Abstract
This study analyzes the diversity of In60, a class 1 integron bearing CR1 and containing blaCTX-M-9, and its association with Tn402, Tn21, and classical conjugative plasmids among 45 CTX-M-9-producing clinical strains (41 Escherichia coli strains, 2 Klebsiella pneumoniae strains, 1 Salmonella enterica strain, and 1 Enterobacter cloacae strain). Forty-five patients in a Spanish tertiary care hospital were studied (1996 to 2003). The diversity of In60 and association of In60 with Tn402 or mercury resistance transposons were investigated by overlapping PCR assays and/or hybridization. Plasmid characterization included comparison of restriction fragment length polymorphism patterns and determination of incompatibility group by PCR-based replicon typing, sequencing, and hybridization. CTX-M-9 plasmids belonged to IncHI2 (n = 26), IncP-1α (n = 10), IncFI (n = 4), and IncI (n = 1) groups. Genetic platforms containing blaCTX-M-9 were classified in six types in relation to the In60 backbone and in eight subtypes in relation to Tn402 derivatives. They were associated with Tn21 sequences when located in IncP-1α or IncHI2 plasmids. Our study identified blaCTX-M-9 in a high diversity of CR1-bearing class 1 integrons linked to different Tn402 derivatives, often to Tn21, highlighting the role of recombination events in the evolution of antibiotic resistance plasmids. The presence of blaCTX-M-9 on broad-host-range IncP-1α plasmids might contribute to its dissemination to hosts that were not members of the family Enterobacteriaceae.
The reasons driving the recent dramatic worldwide dissemination of CTX-M-producing microorganisms are far from understood. Chromosomal β-lactamase genes from different Kluyvera species are considered the ancestors of each of the five CTX-M groups described thus far (CTX-M-1, -2, -8, -9, and -25; http://www.lahey.org/studies/webt.htm). Mobilization of blaCTX-M genes to other bacterial genera seems to have occurred by recombinatorial events mediated by CR1 (a Common Region that includes a putative recombinase named orf513), ISEcp1, or phage-related elements (3, 19, 20, 28). Although dissemination of specific strains or mobile genetic elements has been documented, the general lack of information about the complete genetic context of blaCTX-M genes precludes coming up with a reliable hypothesis about the causes determining their successful spread (3, 5, 19).
The blaCTX-M-9 gene has been found associated with a class 1 integron bearing CR1 (34), which has a modular structure consisting of the conserved segments 5′CS and 3′CS flanking variable gene cassette arrays, CR1, genes that do not resemble gene cassettes, and a second copy of the 3′CS designated 3′CS2 (32, 42). Variations of In60, the integron harboring blaCTX-M-9, have been reported (14, 34), but the complete genetic environment to which In60 or their variants are associated in their turn remain unknown. To date, only a few class 1 integrons bearing CR1 are fully characterized (In6, In34, and In117) (30, 32, 42). They have been found harbored by Tn21-like transposons and, in the case of In34, also by an early antibiotic-resistant conjugative plasmid (32). Indeed, our work supports the hypothesis that the spread of CTX-M enzymes takes advantage of the wide availability in nature of old plasmids, already present in the preantibiotic era, as well as old mercury resistance transposons and classic integrons (5, 12, 21, 25, 31).
On this basis, we have analyzed the diversity of In60 and its association with Tn402, Tn21, and classical conjugative plasmids in clinical enterobacterial isolates identified in our institution since its first isolation in 1996 through 2003 by using different PCR methods (8; this study) designed on the basis of available sequences in the GenBank database.
MATERIALS AND METHODS
Bacterial strains and epidemiological background.
Seventy CTX-M-9-producing clinical isolates (66 Escherichia coli isolates, 2 Klebsiella pneumoniae isolates, 1 Enterobacter cloacae isolate, and 1 Salmonella enterica isolate) from 45 patients at Ramón y Cajal Hospital, a 1,200-bed university teaching hospital in northern Madrid, Spain, were studied (1996 to 2003). Isolates from the same individual showing identical susceptibility profiles and isolated within the same month were excluded in order to avoid overrepresentation of particular strains. Patients were located in medical wards (49%), intensive care units (9%), and surgical wards (2%), and 40% were outpatients. Isolates were recovered from urine (60%), blood (11%), wound exudate (11%), rectal swab (7%), sputum (4%), and other samples (7%). Species identification and preliminary susceptibility testing were performed by using the automated PASCO (Difco, Detroit, MI) or WIDER (Fco. Soria Melguizo, Madrid, Spain) systems. Susceptibility analysis to non-β-lactam antibiotics (gentamicin, tobramycin, amikacin, streptomycin, kanamycin, sulfonamide, trimethoprim, tetracycline, chloramphenicol, ciprofloxacin, and nalidixic acid) was performed by the disk diffusion method following CLSI (formerly NCCLS) guidelines (27). For the purposes of this work, strains with intermediate susceptibility were considered resistant. Characterization of blaCTX-M-9 was performed by isoelectric focusing, PCR, and further sequencing (24).
Clonal analysis.
Genetic relationships among isolates were established by pulsed-field gel electrophoresis (PFGE) as previously described (18, 40). The assignation of phylogenetic groups among E. coli isolates was performed by the classic multiplex PCR assay of Clermont et al. (10) amplifying chuA and yjaA genes and using an anonymous DNA fragment (TspE4C2) which has been found to be specific as phylogenetic group markers.
Conjugation of blaCTX-M-9 elements.
It was tested by broth and/or filter mating at a 1:10 donor/recipient ratio using E. coli K-12 strain BM21R (nalidixic acid and rifampin resistant, lactose fermentation positive, and plasmid free) as a recipient (24). Selection was performed on MacConkey agar plates containing cefotaxime (1 mg/liter) and rifampin (100 mg/liter). Conjugation plates were incubated at both 24°C and 37°C and analyzed at 5 h and 24 h.
Analysis of plasmids.
The content and size of the plasmids carrying blaCTX-M-9 were determined on E. coli transconjugants (or wild-type strains in the absence of transfer) by the technique described by Barton et al. (2, 41). Location of blaCTX-M-9 genes was assessed by hybridization of I-CeuI-digested genomic DNA with blaCTX-M-9 and 16S rRNA gene probes as previously described (23).
