column with a binary solvent system. Further analyses of the polymers were performed with a Voyager MALDI-TOF mass spectroscopy apparatus (Applied Biosystems, Foster City, CA) and amino acid analysis system (AAA Laboratory Service, Boring, OR).
TABLE 1.
Structural formulas for linear and branched histidine-containing peptides
| Peptide | Sequence | No. of amino acids | No. of branches | Mol. mass (Da) |
|---|---|---|---|---|
| H2K | KHKHHKHHKHHKHHKHHKHK | 20 | Linear | 2,688 |
| H2K2b | K(KHKHHKHHKHHKHHKHHKHK)2 | 43 | 2 | 5,505 |
| H2K3b | KK(KHKHHKHHKHHKHHKHHKHK)3 | 63 | 3 | 8,320 |
| H2K4b | KKK(KHKHHKHHKHHKHHKHHKHK)4 | 83 | 4 | 11,137 |
| H3K4b | KKK(KHHHKHHHHKHHHKHHHK)4 | 72 | 4 | 10,028 |
| H2K(L4)4b | KKK(KLKLHKLHKHHKHHKHHKLK)4 | 83 | 4 | 10,759 |
| H2K(L5)4b | KKK(KLKLHKLHKHHKHHKLHKLK)4 | 83 | 4 | 10,663 |
| H2L4b | KKK(KLKLLKLLKLLKLLKLKLK)4 | 83 | 4 | 9,985 |
| Histatin 5 | DSHAKRHHGYKRKFHEKHHSHRGY | 24 | Linear | 3,035 |
Antifungal assay.
The antifungal effects of different polymers were assessed in cultures of several fungi. Various amounts of the polymer (in 0.01% acetic acid and 0.2% bovine serum albumin in a volume of 100 μl) were added to 900 μl of YM medium containing approximately 1 × 105 CFU of yeast. The culture was then grown at room temperature on a rocker for about 48 h until the A600 of the untreated control was between 0.5 and 1.0. The A600 of samples was measured by using a microplate reader (Shimadzu, Japan), and growth inhibition was then determined as follows: percent fungi survival = 100 × (A600 of test sample)/(A600 of negative control). Each data point was obtained in triplicate. The MIC dose was determined by a slight modification of the NCCLS broth dilution method as previously described (29, 41). The concentration of polymer incubated with fungi is given in μg/ml and/or μM units. At selected dosages of the polymers, the fungi were plated on selected medium plates and numbers of CFU were counted.
We then examined the antifungal activity of the histidine-containing peptides at various time points. After C. albicans was diluted to approximately 105 CFU per ml, H2K4b (100 μg/ml; 9 μM) or histatin 5 (100 μg/ml; 33 μM) was added to the fungal culture. At specified time points of 0, 6, and 24 h, the cultures from the different treatment groups were spread onto a plate, and the colonies were counted after 40 h at room temperature.
LDH cytotoxicity assay.
Cells were subcultured into 96-well plates with 1 × 104 to 2 × 104 cells in 100 μl of medium. After cells reached 70 to 80% confluence, increasing amounts of the polymers (range, 0 to 200 μg/ml) were added to duplicate wells. After incubation at 37°C with 5% CO2 and 90% humidity, 100 μl of supernatant from each well was transferred into the corresponding well of an optically clear 96-well plate. To evaluate the cytotoxicity of polymers, a lactate dehydrogenase (LDH) cytotoxicity assay kit (BioVision, Mountain View, CA) was used according to the instructions of the manufacturer. In brief, the cellular supernatant fraction was added to each well containing lactate and formazan for 30 min at room temperature. The absorbance of all samples was measured at 500 nm by using a microtiter plate reader with reference absorbance set at 650 nm. After total cellular toxicity was induced by adding 1% Triton X-100 to the cells, the background was subtracted and the percent cellular toxicity was determined.
Intracellular accumulation of H2K4b.
Fluorescein-labeled H2K4b at a concentration of 5 μg/ml was incubated with C. albicans for 4 h in YM medium. After the cells were centrifuged and the supernatant discarded, the nucleic acid binding SYTO 59 fluorophore (20 μM; Invitrogen, Carlsbad, CA) in 1 ml of isotonic saline was added to the cells for 30 min. The yeast cells were then centrifuged again, resuspended in 150 mM NaCl, and mounted on a glass slide with a coverslip. The living cells were observed with an LSM 510 Meta confocal microscope (Zeiss, Thornwood, NY) using 488-nm excitation and 515- to 545-nm emission filters for fluorescein and a 543-nm excitation and 590-nm long pass emission filter for SYTO 59.
Bioluminescence ATP assay.
ATP was assayed as previously described (22). After 106 CFU of C. albicans in 1 ml of 10 mM sodium phosphate buffer (pH 7.4; Sigma) were incubated with or without DIDS (2 mM) at room temperature for 2 h, histatin 5 (final concentration, 50 or 100 μg/ml) or H2K4b (final concentration, 50 μg/ml) was added. The preparations were incubated at room temperature for 30 min with shaking, followed by centrifugation at 2,000 × g; 25 μl of the supernatant was added to 225 μl of boiling Tris-EDTA buffer, pH 8.0, boiled further for 3 min, and stored on ice until the ATP assay was performed. To measure the amount of ATP release, 50 μl of the luciferin-luciferase assay mixture (Sigma) was added to 25 μl of the boiled mixture and bioluminescence was measured on a luminometer (model TD-20/20; Turner Designs). Data are presented as means ± standard errors for four separate experiments.
Temperature-dependent and colocalization experiments.
Fluoresceinylated H2K4b (10 μg/ml) was incubated with C. albicans for 2 h at 4°C or at room temperature (RT; approximately 22°C). These yeast cells were then washed, suspended in isotonic NaCl, and mounted on a glass slide with a coverslip. The living cells were observed with a fluorescence microscope (Diaphot-TMD; Nikon, Tokyo, Japan) using a 488-nm excitation filter and an emission filter of 520 nm.