Plasmids were classified according to their incompatibility group using the PCR replicon-typing scheme described by Carattoli et al. (8). This assay discriminates 18 types of plasmids of the classical incompatibility groups by the presence of specific genes involved in plasmid maintenance. Positive-control strains were E. coli strain DH5α derivatives containing replicons of the different incompatibility groups cloned into a TA cloning vector (8). PCR products were sequenced in order to confirm the specificity of the method and to analyze similarities with well-characterized plasmids. Correspondence of the replicons amplified with plasmids containing blaCTX-M-9 was validated when probes for both blaCTX-M-9 and a given replicon hybridized with the same plasmid band. Plasmids of the same size and the same incompatibility group were digested with different restriction enzymes in order to establish their relationship.
Characterization of integrons carrying blaCTX-M-9.
Class 1 integrons are associated with defective transposons of the Tn402 family that differ by the presence and type of insertion sequences located downstream of 3′CS and within the truncated tni module (IS1326 and/or IS1353 are associated with the In0-In2-In5-In31 lineage, and IS6100 is associated with the In4 lineage) (31, 33). Thus, the characterization of blaCTX-M-9 integrons included both analysis of In60 backbone structure by an overlapping PCR assay and screening of sequences related to Tn402 derivatives (orf5, IS1326, IS1353, and IS6100) by dot blot hybridization and/or PCR, further linked by an overlapping PCR assay (Fig. 1). Isolates lacking orf5 were screened for the presence of the entire transposition module of Tn402 (tniR-tniQ-tniB-tniA) which has been detected among contemporary plasmids of different incompatibility groups as IncP-1α and/or different mercury resistance transposons as Tn5058 (25, 39). Control strains for In0, In2, and In4 integrons and Tn21 were kindly provided by Hatch Stokes (Macquarie University, Sydney, Australia).
FIG. 1.
Strategy for characterization of blaCTX-M-9-containing elements on the basis of In60, Tn402, and Tn21 sequences. The locations of the primers used are indicated by gray arrows. Vertical bars symbolize inverted repeats of the integron (gray) or Tn21 (dark gray). Small white circles represent 59-bp elements of the corresponding gene cassettes. Known In60 sequence is shown within the gray rectangle. Preliminary classification of the blaCTX-M-9 elements is shown in the table at the bottom of the figure and was performed in relation to the In60 backbone by an overlapping PCR assay and screening of sequences related to Tn402 such as orf5, IS1326, IS1353, and IS6100 (not shown) by PCR and/or hybridization. Linkage of these sequences with Tn21 was performed by an overlapping PCR assay in a subset of representative isolates. PCR with primers P15 and P22 was performed for isolates lacking IS1353; the dotted line indicates the absence of this region. *, PCR product had molecular size higher than expected.
Integrons containing blaCTX-M-9 were classified as types that indicate In60 basic diversity (designated by capital letters) and subtypes that reflect the content of orf5, IS1326, IS1353, and IS6100 (designated by numbers) (Fig. 1). The presence of IS26, often associated with blaCTX-M genes and plasmids of some incompatibility groups (19, 17, 38) was screened by PCR using the primers listed in Table 1.
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotide | Primer | Oligonucleotide sequence | GenBank accession no. | Positions | Reference(s) |
|---|---|---|---|---|---|
| 1 | 5′-CS | 5′-GGC ATC CAA GCA GCA AG-3′ | AF174129 | 1236-1252 | 42 |
| 2 | aadA2R | 5′-TGA CTT GAT GAT CTC GCC-3′ | AF174129 | 2709-2692 | This study |
| 3 | aadA2F | 5′-GCT GGC CGT GCA TTT GTA CG-3′ | AF174129 | 2013-2032 | This study |
| 4 | sul 1-R | 5′-GC AAG GCG GAA ACC CGC G-3′ | AF174129 | 3704-3687 | This study |
| 5 | sul 1-F | 5′-GCG CGG GTT TCC GCC TTG GGA-3′ | AF174129 | 3687-3703 | This study |
| 6 | blaCTXM-9-Rf | 5′-CCG TTG CAC TCT CTT TGT CA-3′ | AF174129 | 6359-6339 | This study |
| 7 | blaCTXM-9-Fr | 5′-GGC TTC AGC GGC GAG AAT CAT-3′ | AF174129 | 7175-7195 | This study |
| 8 | TnpA-R | 5′-C GCT CAA TCG AGG GAT ATT TAC-3′ | AF174129 | 9313-9292 | This study |
| 9 | TnpA-F | 5′-GTA AAT ATC CCT CGA TTG AGC G-3′ | AF174129 | 9292-9313 | This study |
| 10 | IS3000 R | 5′-GCC GTC TGT GGC CTC CAG-3′ | AF174129 | 12383-12366 | This study |
| 11 | blaCTXM-9-F | 5′-GT GAC AAA GAG AGT GCA ACG G-3′ | AF174129 | 6339-6359 | 24 |
| 12 | blaCTXM-9-R | 5′-ATG ATT CTC GCC GCT GAA GCC-3′ | AF174129 | 7195-7175 | 24 |
| 13 | orf5F | 5′-CGA TAT CGA CGA GGT TGT GC-3′ | AF071413 | 7712-7730 | 42 |
| 14 | orf5R | 5′-AGT TCT AGG CGT TCT GCG-3′ | AF071413 | 8157-8140 | 42 |
| 15 | IS1326R | 5′-ACT GTC ATA GCG GTT CAC GTT-3′ | AF071413 | 9141-9161 | 42 |
| 16 | IS1326F | 5′-TAC CGG GTC TTA TGA CCG AGT-3′ | AF071413 | 10357-10337 | 42 |
| 17 | IS1353R | 5′-ACA CTA CGG CAG CTG GGA TA-3′ | AF071413 | 10830-10849 | This study |
| 18 | IS1353F | 5′-TGC AGC ATT GTC TTG CGA GCA-3′ | AF071413 | 12113-12093 | This study |
| 19 | IS1353Rf | 5′-TGC TCG CCA GAC AAT GCT GCA-3′ | AF071413 | 12093-12113 | This study |
| 20 | IS6100 F | 5′-GGC TCT GTT GCA AAA ATC GTG AAG-3′ | AY463797 | 4669-4692 | 13 |
| 21 | IS6100 R | 5′-GGC TCT GTT GCA AAG ATT GGC-3′ | AY463797 | 5548-5528 | 13 |
| 22 | tniBΔ1F | 5′-AT CAT CGA CCT GTC CCA CCT-3′ | AF071413 | 13201-13182 | 42 |
| 23 | tniARF | 5′-TCG TGC GGA GAT CAT CAG TCC-3′ | AF071413 | 14821-14801 | 42 |
| 24 | merA1 | 5′-ACC ATC GGC GGC ACC TGC GT-3′ | AF071413 | 17597-17578 | 22 |
| 25 | merA5 | 5′-ACC ATC GTC AGG TAG GGG AAC AA-3′ | AF071413 | 16360-16382 | 22 |
| 26 | merT1 | 5′-CCA GGC AGC AGG TCG ATG CAA G-3′ | AF071413 | 19055-19076 | 22 |
| 27 | merR1 | 5′-GCG GAT TTG CCT CCA CGT TGA-3′ | AF071413 | 19278-19260 | 22 |
| 28 | Tn21IR/38 | 5′-GGG CAC CTC AGA AAA CGG AAA-3′ | AF071413 | 19669-19649 | 42 |
| 29 | IR1Tn21F | 5′-GGG TCG TCT CAG AAA ACG G-3′ | AF071413 | 1-38 | 42 |
| 30 | TnpR-R | 5′-CCG TGG TGG TGC ATA GCA T-3′ | AF071413 | 3394-3376 | This study |
| 31 | TnpR-F | 5′-ATG CTA TGC ACC ACC ACG G-3′ | AF071413 | 3376-3394 | This study |
| 32 | intF1 | 5′-GGG TCA AGG ATC TGG ATT TCG-3′ | AF071413 | 4775-4755 | 42 |
| 33 | intR1 | 5′-ACA TGC GTG TAA ATC ATC GTC G-3′ | AF071413 | 4312-4333 | 42 |
| 34 | 5′-CSR | 5′-CT TGC TGC TTG GAT GCC-3′ | AF174129 | 1252-1236 | 42 |
| 35 | 3′-CS | 5′-AAG CAG ACT TGA CCT GAT-3′ | AF174129 | 2813-2830 | 42 |
| 36 | tniAR | 5′-GGA CTG ATG ATC TCC GCA CGA-3′ | AF071413 | 14801-14821 | 42 |
| 37 | IRIn2F | 5′-TTT CAG AAG ACG GCT GCA CTG-3′ | AF071413 | 4046-4066 | This study |
| 38 | qacEΔ1B | 5′-CAA GCT TTT GCC CAT GAA GC-3′ | AF174129 | 3132-3113 | This study |
| 39 | qacEΔ2 | 5′-ATC GCA ATA GTT GGC GAA GT-3′ | AF174129 | 2906-2926 | This study |
| 40 | orf513R | 5′-C TCG CTT GAG GCG TTG CAT-3′ | AF174129 | 5791-5773 | This study |
| 41 | IS26F | 5′-AGC GGT AAA TCG TGG AGT GA-3′ | AF205943 | 324-344 | This study |
| 42 | IS26R | 5′-AG GCC GGC ATT TTC AGC GTG-3′ | AF205943 | 960-979 | This study |
Analysis of flanking sequences of the integron carrying blaCTX-M-9.
Since class 1 integrons bearing CR1 have been linked to mercury resistance transposons (33), we tested the occurrence of merA, a gene that is highly conserved in a variety of these genetic elements (22). The presence of backbone structures associated with Tn21 subgroup transposons was investigated by a overlapping PCR assay based on Tn21 and Tn1696 sequences (GenBank accession numbers AF071413 and AY223253, respectively; 31) in a subset of isolates containing merA and representing different In60 variants (see above) (Fig. 1).
Sequencing of integron-specific PCR products.
Sequencing of the amplified DNA fragments corresponding to different functional modules of the genetic elements containing blaCTX-M-9 (integrase, 5′CS1-3′CS1, 3′CS1-orf513, 3′CS2-tniA, and tniB-tniA) was performed for selected isolates by using ABI Prism 377 automated sequencer (Applied Biosystems PE, Foster City, CA). Nucleotide sequences were compared with sequences in the GenBank and EMBL databases by using the BLASTN local alignment search tools. Information about primers used for sequencing can be supplied on request.
DNA methodology.
Overlapping PCR assays were performed in volumes of 50 μl under the following conditions: 1.5 mM MgCl2, 0.2 mM of each deoxynucleoside triphosphate, 0.1 μM of each primer, and 1.5 units of Taq DNA polymerase (AmpliTaqGold; PE Applied Biosystems, Norwalk, Conn.) for 12 min at 94°C; 35 cycles, with 1 cycle consisting of 1 min at 94°C, 1 to 2 min at 56 to 65°C, and 1 to 3 min at 72°C, and a final step of 10 min at 72°C for standard PCR assays; and 2.5 mM MgCl2, 5% dimethyl sulfoxide (when necessary), 0.1 μM of each primer, and 2.5 units of Takara LA Taq polymerase (Takara Bio Inc., Shiga, Japan) for 1 min at 94°; and 35 cycles, with 1 cycle consisting of 20 s at 96°C, 1 min at 55 to 64°C, and 3 min at 72°C, followed by a final step of 10 min at 72°C, for long PCRs (>3 kb). DNA transfer and hybridization were performed by standard procedures (35). All probes were generated by PCR from the appropriate DNA controls as templates using the primers listed in Table 1. Labeling and detection were carried out using ECL kits following the manufacturer's instructions (Amersham Life Sciences, Uppsala, Sweden). PFGE was performed as described previously (18, 40) using the following conditions: 5- to 25-s pulses for 23 h and 60- to 120-s pulses for 10 h, 14°C, 6 V/cm2 (I-Ceu-I) and 10- to 40-s pulses for 24 h, 14°C, 6 V/cm2 (XbaI).
RESULTS
CTX-M-9-producing isolates have a heterogeneous epidemiological background.