For colocalization studies H2K4b with an endocytic marker, AlexaFluor 594-labeled dextran (Invitrogen), and fluoresceinylated H2K4b were incubated with C. albicans for 2 h in YM medium. The cells were then washed, and the probes were observed with an LSM 510 microscope using 488-nm excitation and 515- to 545-nm emission filters for fluorescein and 543-nm excitation and 560-nm long pass emission filters for AlexaFluor 594.
Inhibition of ATP release by bafilomycin from C. albicans.
The above protocol to measure ATP levels was followed except that bafilomycin A1 (50 ng/ml; Sigma, St. Louis, Mo.) was first added to C. albicans overnight. Then, C. albicans was incubated with H2K4b at a concentration of 20 μg/ml (1.8 μM) for 0.5 or 2 h and ATP levels were measured.
RESULTS
Antifungal efficacy of linear and branched HK peptides.
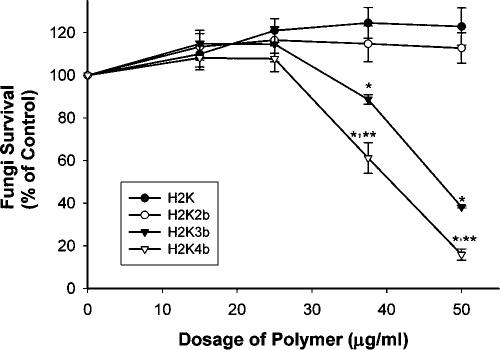
In previous studies, branched HK polymers were more effective in delivering genes into cells than were their linear counterparts (9, 24, 25). To determine whether the branched HK polymers also have more effective antifungal activity than linear ones, we compared different HK polymers that varied in the number of branches for their ability to inhibit the growth of C. albicans (Table 1; Fig. 1) (if not specified, we are referring to the ATCC 10231 isolate in the remainder of this report). These polymers included the four-branched HK polymer H2K4b, the three-branched polymer H2K3b, the two-branched polymer H2K2b, and the linear polymer H2K. Whereas at 50 μg/ml the linear H2K (18.6 μM) and the two-branched H2K2b (9.1 μM) had no effect on the growth of C. albicans, the three-branched H2K3b (6 μM) had an intermediate inhibitory effect. With H2K3b, the growth of C. albicans was reduced by 11.3% at 37.5 μg/ml (4.5 μM) and by 61.7% at 50 μg/ml (6.0 μM). The four-branched HK polymer, H2K4b, had the strongest antifungal effect. The growth of C. albicans was reduced by H2K4b to nearly 40% at 37.5 μg/ml (3.4 μM) and 85% at 50 μg/ml (4.5 μM). Thus, more highly branched HK polymers were more effective at inhibiting the growth of C. albicans than their lesser-branched counterparts.
FIG. 1.
Effect of various degrees of branching HK peptides on growth of C. albicans. Increasing amounts (final concentration: 0, 15, 25, 37.5, and 50 μg/ml) of the polymers, H2K, H2K2b, H2K3b, or H2K4b, were added to C. albicans (5X105 CFU/ml). At 50 μg/ml, the μmolar concentration of H2K is 18.6, H2K2b is 9.1, H2K3b is 6, and H2K4b is 4.5. After the fungi were rotated at room temperature for about 24 h in the presence or absence of these polymers, growth inhibition was then determined. Significance was determined by the Student-Newman-Keuls multiple comparisons test: *, P < 0.05, H2K4b, H2K3b versus. H2K2b, HK; **, P < 0.05, H2K4b versus. H2K3b.
Comparison of histatin 5 and H2K4b.
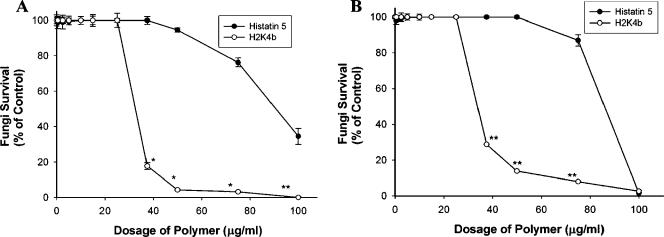
Because histatin 5 was effective in reducing the growth of fungi, we initially compared H2K4b with histatin 5 at two time points (6 and 24 h) (Table 2). At 100 μg/ml, H2K4b (9 μM) significantly reduced the CFU of C. albicans compared to histatin 5 (33 μM). Furthermore, at this concentration of H2K4b (100 μg/ml; 9 μM), there were fewer CFU at 24 h than at 0 or 6 h, indicating fungicidal activity. We then examined the antifungal effect of H2K4b on C. albicans and C. kefyr in greater detail. At 50 μg/ml, H2K4b (4.5 μM) markedly inhibited the growth of C. albicans by approximately 90%, but histatin 5 (16.5 μM) did not inhibit growth (Fig. 2A). At 100 μg/ml, H2K4b (9 μM) completely inhibited C. albicans, while histatin 5 (33 μM) inhibited growth by only 65.5%. Similarly, H2K4b inhibited the growth of C. kefyr significantly more than histatin 5 at comparable concentrations (Fig. 2B), which established that H2K4b was more effective than histatin 5 at inhibiting growth in these fungi. Although the peptide concentrations in Fig. 2 are expressed in μg/ml, the difference between histatin 5 and H2K4b in their antifungal efficacies is more striking when they are compared on a molar basis. For example, against C. kefyr, the MIC of H2K4b was 9 μM, while the MIC of histatin 5 was 33 μM.
TABLE 2.
Effect of time of treatment with H2K4b polymer on growth of C. albicans
| Treatment group | CFU (102)a
|
|
|---|---|---|
| 6 h | 24 h | |
| Control | 1.93 ± 0.06 | >104 |
| Histatin 5 | 1.00 ± 0.08 | 5 × 103 |
| H2K4b | 1.07 ± 0.03 | 0.60 ± 0.04* |
Fold increase in CFU in different treatment groups compared to initial CFU in the untreated group. The mean CFU of the untreated group at time zero was 161 ± 12.9, and this was given a value of 1. *, P < 0.025 for H2K4b (24 h) versus H2K4b (6 h) or control at time zero. Histatin 5 was used at 100 μg/ml (33 μM), and H2K4b was used at 100 μg/ml (9 μM).