Forty-five isolates with different PFGE types (41 E. coli isolates, 2 K. pneumoniae isolates, 1 S. enterica isolate, and 1 E. cloacae isolate) from 45 patients were studied. One E. coli isolate was recovered from two individuals, and two different E. coli isolates were obtained from a single patient. The E. coli phylogenetic groups A and B1 (more related to animal or commensal strains) were found at a higher proportion than were groups D and B2 (associated with extraintestinal pathogenic E. coli) among CTX-M-9-producing isolates (56% versus 44%). Most of the strains were resistant to sulfonamides (98%), trimethoprim (96%), streptomycin (96%), tetracycline (93%), and nalidixic acid (73%); a lower percentage showed resistance to ciprofloxacin (49%), gentamicin (20%), kanamycin (29%), and chloramphenicol (22%). Interestingly, resistance to sulfonamide, trimethoprim, or streptomycin that was expected to be related to the In60 integron was not expressed in all transconjugants despite the presence of the corresponding genes in the plasmid (only 41%, 79%, and 71% of transconjugants were resistant to sulfonamide, trimethoprim, or streptomycin, respectively). The epidemiological background of the strains is shown in Table 2.
TABLE 2.
Epidemiological features (clinical, clonal, plasmid, and integron data) of CTX-M-9-producing Enterobacteriaceae isolates at the Ramón y Cajal Hospital shown in relation to plasmid types (1996 to 2003)
| Plasmid Inc groupa | Approximate plasmid size (kb)b | Replicon type by PCRc | Typed | Subtyped | Isolatee | No. of patients | Species | Wardf | Specimen source | E. coli phylogenetic group | Date (mo/yr) | Presence ofg:
|
Antibiotic resistanceh | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IS26 | merA | |||||||||||||
| P-1α | 100 | P, I1, FIB | In60-A | 1 | D47 | 1i | E. coli | Outpatient | Wound | A | 3/02 | + | + | Sm, Su, Tp, Te |
| P-1α | 100 | P, I1, FIB | In60-A | 1 | D72 | 1i | E. coli | Int. Med. | Rectal swab | B2 | 4/02 | − | + | Sm, Su, Tp, Te, Cm, Na, Ak |
| P-1α | 100 | P, I1 | In60-A | 1 | D80 | 1 | E. coli | Nephrology | Urine | B1 | 5/02 | − | + | Sm, Su, Tp, Te, Km |
| P-1α | 100 | P, I1, FIB, F | In60-A | 1 | F18 | 1 | E. coli | Gastroent. | Urine | D | 10/02 | + | + | Sm, Su, Tp, Te, Na, Ak |
| P-1α | 100 | P | In60-A | 3 | EC40 | 1 | E. coli | Urology | Urine | A | 6/98 | − | + | Sm, Su, Tp, Te, Na |
| P-1α | 100 | P, I1, FIB, F | In60-A | 4 | F5 | 1 | E. coli | Outpatient | Blood | A | 9/02 | + | + | Sm, Su, Tp, Te, Na, Gm, Ak |
| P-1α | 100 | P, I1 | In60-A | 4 | G34 | 1 | K. pneumoniae | Outpatient | Urine | 6/03 | − | + | Sm, Su, Tp, Te, Km | |
| P-1α | 100 | P, I1 | In60-B | 3 | C77 | 1 | E. coli | Outpatient | Urine | A | 9/01 | − | + | Sm, Su, Tp, Te |
| P-1α | 100 | P, I1, FIB | In60-B | 3 | E27 | 1 | E. coli | Outpatient | Urine | B2 | 5/02 | − | + | Sm, Su, Tp, Te |
| P-1α | 100 | P, I1, FIB, F | In60-B | 4 | EC72 | 1 | E. coli | Nephrology | Wound | A | 10/00 | + | + | Sm, Tb, Su, Tp, Te, Cp, Na, Km, Gm |
| I1 | 120j | I1 | In60-A | 5 | EC44 | 1 | E. coli | Dermatology | Urine | A | 1/99 | + | + | Sm, Su, Tp, Te, Cp, Na |
| HI2 | 280 | HI2, FIB, F | In60-A | 1 | EC28 | 1 | E. coli | Nephrology | Blood | B1 | 10/97 | + | − | Sm, Su, Tp, Te, Cm, Cp, Na |
| HI2 | 280 | HI2, FIB, F | In60-A | 1 | EC76 | 1 | E. coli | Outpatient | Urine | A | 12/00 | + | − | Sm, Su, Tp, Te, Cm, Cp, Na |
| HI2 | 280 | HI2, FIB, F | In60-A | 1 | D4 | 1 | E. coli | Outpatient | Urine | B1 | 10/01 | + | − | Sm, Su, Tp, Te, Cp, Na |
| HI2, F1 | 320 | HI2, FIB | In60-A | 1 | D36 | 1 | E. coli | Gastroent. | Blood | B2 | 3/02 | + | − | Sm, Su, Tp, Na, Cp, Km |
| HI2 | 320 | HI2, FIB | In60-A | 2 | EC29 | 1 | E. coli | Urology | Urine | B2 | 3/97 | + | + | Sm, Su, Tp, Te, Cm, Na |
| HI2 | 270 | HI2 | In60-A | 2 | EC63 | 1 | E. coli | Urology | Urine | A | 4/00 | + | + | Sm, Su, Tp, Te, Cp, Na |
| HI2 | 290 | HI2 | In60-A | 2 | EC62 | 1 | E. coli | Nephrology | Urine | A | 4/00 | + | − | Sm, Su, Tp, Te, Cp |
| HI2 | 280 | HI2 | In60-A | 2 | EC68 | 1 | E. coli | Surgery ICU | Drainage | B1 | 7/00 | + | + | Sm, Su, Tp, Te, Cp, Na |
| HI2 | 260 | HI2, FIB, F | In60-A | 3 | EC25 | 2 | E. coli | Surgery ICU | Respiratory | D | 10/96 | + | + | Sm, Su, Tp, Te, Cp, Na, Km |
| Urology | Urine | 12/96 | ||||||||||||
| HI2 | 280 | HI2, F | In60-A | 4 | EC24 | 1 | E. coli | Urology | Urine | A | 10/96 | + | − | Sm, Su, Tp, Te, Cm, Na, Km |
| HI2 | 260 | HI2, I1 | In60-A | 4 | EC57 | 1 | E. coli | Neurosurg. ICU | Respiratory | A | 2/00 | + | − | Sm, Su, Tp, Te, Cp, Na, Gm, Km, Tb |
| HI2 | 280 | HI2, FIB, F | In60-A | 4 | EC39 | 1 | E. coli | Neurosurg. ICU | Catheter | D | 2/98 | + | + | Sm, Su, Tp, Te |
| HI2 | 280 | HI2, FIB | In60-A | 4 | EC41 | 1 | E. coli | Outpatient | Urine | A | 12/98 | + | + | Sm, Su, Tp, Te |
| HI2 | 280 | HI2, FIB | In60-A | 4 | EC46 | 1 | E. coli | Outpatient | Urine | A | 3/99 | + | − | Sm, Tb, Su, Tp, Te, Cm, Cp, Na, Km, Gm |
| HI2 | 280 | HI2, FIB, F | In60-A | 4 | EC42 | 1 | E. coli | Outpatient | Blood | D | 8/99 | + | − | Sm, Su, Tp, Na |
| HI2, F1 | 330j | HI2, FIB | In60-A | 4 | EC66 | 1 | E. coli | Outpatient | Urine | B2 | 5/00 | + | − | Sm, Su, Tp, Te, Cm, Na |
| HI2 | 290 | HI2, Y | In60-A | 4 | D7 | 1 | E. coli | Urology | Urine | B1 | 11/01 | + | − | Sm, Su, Tp, Te, Cm, Cp, Na, Gm |
| HI2 | 290 | HI2 | In60-A | 4 | F25 | 1 | E. cloacae | Outpatient | Urine | 10/02 | + | − | Sm, Su, Tp, Te | |
| HI2 | NDk | HI2, FIB | In60-A | 4 | F34 | 1 | E. coli | Nephrology | Urine | B1 | 11/02 | + | − | Su, Tp, Te |
| HI2 | 280 | HI2 | In60-A | 4 | F38 | 1 | E. coli | Nephrology | Wound | B2 | 12/02 | + | − | Sm, Su, Tp, Te |
| HI2 | 310 | HI2 | In60-A | 4 | H65 | 1 | S. enterica | Hematology | Rectal swabs | 6/03 | + | − | Sm, Su, Tp, Te, Na | |
| HI2 | 280 | HI2, FIA, FIB | In60-A | 6 | EC69 | 1 | E. coli | Outpatient | Urine | D | 8/00 | + | − | Sm, Su, Tp, Te, Cp, Na |
| HI2 | 290 | HI2 | In60-C | 1 | EC34 | 1 | E. coli | Nephrology | Urine | D | 12/97 | + | + | Sm, Su, Tp, Te, Cp, Na |
| HI2 | 290 | HI2, Y | In60-C | 2 | EC33 | 1 | E. coli | Gastroent. | Peritoneal | D | 11/97 | + | + | Sm, Su, Tp, Te, Cm, Cp, Km, Na, Gm, Tb |
| HI2 | 260 | HI2, Y | In60-E | 4 | EC50 | 1 | E. coli | Urology | Urine | A | 12/99 | + | − | Sm |
| HI2 | 240 | HI2, FIB, F | In60-F | 4 | D61 | 1 | E. coli | Nephrology | Urine | B2 | 4/02 | + | − | Su, Te, Na |
| FIB | 150 | FIB, F | In60-A | 1 | C45 | 1 | E. coli | Outpatient | Blood | B1 | 1/01 | + | − | Sm, Su, Tp, Te, Cp, Na |
| FIB | 140 | FIB, F | In60-A | 1 | D10 | 1 | E. coli | CV surgery | Wound | A | 11/01 | + | − | Sm, Su, Tp, Te, Na |
| FIB | 160 | FIB, F | In60-C | 7 | D79 | 1 | E. coli | Outpatient | Urine | D | 5/02 | + | − | Sm, Su, Tp, Te, Cp, Na, Gm, Tb |
| FIB | 160 | FIB, F | In60-D | 8 | E79 | 1 | E. coli | Urology | Urine | D | 8/02 | + | − | Sm, Su, Tp, Te, Na, Ak, Gm, Km, Tb |
| NIl | 70 | Y, B/O | In60-A | 1 | C59 | 1 | E. coli | Outpatient | Urine | A | 4/01 | + | − | Su, Tp, Te, Cp, Na, Gm, Km, Tb |
| NI | ND | B/O | In60-A | 1 | D34 | 1 | E. coli | Outpatient | Urine | D | 3/02 | + | + | Sm, Su, Tp, Te, Cm, Cp, Na |
| NI | 120 | Y | In60-A | 4 | EC99 | 1 | E. coli | Outpatient | Rectal swabs | D | 5/02 | + | − | Sm, Su, Tp, Te, Cp, Na, Km |
| NI | 120 | In60-B | 3 | Kp40 | 1 | K. pneumoniae | Gastroent. | Wound | 11/99 | + | + | Sm, Su, Tp, Te, Cp, Na, Km | ||
Incompatibility group of plasmids harboring blaCTX-M-9 determined by PCR, hybridization, and in some cases, sequencing of the replicon.
Plasmid size determined by hybridization of I-Ceu-I- or S1 nuclease-digested genomic DNA of E. coli BM21R transconjugants (or wild type if failed transfer) with an intragenic blaCTX-M-9 probe.
Replicons hybridizing in the same band as that of blaCTX-M-9 are underlined.
Types and subtypes of the blaCTX-M-9 integron were defined in Fig. 1.
Transferability of blaCTX-M-9 is indicated by underlining.
Int. Med., Internal medicine; Gastroent., gastroenterology; ICU, intensive care unit; Neurosurg., neurosurgery; CV, cardiovascular surgery.
+, present; −, absent.
Antibiotic resistance profile corresponding to the original strain; antibiotic resistance patterns of transconjugants are underlined. Chloramphenicol, nalidixic acid, and ciprofloxacin were not tested in the transconjugants. Sm, streptomycin; Gm, gentamicin; Tb, tobramycin; Km, kanamycin; Ak, amikacin; Su, sulfonamide; Tp, trimethoprim; Te, tetracycline; Cp, ciprofloxacin; Na, nalidixic acid; Cm, chloramphenicol.
Isolates recovered from the same patient.
Chromosomal location of blaCTX-M-9.
ND, not determined since DNA extraction repeatedly failed.
NI, not identified.
The blaCTX-M-9 gene is often located in IncHI2, IncP1-α, and IncFI conjugative plasmids.
Transfer of cefotaxime resistance to E. coli BM21R was achieved by conjugation in 76% of the strains. We identified blaCTX-M-9 on plasmids ranging from approximately 70 to 320 kb and belonging to incompatibility groups IncHI2 (n = 26), IncP-1α (n = 10), IncFI (n = 4), and IncI (n = 1).