FIG. 2.
Comparison of H2K4b and histatin 5 against C. albicans (A) and C. kefyr (B). Several doses of H2K4b or histatin 5 (0, 0.5, 2.5, 5, 10, 15, 25, 37.5, 50, 75, and 100 μg/ml) were added to YM medium containing either C. albicans or C. kefyr. At 100 μg/ml, the μmolar concentrations of histatin 5 and H2K4b are 33 and 9 μM, respectively. The fungi were then rotated at room temperature for 24 h and growth inhibition by the polymer was determined as indicated in Fig. 1. Experiments were performed in triplicate and the data are means and standard errors. *, P < 0.001, **, P < 0.01; H2K4b versus. Histatin 5.
Antifungal efficacy of H2K4b.
Since the four-branched HK polymer, H2K4b, had significant antifungal activity against C. albicans and C. kefyr, we assessed the efficacy of this polymer at several concentrations against several additional fungi, primarily Candida species. In Table 3, the MICs of H2K4b are listed, and H2K4b effectively inhibited all fungi, with MICs ranging from 37.5 μg/ml (3.4 μM) to 125 μg/ml (10.8 μM) (C. albicans, C. parapsilosis, C. glabrata, C. tropicalis, C. krusei, C. kefyr, and T. cutaneum). H2K4b was most effective at inhibiting the growth of C. tropicalis and C. krusei, with a MIC of 37.5 μg/ml (3.4 μM). Several different strains of C. albicans, including one that was resistant to fluconazole, were also sensitive to H2K4b, with all having MICs of 100 μg/ml (9 μM). Thus, the efficacy of H2K4b against all fungal species based on MIC determinations was as follows (from most to least effective): C. tropicalis = C. krusei > C. glabrata = T. cutaneum > C. albicans (ATCC 10231, ATCC 76615, ATCC 90259, ATCC MYA-1237, and ATCC MYA-576) = C. kefyr > C. parapsilosis.
TABLE 3.
Antifungal effects of H2K4b
| Fungal species | MICa
|
|
|---|---|---|
| μg/ml | μM | |
| C. albicans 10231 | 100 | 9 |
| C. albicans 76615 | 100 | 9 |
| C. albicans 90259 | 100 | 9 |
| C. albicans MYA-1237 | 100 | 9 |
| C. albicans MYA-576 | 100 | 9 |
| C. parapsilosis | 125 | 10.8 |
| C. glabrata | 75 | 6.7 |
| C. tropicalis | 37.5 | 3.4 |
| C. krusei | 37.5 | 3.4 |
| C. kefyr | 100 | 9 |
| T. cutanuem | 75 | 6.7 |
To determine the MIC of H2K4b, increasing amounts of the peptide (0, 0.5, 2.5, 5, 10, 15, 25, 37.5, 50, 75, 100, 125, and 150 μg/ml) were added to the YM culture containing 5 × 105 CFU/ml of the fungal species. The fungi were rotated at room temperature until the optical density at 600 nm of the untreated control group was between 0.5 and 1.0.
Comparison of H2K4b with its analogs.
Because increased hydrophobicity of peptides may increase their association with membranes and their antimicrobial activity (12, 19), we increased the hydrophobicity of the H2K4b polymer by replacing one or more of its lysines with leucines in the terminal branches. The MICs of these polymers with increased hydrophobicity [H2K(L4)4b, H2K(L5)4b, and H2L4b] (Table 1) were then compared with H2K4b. Although these hydrophobic alterations did not improve (and frequently reduced) the efficacy of the polymer against the majority of fungi, the modified H2K(L5)4b was more effective at inhibiting the growth of T. cutaneum compared to H2K4b (Table 4). Against T. cutaneum, the MIC of H2K4b was 75 μg/ml (6.7 μM), while the MIC of H2K(L5) was 15 μg/ml (1.4 μM). Nevertheless, H2K4b was clearly the most effective polymer against most fungi. Furthermore, when H2K4b was compared with another four-branched histidine-lysine polymer with different sequences, H2K4b was more effective than H3K4b as a fungistatic agent against C. albicans (Table 4); the MIC of H2K4b was 9 μM, while the MIC of H3K4b was 15 μM. Notably, H3K4b has the same number of histidines as H2K4b but fewer lysines (Table 1).
TABLE 4.
Effects of patterns of branched polymers
| Fungal species | MIC [μg/ml (μM)]a
|
||||
|---|---|---|---|---|---|
| H2L4b | H2K(L4)4b | H2K(L5)4b | H3K4b | H2K4b | |
| T. cutaneum | 50 (5) | 50 (4.6) | 15 (1.4) | NDb | 75 (6.7) |
| C. albicans | —c | — | — | 150 (15) | 100 (9) |
| C. tropicalis | 15 (1.5) | 15 (1.4) | 15 (1.4) | ND | 37.5 (3.4) |
| C. kefyr | — | — | — | 125 (12.5) | 100 (9) |
The MICs of several polymers [H2L4b, H2K(L4)4b, H2K(L5)4b, H3K4b, and H2K4b] against several fungi were determined The polymers at concentrations between 15 μg/ml and 150 μg/ml were added to YM cultures containing fungi (5 × 105 CFU/ml), and the tubes were rotated at room temperature as described in Materials and Methods.
ND, not done.
—, MIC > 150 μg/ml.
Toxicity of H2K4b against human cell lines.