Plasmids identified as belonging to the IncHI2 group were recovered from patients in different areas of the hospital and from different sources during an extended period of time (1996 to 2003). They showed variability in molecular size (240 to 320 kb) and in the presence of both merA and antibiotic resistance markers (Table 2). The presence of common bands among a variable number of restriction fragment length polymorphism patterns from a representative number of IncHI2 plasmids and the 99% homology (1 nucleotide change) of amplified iteron regions from six IncHI2 plasmids of different sizes and merA contents with sequences of pR478 (GenBank accession number BX664015) seem to reflect that they were all derived from a single IncHI2 ancestor plasmid suffering different rearrangement events. A large number of transconjugants containing more than one plasmid (19 out of 26) amplified with primers for the IncHI2 replicons and other Inc replicons (mainly those of IncFI). In two cases, a positive hybridization signal within a single band was obtained with probes for both IncHI2 and IncFI (isolates D36 and EC66), suggesting the formation of cointegrates in the transconjugant. This hypothesis is strengthened by the larger size of the plasmid carried by D36 (320 kb). For EC66, these probes hybridized with a chromosomal fragment of approximately 330 kb (Table 2).
The IncP-1α plasmids were isolated from patients in the hospital from 1998 through 2003, both from outpatients and patients in medical areas, and all had a molecular size of approximately 100 kb (Table 2). The highly related restriction fragment length polymorphism patterns and the 100% homology of the amplified replicon with the sequence of pRK2 (GenBank accession number M20134), the prototype of IncP-1α plasmids, suggest the spread of a single plasmid species. As with the IncHI2 plasmids, the CTX-M-9 IncP-1α plasmids usually coexisted with IncF plasmids in most isolates. Although this plasmid type was frequently detected in CTX-M-9-producing strains, the location of blaCTX-M-9 in IncF plasmids was demonstrated in only four cases from 2001 to 2002. These four plasmids, classified as IncFI on the basis of sequences of repFIB and repF PCR products, had a variable molecular size (140 to 160 kb) and did not contain merA.
IncI amplicons were also detected among the CTX-M-9 producers of our collection, but there was evidence of blaCTX-M-9 location in an IncI plasmid in only one case. We could not identify the Inc plasmid group in four cases: two plasmids of approximately 120 kb, one of approximately 70 kb, and one of undetermined size (Table 2). The results of hybridization with probes for IncP-1α, IncI, IncHI2, and IncFI were all negative in these four cases.
blaCTX-M-9 is located in a variety of class 1 integrons containing CR1 and associated with Tn402 derivatives.
Integrons containing blaCTX-M-9 were classified as six different In60 variants (A to F) on the basis of the previously described In60 backbone: type A is identical to In60 (n = 35), type B has differences in sequences downstream of blaCTX-M-9 (n = 4), types C and D contain different gene cassette arrays within 5′CS-3′CS1 (aadA1 or dfrA12-orfX-aadA8, respectively, versus dfrA16-aadA2 in In60) (n = 4), and types E and F lack the first 5′CS-3′CS (n = 2). Subtypes, defined according to the content of IS1326, IS1353, IS6100, and orf5, reflect association of In60 variants with different Tn402 derivatives. The distribution of types and subtypes appears in Fig. 1.
A high degree of homology was found among sequences from all of the identified gene cassette arrays: dfrA16-aadA2 (indistinguishable among all our isolates and those carrying qnr from China and North America, GenBank accession number AY259085), aadA1 (indistinguishable from that of the worldwide disseminated Tn21, GenBank accession number AF071413), and dfrA12-orfX-aadA8b from our E79 isolate (identical to that described in fecal isolates from Australia and clinical isolates from the United Kingdom) (15, 21, 45) (GenBank accession number AM040708). Analysis of the integrase intI1 of one representative isolate of each integron type revealed the presence of Pc promoter sequences that correspond to the weak and intermediate versions of the Pc promoter (33).
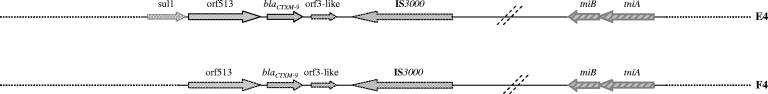
The 405-bp intergenic region between sul1F and orf513 described in other class 1 integrons bearing CR1 as In35, In36, In37, or In117 (GenBank accession numbers AY079169, AY259085, AY259086, and AY162283, respectively) was identified in all cases studied. Although the region immediately downstream of IS3000 of In60 (Fig. 2) could not be identified, positive hybridization of IS3000 and Tn402/tni sequences for specific isolates in the same DNA fragment suggest an eventual linkage between them. A 3′CS2-tni region similar to that of In0 (isolates EC29 and EC33) or that of In2 (isolates D72 and EC34) was detected in isolates harboring blaCTX-M-9 located in different integron types (subtypes 1 and 2). In these cases, sequencing of IRi regions confirmed that they belonged to the In0-In2 lineage of Tn402 (data not shown). The upstream and downstream regions of subtypes 4, 5, 7, and 8 could not be identified by our overlapping PCR assay. Interestingly, the tniA-tniB region was amplified in two isolates of subtype 4 (In60E-4 and In60F-4 corresponding to isolates EC50 and D61) harboring plasmids of the IncHI2 group. These sequences were identical to those of Tn5058, a mercury resistance transposon derivative of Tn5053, although a genetic linkage of this region with In60 was not established.
FIG.2.
Schematic representation of the genetic elements characterized by overlapping PCR, hybridization, and sequencing (see text). The horizontal doted lines indicate regions not linked under our PCR conditions. tniB-tniA sequences showed homology with Tn21 (gray arrows) or Tn5058 (hatched arrows).
Integrons containing blaCTX-M-9 are frequently associated with transposons of the Tn21 subgroup.
Forty-seven percent of the isolates containing blaCTX-M-9 studied carried merA. The presence of the complete left arm of Tn21 (tnpA-tnpR-tnpM) was demonstrated in isolates harboring different integron platforms (types In60 A-1, B-3, C-1, and C-2) located on plasmids of either the IncP-1α or IncHI2 group. However, the presence of the left branch of Tn21 was variable in isolates containing the same integron type (isolates KP40 and E. coli E27 harboring type In60 B-3). Tn21-mer sequences were detected in all isolates carrying blaCTX-M-9 on an IncP-1α plasmid, in a variable number of isolates carrying IncHI2 plasmid, and were absent in those on IncFI plasmids.
DISCUSSION
We describe the association of the CR1 integron containing blaCTX-M-9 with different Tn402 derivatives, often associated with Tn21 and mostly located in early antibiotic resistance IncHI2, IncP1α, and IncFI plasmids. The high diversity found in every functional module of the genetic element containing blaCTX-M-9 (5′CS-3′CS1 and CR1 regions and the Tn402-tni module) was not surprising, since recombinatorial exchange between these regions and the corresponding homologous regions in other elements has been reported. Moreover, the multiplicity of hot spots for recombination (intI1, 3′CS, Tn402-tni module on class 1 integrons or res site and mer operon in Tn21 derivatives) may yield chimeric structures and localized deletions (1, 4, 32, 33, 36-38, 43, 47). Plasticity of gene cassettes was also observed. The dfrA12-orfX-aadA8b array from our E79 isolate seems to have arisen by recombination events involving the aadA2 gene from the globally disseminated dfrA12-orfX-aadA2 array first described in Finland in 1969 (15). Our results are in agreement with other studies and show that the propensity for recombination and genetic exchange in nature seems to be more frequent than previously believed, playing a relevant role in the adaptation of specific plasmids to different cellular environments (9, 29). They also reflect the difficulties in understanding the spread of specific antibiotic resistance genes in the absence of a detailed characterization of their genetic environment, thus hiding the relevance of widespread key genetic elements in gene dispersal (36, 37).
Defective Tn402 transposons belong to the In0-In2-In5 lineage and are often associated with mercury resistance transposons such as Tn21 (30-32). To date, such mercury resistance transposons have been detected on narrow-host-range plasmids of incompatibility group F, such as pR100, pC15-1a, pRMH760, and pRSB107, or on IncHI1 plasmids, such as pHCM1 (GenBank accession numbers NC_002134, AY458016, AY123253, AJ851089, and AL513383, respectively; 16, 21, 32, 36-38, 43). Our study highlights the current wide spread of mercury resistance transposons in other groups of early antibiotic resistance plasmids such as IncHI2, IncP-1α, or IncI. IncHI2 plasmids, frequently harboring blaCTX-M-9 in our study, were first isolated in Serratia marcescens in the United States in 1969 and later recovered from environmental and human Salmonella enterica serovar Panama isolates from Chile in the 1980s and 1990s (11, 17), but recent reports remain scarce. IncHI2 plasmids possess a large segment named the “principal plasticity zone” that encodes the majority of resistance determinants such as ter, cat, aphA, mer, sil, cop, and Tn7 and also a large number of IS26 sequences (17). The widespread presence of IS and transposons in these plasmids would enable intra- and interplasmid recombinatorial events and might explain the variability in size and presence of mer and resistance markers in IncHI2 plasmids detected in our work. IncP-1α plasmids, also frequently associated with blaCTX-M-9, were first isolated in 1969 from Pseudomonas aeruginosa and enterobacterial clinical isolates from Birmingham, United Kingdom, and they have recently been recovered from wastewater in Germany (39). In our series, IncP-1α plasmids were mainly isolated from community isolates and, unlike those previously described, contained merA mostly associated with Tn21-like structures. It is of interest to note that IncF plasmids, often linked withTn21 in the literature (7, 16, 21, 30, 36-38, 43), did not contain sequences related to mercury resistance transposons. IncI plasmids have been shown to contain class 1 integrons and transposases similar to those of Tn21, although to our knowledge, the presence of the mer operon has not been demonstrated thus far (46). Defective Tn402 derivatives containing orf5 and IS6100 (class 1 integrons of In4 lineage, subtype 5) were rare in our collection despite having been previously found in a variety of plasmids, transposons, and bacterial chromosomes (26, 31). The presence of IS6100 detected alone (subtype A-5 and A-6) or in combination with IS1353 and IS1326 (type D-8) was not surprising, since this insertion sequence can be located in a wide genetic background without major specific associations.
Resistance to sulfonamide, trimethoprim, and streptomycin in wild clinical strains was not detected in a percentage of transconjugants, even though In60 contains gene cassettes presumptively responsible for these resistance phenotypes. The presence of additional genes encoding resistance to these antibiotics in some CTX-M-9-producing isolates as sulII, present in most sulfonamide-resistant transconjugants (unpublished results), or Tn7 (dhfrA1-sat-aadA1-orfX) present in some CTX-M-9-producing isolates (24) and also in IncHI2 plasmids (17) might have caused this phenomenon. Other hypotheses, such as gene inactivation or silencing, cannot be discarded and also deserve to be studied further.
CTX-M enzymes remain confined to members of the family Enterobacteriaceae, whereas other widely disseminated extended-spectrum beta-lactamases or metallo-β-lactamases have been found in different bacterial families (6, 44). It is tempting to suggest that this species selectivity might be related to the host range of the plasmids involved in their dissemination, but the identification of blaCTX-M-9 on plasmids of both narrow host range (IncHI2, IncI, and IncF) and broad host range (IncP-1α group) indicates the necessity of further studies. Finally, the results of this study contribute to increasing the list of fully characterized integrons bearing CR1 (In117, In34, and integrons of epidemic Salmonella IncF plasmids) associated in all cases with Tn21 and highlight the role of mercury resistance transposons frequently located in classical conjugative plasmids in fuelling antibiotic resistance genes (12, 30, 32, 33, 37, 42).
In summary, this study highlights the relevance of classical conjugative plasmids found in early antibiotic-resistant isolates in the dissemination of contemporary antibiotic resistance genes. The presence of similar plasmid backbones containing a high diversity of genetic elements harboring blaCTX-M-9 suggests the intraplasmid evolution of these elements by multiple recombinatorial events. The modular plasticity of plasmid-contained mobile genetic elements is of concern, since widely disseminated antibiotic resistance genes located on integrons might be incorporated into a variety of these plasmid-located modular platforms, as previously happened for the SG1 genetic island of Salmonella or antibiotic resistance IncF plasmids of Salmonella (4, 7). In addition, the presence of blaCTX-M-9 on broad-host-range IncP-1α plasmids might contribute to its dissemination to hosts other than Enterobacteriaceae.
Acknowledgments
Ângela Novais was supported by a fellowship from the Ministry of Science and Technology of Spain (SAF 2003-09285). This work was partially funded by research grants from the Ministry of Science and Technology of Spain (SAF 2003-09285) and the European Commission (grants LSHM-CT-2003-503335 and LSHM-CT-2005-018705).