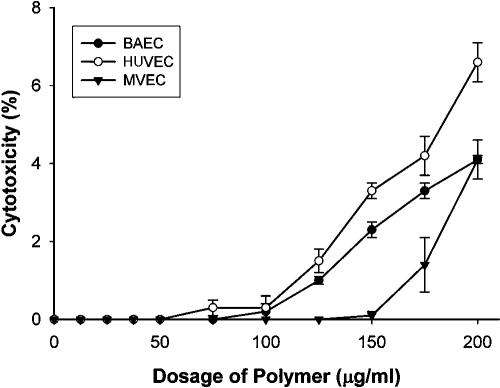
To determine whether H2K4b was toxic to human cells, we examined its cytotoxic effects on three cell lines: large vessel endothelial cells, bovine aortic endothelial cells, and microvascular endothelial cells. At a concentration of 100 μg/ml, at which H2K4b markedly inhibited fungal growth, this polymer had minimal cytotoxicity and caused cell death in less than 1% in all cell lines tested (Fig. 3). On exposure to the polymer at 200 μg/ml, cytotoxicity was observed in 6.1% of large vessel endothelial cells, 4.5% of bovine aortic endothelial cells, and 4.7% of microvascular endothelial cells. In addition to these in vitro studies, we have observed no acute toxicity in mice administered H2K4b at dosages up to 400 μg intravenously over a 10-second period. Histology of major tissues (heart, lung, liver, and kidney) 24 h after injection did not reveal toxicity (data not shown).
FIG. 3.
LDH-cytotoxicity assay of H2K4b on several cell lines. Polymers were added to medium of human umbilical vein endothelial cells (HUVEC), microvascular endothelial cells (MVEC), or bovine endothelial cells (BAEC). Cytotoxicity was assessed by the LDH-cytotoxicity assay kit. Experimental values represent the average of three separate experiments.
Intracellular accumulation of H2K4b.
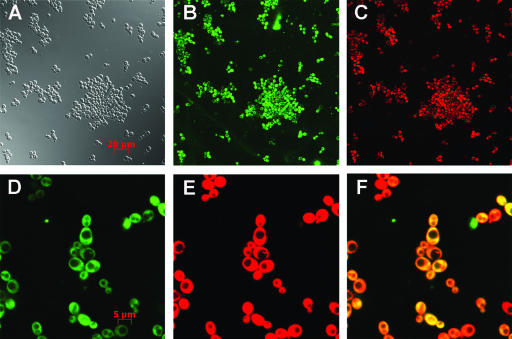
Previous investigations have provided evidence that internalization of histatin 5 is necessary for its antifungal toxicity (2, 26). As a result, we determined if H2K4b accumulated intracellularly. After C. albicans was incubated for 4 h with a fluorescent-labeled conjugate of H2K4b peptide, there was substantial intracellular colocalization of the polymer with a nuclear and cytosolic staining fluorophore (Syto 59) (Fig. 4). Furthermore, nearly 100% of yeast cells accumulated H2K4b intracellularly (Fig. 4A, B, and C). For these experiments of short duration, we selected a concentration of H2K4b (5 μg/ml) that was significantly lower than its inhibitory activity for C. albicans. Notably, labeled H2K4b also efficiently accumulated intracellularly in other Candida species (data not shown).
FIG. 4.
Intracellular Accumulation of H2K4b. Fluorescein-labeled H2K4b was incubated with C. albicans for 4 h in YM medium, Syto 59 was incubated with cells for 20 min to localize nucleic acids. The living cells were then examined with an LSM 510 confocal microscope. Differential interference contrast image (DIC) (A) was shown with fluorescein-labeled H2K4b staining image (B) and SYTO 59 dye staining image (C). Enlarged fluorescein-labeled H2K4b staining image (D) and SYTO 59 dye staining image (E) were shown with the overlaid image (F) to visualize colocalization.
Comparison of antifungal mechanisms of H2K4b and histatin 5.
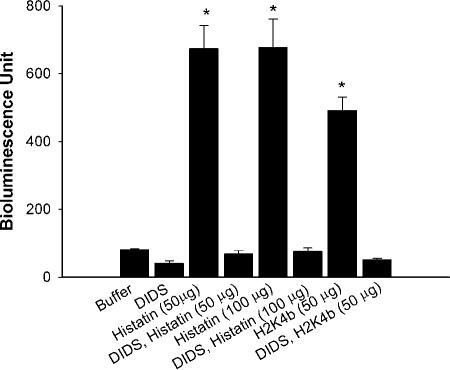
We next determined if histatin 5 and H2K4b had a similar fungicidal mechanism for C. albicans. Release of ATP from C. albicans by histatin 5 has been shown to be an early manifestation of toxicity. The release of ATP is not due to lysis of the Candida cells, but ATP is released from ion channels into the medium before the yeast cells are killed (22). Prior incubation with a small anion channel blocker such as DIDS, which is known to block the K+ ion channels and ATP efflux, significantly reduced toxicity by histatin 5. Similar to histatin 5, we found that DIDS blocked ATP release from C. albicans in the presence of H2K4b by 10-fold (Fig. 5). This indicates that H2K4b and histatin 5 share at least some fungicidal mechanistic properties.
FIG. 5.
Release of ATP from Candida caused by incubation with histatin 5 and H2K4b. After C. albicans were incubated for 30 min with and without DIDS, the cells were washed, and then histatin 5 (50 or 100 μg/ml) or H2K4b (50 μg/ml) was added to the culture for 30 min. The amount of released ATP was then measured in each treatment group. Student t test, *, P < 0.05, peptide treatment without DIDS versus. peptide treatment with DIDS.
Role of endocytosis in the uptake of H2K4b.
Three studies were done that supported that H2K4b uptake into the cytosol was by endocytosis.
(i) Temperature dependence study.
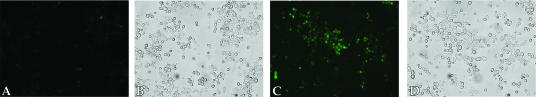
Temperature dependency has been used by several groups to suggest that uptake of macromolecules is by energy-dependent endocytosis (14, 28, 33, 44). At 4°C, there was little association of the labeled H2K4b polymer with the cells during the 2-h incubation (Fig. 6). There was no difference between yeast cells exposed to labeled H2K4b for brief periods of time (<1 min) and for 2 h at 4°C (data not shown). In contrast, there was significant accumulation of the labeled H2K4b polymer in yeast after a 2-h incubation at room temperature. The uptake difference between RT and 4°C of H2K4b indicates a transport system dependent on elevated energy levels. Although temperature dependency is consistent with endocytosis, we carried out further experiments to identify endocytosis specifically as the primary mode of transport.
FIG. 6.
Uptake of H2K4b is Temperature -Dependent. C. albicans was incubated with H2K4b at 4°C (A, fluorescence; B, DIC) and at RT for 2 h (C, fluorescence; D, DIC). After washing the cells, they were observed with a Diaphot-TMD fluorescent microscope.
(ii) Colocalization studies.
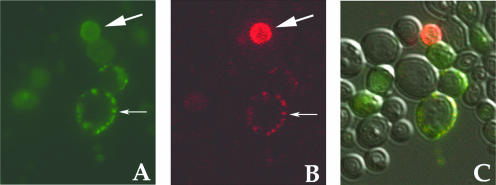
AlexaFluor 594 dye conjugated to dextran utilizes endocytosis for its uptake into cells and, consequently, it is used as an endocytosis marker. We investigated whether fluoresceinylated H2K4b colocalized with the AlexaFluor 594 dye-dextran within yeast (Fig. 7). There was considerable colocalization of the two dyes in small vesicles consistent with endosomes. Furthermore, in some cells, there was diffuse fluorescence of the two dyes, indicating that HK polymer may have had a role in disrupting endosomes in these cells.
FIG. 7.
Colocalization of Dextran and H2K4b in yeast AlexaFluor 594 dexran and floresceinylated H2K4b were incubated with yeast for 2 h, the yeast were then washed with PBS X 2 and then these probes were observed with the LSM510 using 488 nm excitation and 515-545 nm emission filters for fluorescein and 543 nm excitation and 560 nm long pass emission filter for AlexaFluor 594. The fluorescein-labeled polymer (A) and AlexaFluor 594 dextran (B) were shown with the overlaid image (C) to visualized colocalization. The polymer and dextran colocalized to small peripheral endosomal-like vesicles (thin arrows) or to the cytosol compartment (thick arrows).
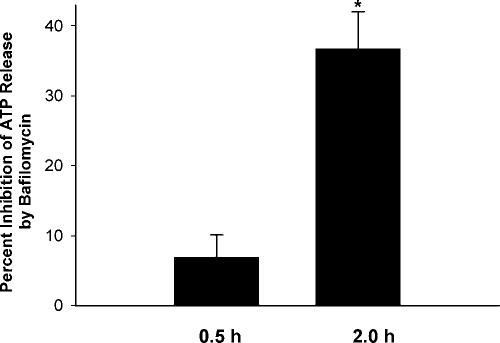
(iii) Bafilomycin A1.
If H2K4b were taken up by endocytotic vesicles into the yeast interior, bafilomycin A1, an inhibitor of the proton pump that acidifies the endosomes, would be expected to inhibit the release of H2K4b from endocytotic vesicles into the cytosol. By inhibiting endosomal acidification in mammalian cells, bafilomycin A1 reduces endosomal disruption by titrable polymers (e.g., H2K4b) and, consequently, inhibits the release H2K4b and its nucleic acid cargo into the cytosol (9). Thus, if uptake of H2K4b occurs by endocytosis in Candida, this polymer in the presence of bafilomycin A1 should show reduced antifungal activity. We compared the antifungal efficacy of H2K4b in the presence and absence of bafilomycin A1 in a bioluminescence ATP assay. Bafilomycin A1 significantly reduced the ATP released from C. albicans by H2K4b (P < 0.05) (Fig. 8).
FIG. 8.
Bafilomycin A1 inhibits ATP Release by H2K4b from Candida. After C. albicans was incubated with the proton pump inhibitor, bafilomycin A1 (50 ng/ml; added to half the cultures), the medium was changed and H2K4b (20 μg/ml; 1.8 μM) was added to the yeast for 0.5 or 2 h. The amount of released ATP was then measured. *, P < 0.05, bafilomycin-free control versus bafilomycin treated.
DISCUSSION
In contrast to histatins that specifically act as antifungal agents, cationic peptides such as defensins have widespread antimicrobial activities against gram-positive and gram-negative bacteria, fungi, and viruses (18). The surface proteins Ssa1/2, found on a number of fungi, play an important role in the selective antifungal activity and the likely intracellular accumulation of histatins (26). Since gram-positive and -negative bacteria lack this protein, the histatin family has limited activity against them. Similar to histatins, we found that branched histidine-containing peptides accumulated intracellularly in yeast and had antifungal activity but that these branched peptides had limited activity on gram-positive or -negative bacteria (data not shown). More important, a hallmark of the killing of C. albicans by histatin 5 is the release of small molecules, such as K+ and ATP, which can be blocked by the anion channel inhibitor DIDS (3). Our results suggest that H2K4b shares this fungicidal mechanism with histatin 5. Thus, because of the selective antifungal activity, the intracellular accumulation, the high content of histidine in the peptides, and the specific blockade of toxicity by the anion channel inhibitor DIDS, branched polymers and histatins are likely to share at least some mechanistic properties in reducing fungal growth.
In this study, we examined the antifungal efficacies of several linear and branched histidine-lysine polymers. We determined that HK polymers with more branches were more effective than those with fewer branches. Most of the branched polymers (e.g., H2K, H2K2b, H2K3b, and H2K4b) used in this study had a repeating sequence pattern of -HHK-. Interestingly, branched HK polymers are also more effective as gene therapy carriers (9, 24); in particular, H2K4b is one of the most efficient branched polymer carriers of DNA in mammalian cells. There are additional associations of interest between antifungal and gene delivery therapies. For example, sequence patterns of histidine and lysine were extremely important in determining the optimal agent for gene therapy delivery (24, 25). Although the most effective pattern of HK polymer as an antifungal agent has not been determined, we anticipate that variations in the patterns of histidine, lysine, and other amino acids may affect and possibly improve the efficacy of these branched polymers. Two lines of evidence from our laboratory support this hypothesis. First, branched peptides with a higher ratio of histidine to lysine (e.g., H3K4b) were less effective than H2K4b. The higher content of lysine in the H2K4b polymers may have a role in binding to the yeast cell surface. Second, a change in the amino acid pattern may improve the efficacy of these branched polymers, as suggested by replacing some of the histidines with leucines (Table 3). These amino acid sequence changes in the branched polymers significantly improved their antifungal efficacies, particularly against T. cutaneum.
That H2K4b is an effective gene therapy carrier and antifungal agent may be due, at least in part, to similar mechanisms. With gene delivery systems, the histidine component in the polymer plays a key role in buffering and lysis of acidic endosomes (8). In mammalian cells, endosomes become progressively acidified, and the pH-buffering component of the polymer (e.g., histidine) may act as a “proton sponge,” resulting in endosomal swelling and disruption (4). As a result of these properties, histidine-containing polymers and their cargo are likely to escape enzymatic degradation by lysosomes. Furthermore, the endocytic pathways of yeast and mammalian cells have many similarities (21, 30, 35, 36, 38). Although the role of histidine has not been previously appreciated in buffering and lysing yeast endosomes, we think this mechanism has a significant role in transporting histidine-rich peptides (e.g., histatin 5 and H2K4b). Similar to mammalian cells, yeasts have a receptor-mediated endocytic pathway, and their endosomes become progressively more acidic. Consequently, after histidine-rich peptides specifically bind to the cell surface Ssa1/2 proteins (26) and enter the cell via endosomes, the histidine component of these peptides may have a significant role in lysing these organelles, thereby allowing an intact peptide to reach its intracellular target. In previous studies with mammalian cells, bafilomycin A1, which inhibits the vacuolar-type proton pump and endosomal acidification, markedly reduced the ability of H2Kb to carry DNA into the cytosol (10). Bafilomycin 1 not only inhibits the vacuolar-type proton pump in mammalian cells but also potently inhibits the proton pump of vacuoles in a wide range of organisms (7). Notably, binding sites for bafilomycin on the proton pump have been identified in yeast and/or fungi (5, 6, 43). Because vacuolar proton pump inhibition by bafilomycin is apparently ubiquitous in eukaryotes, we examined the effects of bafilomycin A1 on release of ATP by H2K4b in the current study. The findings with bafilomycin A1 support the idea that endosomal lysis is an important mechanism by which these titrable HK peptides function in C. albicans. Furthermore, colocalization studies not only suggest that H2K4b uptake is by endocytosis but also that H2K4b induces endosomal lysis. The intense and diffuse intracellular signal of AlexaFluor-dextran only occurs in yeast cells in which increased levels of H2K4b have also accumulated; this finding is consistent with endosomal lysis with release of its contents (including dextran), once H2K4b achieves a critical concentration within the endosome. Since branched histidine-containing polymers have a higher content of pH-buffering histidines compared to lesser-branched and linear polymers, they are likely to be more effective at disrupting yeast endosomes, thereby allowing these polymers to be released into the cytoplasm. Indeed, branched HK peptides may be effective carriers of other antifungal agents into Candida species, thereby synergizing the activity of both agents. As a result, we have initiated experiments utilizing H2K4b as a carrier, and preliminary data indicate that H2K4b transports labeled plasmid DNA effectively into the cytosol of C. albicans.
Notably, although disruption of endosomes by histidine-rich polymers with their intracellular accumulation may be necessary, it is likely not a sufficient condition to kill the yeast. Mammalian cells tolerate endosomolytic peptides with little toxicity, and disruption of the endosomal membranes may be transient. Once they escape the endosomes of C. albicans (and other fungi), these peptides hone in on an intracellular target (e.g., Trk1p) responsible for their fungicidal activity. Consistent with this, histatin 5 expressed intracellularly by means of plasmid-based therapy effectively lyses and kills yeast (1). The lack of a homologous Trk1p ion channel in the mammalian cells studied may explain reduced toxicity to the endothelial cells. Moreover, the increased toxicity of histidine-rich peptides to fungi in contrast to mammalian cells may also be related to their high intracellular concentration, perhaps related to their binding to SSA1/2 surface proteins.
A recent focus of our laboratory that may enable development of more effective nucleic acid carriers or antifungal peptides has been to determine the structure of branched histidine-rich peptides. Currently, little is known about the structure of HK peptides either alone or in complex with DNA. Nevertheless, a few general properties are known about polylysine and polyhistidine peptides that are likely to be applicable to the structure of HK peptides. Both polyhistidine (at a pH of less than 6) and poly-l-lysine (pH < 9) form random coils (15, 31). Consequently, in acidic environments (e.g., endosomes), HK peptides with a pKa of approximately 6.0 probably will form random coils because of protonation of imidazole groups. While the pH buffering and chelation properties of histidine are widely known and can affect the structure of histidine-rich peptides (34), less appreciated is the hydrogen bonding that occurs between the imidazole groups at physiologic pH (20). The attraction of imidazole groups within and between polymers may play a role in its native structure and its ability to bind DNA stably in serum. Nucleic magnetic resonance experiments are ongoing to understand more clearly the structure of HK polymers.
In summary, we have found that certain branched HK peptides have greater antifungal activity compared to lesser-branched HK peptides or to the naturally occurring histatin 5. The uptake mechanism of the polymer and the therapeutic implications from this study are perhaps more important than the antifungal activity of H2K4b. First, the findings that histidine-rich peptides enter yeast through the endosomal pathway may provide insight as to why histidines, which have an endosomal-disrupting property, are incorporated within histatins. Similar to methods that we have used in HK gene therapy studies (25), the judicious addition of histidines to synthetic HK peptides or to one of the histatins may further augment their antifungal activities. Second, the antifungal properties of H2K4b may not lie solely with its ability to reduce fungal growth but also with its ability to serve as a carrier for other antifungal agents. Indeed, the efficient transport of fluorescein into the yeast interior by H2K4b at 0.18 μM (1/50 the MIC for C. albicans) suggests that H2K4b may be an effective carrier of low-molecular-weight chemotherapeutic agents. Future studies will focus on combining these strategies to develop more effective antifungal therapy utilizing histidine-rich peptides.
Acknowledgments
We are grateful to Pamela Talalay, Alan Cross, and John Sacci for their reading and useful comments concerning the manuscript. We thank Nicholas Ambulos of the Maryland Biopolymer lab for synthesizing the peptides in this study.
This study was supported by National Cancer Institute grants CA70394 and CA96984 and by a grant from the Maryland Technology Development Corporation.
REFERENCES
- 1.Baev, D., X. Li, and M. Edgerton. 2001. Genetically engineered human salivary histatin genes are functional in Candida albicans: development of a new system for studying histatin candidacidal activity. Microbiology 147:3323-3334. [DOI] [PubMed] [Google Scholar]
- 2.Baev, D., A. Rivetta, X. S. Li, S. Vylkova, E. Bashi, C. L. Slayman, and M. Edgerton. 2003. Killing of Candida albicans by human salivary histatin 5 is modulated, but not determined, by the potassium channel TOK1. Infect. Immun. 71:3251-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baev, D., A. Rivetta, S. Vylkova, J. N. Sun, G. F. Zeng, C. L. Slayman, and M. Edgerton. 2004. The TRK1 potassium transporter is the critical effector for killing of Candida albicans by the cationic protein, histatin 5. J. Biol. Chem. 279:55060-55072. [DOI] [PubMed] [Google Scholar]
- 4.Boussif, O., F. Lezoualc'h, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix, and J. P. Behr. 1995. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA 92:7297-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman, B. J., and E. J. Bowman. 2002. Mutations in subunit C of the vacuolar ATPase confer resistance to bafilomycin and identify a conserved antibiotic binding site. J. Biol. Chem. 277:3965-3972. [DOI] [PubMed] [Google Scholar]
- 6.Bowman, E. J., L. A. Graham, T. H. Stevens, and B. J. Bowman. 2004. The bafilomycin/concanamycin binding site in subunit c of the V-ATPases from Neurospora crassa and Saccharomyces cerevisiae. J. Biol. Chem. 279:33131-33138. [DOI] [PubMed] [Google Scholar]
- 7.Bowman, E. J., A. Siebers, and K. Altendorf. 1988. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. USA 85:7972-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Q. R., L. Zhang, P. W. Luther, and A. J. Mixson. 2002. Optimal transfection with the HK polymer depends on its degree of branching and the pH of endocytic vesicles. Nucleic Acids Res. 30:1338-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, Q. R., L. Zhang, S. A. Stass, and A. J. Mixson. 2001. Branched co-polymers of histidine and lysine are efficient carriers of plasmids. Nucleic Acids Res. 29:1334-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Q. R., L. Zhang, S. A. Stass, and A. J. Mixson. 2000. Co-polymer of histidine and lysine markedly enhances transfection efficiency of liposomes. Gene Ther. 7:1698-1705. [DOI] [PubMed] [Google Scholar]
- 11.Coleman, D. C., D. E. Bennett, D. J. Sullivan, P. J. Gallagher, M. C. Henman, D. B. Shanley, and R. J. Russell. 1993. Oral Candida in HIV infection and AIDS: new perspectives/new approaches. Crit. Rev. Microbiol. 19:61-82. [DOI] [PubMed] [Google Scholar]
- 12.Dathe, M., and T. Wieprecht. 1999. Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells. Biochim. Biophys. Acta 1462:71-87. [DOI] [PubMed] [Google Scholar]
- 13.Epand, R. M., and H. J. Vogel. 1999. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta 1462:11-28. [DOI] [PubMed] [Google Scholar]
- 14.Farrera-Sinfreu, J., E. Giralt, S. Castel, F. Albericio, and M. Royo. 2005. Cell-penetrating cis-gamma-amino-l-proline-derived peptides. J. Am. Chem. Soc. 127:9459-9468. [DOI] [PubMed] [Google Scholar]
- 15.Greenfield, N., B. Davidson, and G. D. Fasman. 1967. The use of computed optical rotatory dispersion curves for the evaluation of protein conformation. Biochemistry 6:1630-1637. [DOI] [PubMed] [Google Scholar]
- 16.Gyurko, C., U. Lendenmann, E. J. Helmerhorst, R. F. Troxler, and F. G. Oppenheim. 2001. Killing of Candida albicans by histatin 5: cellular uptake and energy requirement. Antonie Leeuwenhoek. 79:297-309. [DOI] [PubMed] [Google Scholar]
- 17.Hancock, R. E. 1999. Host defence (cationic) peptides: what is their future clinical potential? Drugs 57:469-473. [DOI] [PubMed] [Google Scholar]
- 18.Hancock, R. E., and R. Lehrer. 1998. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 16:82-88. [DOI] [PubMed] [Google Scholar]
- 19.Helmerhorst, E. J., W. van't Hof, P. Breeuwer, E. C. Veerman, T. Abee, R. F. Troxler, A. V. Amerongen, and F. G. Oppenheim. 2001. Characterization of histatin 5 with respect to amphipathicity, hydrophobicity, and effects on cell and mitochondrial membrane integrity excludes a candidacidal mechanism of pore formation. J. Biol. Chem. 276:5643-5649. [DOI] [PubMed] [Google Scholar]
- 20.Henig, M., and B. Geierstanger. 1999. Direct detection of a histidine-histidine side chain hydrogen bond important for folding of apomyoglobin. J. Am. Chem. Soc. 121:5123-5126. [Google Scholar]
- 21.Kail, M., M. Hollinshead, M. Kaufmann, J. Bottcher, D. Vaux, and A. Barnekow. 2005. Yeast Ypt11 is targeted to recycling endosomes in mammalian cells. Biol. Cell 97:651-658. [DOI] [PubMed] [Google Scholar]
- 22.Koshlukova, S. E., T. L. Lloyd, M. W. B. Araujo, and M. Edgerton. 1999. Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J. Biol. Chem. 274:18872-18879. [DOI] [PubMed] [Google Scholar]
- 23.La Rocca, P., P. C. Biggin, D. P. Tieleman, and M. S. Sansom. 1999. Simulation studies of the interaction of antimicrobial peptides and lipid bilayers. Biochim. Biophys. Acta 1462:185-200. [DOI] [PubMed] [Google Scholar]
- 24.Leng, Q., and A. J. Mixson. 2005. Modified branched peptides with a histidine-rich tail enhance in vitro gene transfection. Nucleic Acids Res. 33:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leng, Q., P. Scaria, J. Zhu, N. Ambulos, P. Campbell, and A. J. Mixson. 2005. Highly branched HK peptides are effective carriers of siRNA. J. Gene Med. 7:977-986. [DOI] [PubMed] [Google Scholar]
- 26.Li, X. S., M. S. Reddy, D. Baev, and M. Edgerton. 2003. Candida albicans Ssa1/2p is the cell envelope binding protein for human salivary histatin 5. J. Biol. Chem. 278:28553-28561. [DOI] [PubMed] [Google Scholar]
- 27.Lupetti, A., R. Danesi, J. W. van 't Wout, J. T. van Dissel, S. Senesi, and P. H. Nibbering. 2002. Antimicrobial peptides: therapeutic potential for the treatment of Candida infections. Expert Opin. Investig. Drugs 11:309-318. [DOI] [PubMed] [Google Scholar]
- 28.Massodi, I., G. L. Bidwell III, and D. Raucher. 2005. Evaluation of cell penetrating peptides fused to elastin-like polypeptide for drug delivery. J. Control Release 108:396-408. [DOI] [PubMed] [Google Scholar]
- 29.Mosca, D. A., M. A. Hurst, W. So, B. S. Viajar, C. A. Fujii, and T. J. Falla. 2000. IB-367, a protegrin peptide with in vitro and in vivo activities against the microflora associated with oral mucositis. Antimicrob. Agents Chemother. 44:1803-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson, N., N. Perzov, A. Cohen, K. Hagai, V. Padler, and H. Nelson. 2000. The cellular biology of proton-motive force generation by V-ATPases. J. Exp. Biol. 203:89-95. [DOI] [PubMed] [Google Scholar]
- 31.Norland, K. S., G. D. Gasman, E. Katchalsky, and E. R. Blout. 1963. Some optical properties of poly-l-bennyl-l-histidine and poly-l-histidine. Biopolymers 1:277-294. [Google Scholar]
- 32.Oppenheim, F. G., T. Xu, F. M. McMillian, S. M. Levitz, R. D. Diamond, G. D. Offner, and R. F. Troxler. 1988. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 263:7472-7477. [PubMed] [Google Scholar]
- 33.Padari, K., P. Saalik, M. Hansen, K. Koppel, R. Raid, U. Langel, and M. Pooga. 2005. Cell transduction pathways of transportans. Bioconjug. Chem. 16:1399-1410. [DOI] [PubMed] [Google Scholar]
- 34.Patchornik, A., A. Berger, and E. Katchalski. 1957. Poly-l-histidine. J. Am. Chem. Soc. 79:5227-5236. [Google Scholar]
- 35.Pelham, H. R. 2002. Insights from yeast endosomes. Curr. Opin. Cell Biol. 14:454-462. [DOI] [PubMed] [Google Scholar]
- 36.Prescianotto-Baschong, C., and H. Riezman. 1998. Morphology of the yeast endocytic pathway. Mol. Biol. Cell 9:173-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rex, J. H., M. G. Rinaldi, and M. A. Pfaller. 1995. Resistance of Candida species to fluconazole. Antimicrob. Agents Chemother. 39:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riezman, H. 1993. Three clathrin-dependent budding steps and cell polarity. Trends Cell Biol. 3:330-332. [DOI] [PubMed] [Google Scholar]
- 39.Sims, C. R., L. Ostrosky-Zeichner, and J. H. Rex. 2005. Invasive candidiasis in immunocompromised hospitalized patients. Arch. Med. Res. 36:660-671. [DOI] [PubMed] [Google Scholar]
- 40.Sitaram, N., and R. Nagaraj. 1999. Interaction of antimicrobial peptides with biological and model membranes: structural and charge requirements for activity. Biochim. Biophys. Acta 1462:29-54. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg, D. A., and R. I. Lehrer. 1997. Designer assays for antimicrobial peptides. Disputing the “one-size-fits-all” theory. Methods Mol. Biol. 78:169-186. [DOI] [PubMed] [Google Scholar]
- 42.Tsai, H., and L. A. Bobek. 1998. Human salivary histatins: promising anti-fungal therapeutic agents. Crit. Rev. Oral Biol. Med. 9:480-497. [DOI] [PubMed] [Google Scholar]
- 43.Wang, Y., T. Inoue, and M. Forgac. 2005. Subunit a of the yeast V-ATPase participates in binding of bafilomycin. J. Biol. Chem. 280:40481-40488. [DOI] [PubMed] [Google Scholar]
- 44.Xing, X., X. He, J. Peng, K. Wang, and W. Tan. 2005. Uptake of silica-coated nanoparticles by HeLa cells. J. Nanosci. Nanotechnol. 5:1688-1693. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, L., N. Ambulos, and A. J. Mixson. 2004. DNA delivery to cells in culture using peptides. Methods Mol. Biol. 245:33-52. [DOI] [PubMed] [Google Scholar]