We thank Hatch Stokes (Macquarie University, Sydney, Australia) for kindly providing control strains for different class 1 integrons and Tn21.
REFERENCES
- 1.Baquero, F. 2004. From pieces to patterns: evolutionary engineering in bacterial pathogens. Nat. Rev. Microbiol. 2:510-518. [DOI] [PubMed] [Google Scholar]
- 2.Barton, B. M., G. P. Harding, and A. J. Zuccarelli. 1995. A general method for detecting and sizing large plasmids. Anal. Biochem. 226:235-240. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet, R. 2004. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd, D., A. Cloeckaert, E. Chaslus-Dancla, and M. R. Mulvey. 2002. Characterization of variant Salmonella genomic island 1 multidrug resistance regions from serovars Typhimurium DT104 and Agona. Antimicrob. Agents Chemother. 46:17314-17322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd, D. A., S. Tyler, S. Christianson, A. McGeer, M. P. Muller, B. M. Willey, E. Bryce, M. Gardam, P. Nordmann, and M. R. Mulvey. 2004. Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob. Agents Chemother. 48:3758-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford, P. A. 2001. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carattoli, A., L. Villa, C. Pezzella, E. Bordi, and P. Visca. 2001. Expanding drug resistance through integron acquisition by IncFI plasmids of Salmonella enterica Typhimurium. Emerg. Infect. Dis. 7:444-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carattoli, A., A. Bertini, L. Villa, V. Falbo, K. L. Hopkins, and E. J. Threlfall. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219-228. [DOI] [PubMed] [Google Scholar]
- 9.Chen, S. L., C. S. Hung, J. Xu, C. S. Reigstad, V. Magrini, A. Sabo, D. Blasiar, T. Bieri, R. R. Meyer, P. Ozersky, J. R. Armstrong, R. S. Fulton, J. P. Latreille, J. Spieth, T. M. Hooton, E. R. Mardis, S. J. Hultgren, and J. I. Gordon. 2006. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc. Natl. Acad. Sci. USA 103:5977-5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cordano, A. M., and R. Virgilio. 1996. Evolution of drug resistance in Salmonella panama isolates in Chile. Antimicrob. Agents Chemother. 40:336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datta, N., and V. M. Hughes. 1983. Plasmids of the same Inc groups from Enterobacteriaceae before and after the medical use of antibiotics. Nature 306:616-617. [DOI] [PubMed] [Google Scholar]
- 13.Dogra, C., V. Raina, R. Pal, M. Suar, S. Lal, K. H. Gartemann, C. Holliger, J. R. van der Meer, and R. Lal. 2004. Organization of lin genes and IS6100 among different strains of hexachlorocyclohexane-degrading Sphingomonas paucimobilis: evidence for horizontal gene transfer. J. Bacteriol. 186:2225-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia, A., F. Navarro, E. Miro, B. Mirelis, S. Campoy, and P. Coll. 2005. Characterization of the highly variable region surrounding the blaCTX-M-9 gene in non-related Escherichia coli from Barcelona. J. Antimicrob. Chemother. 56:819-826. [DOI] [PubMed] [Google Scholar]
- 15.Gestal, A. M., H. W. Stokes, S. R. Partridge, and R. M. Hall. 2005. Recombination between the dfrA12-orfF-aadA2 cassette array and an aadA1 gene cassette creates a hybrid cassette, aadA8b. Antimicrob. Agents Chemother. 49:4771-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert, M. P., and A. O. Summers. 1988. The distribution and divergence of DNA sequences related to the Tn21 and Tn501mer operons. Plasmid 20:127-136. [DOI] [PubMed] [Google Scholar]
- 17.Gilmour, M. W., N. R. Thomson, M. Sanders, J. Parkhill, and D. E. Taylor. 2004. The complete nucleotide sequence of the resistance plasmid R478: defining the backbone components of incompatibility group H conjugative plasmids through comparative genomics. Plasmid 52:182-202. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann, M. E. 1998. Pulsed-field gel electrophoresis. Methods Mol. Med. 15:17-31. [DOI] [PubMed] [Google Scholar]
- 19.Lartigue, M. F., L. Poirel, and P. Nordmann. 2004. Diversity of genetic environment of blaCTX-M genes. FEMS Microbiol. Lett. 234:201-207. [DOI] [PubMed] [Google Scholar]
- 20.Lartigue, M. F., L. Poirel, D. Aubert, and P. Nordmann. 2006. In vitro analysis of ISEcp1B-mediated mobilization of naturally occurring β-lactamase gene blaCTX-M of Kluyvera ascorbata. Antimicrob. Agents Chemother. 50:1282-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liebert, C., R. M. Hall, and A. O. Summers. 1999. Transposon Tn21, flagship of the floating genome. Microbiol. Mol. Biol. Rev. 63:507-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liebert, C. A., J. Wireman, T. Smith, and A. O. Summers. 1997. Phylogeny of mercury resistance (mer) operons of gram-negative bacteria isolated from the fecal flora of primates. Appl. Environ. Microbiol. 63:1066-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, S. L., A. Hessel, and K. E. Sanderson. 1993. Genomic mapping with I-Ceu I, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc. Natl. Acad. Sci. USA 90:6874-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machado, E., R. Cantón, F. Baquero, J. C. Galán, A. Rollan, L. Peixe, and T. M. Coque. 2005. Integron content of extended-spectrum-β-lactamase-producing Escherichia coli strains over 12 years in a single hospital in Madrid, Spain. Antimicrob. Agents Chemother. 49:1823-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mindlin, S., L. Minakhin, M. Petrova, G. Kholodii, S. Minakhina, Z. Gorlenko, and V. Nikiforov. 2005. Present-day mercury resistance transposons are common in bacteria preserved in permafrost grounds since the Upper Pleistocene. Res. Microbiol. 156:994-1004. [DOI] [PubMed] [Google Scholar]
- 26.Miriagou, V., A. Carattoli, E. Tzelepi, L. Villa, and L. S. Tzouvelekis. 2005. IS26-associated In4-type integrons forming multiresistance loci in enterobacterial plasmids. Antimicrob. Agents Chemother. 49:3541-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. 2000. Methods for diffusion disk antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 28.Oliver, A., T. M. Coque, D. Alonso, A. Valverde, F. Baquero, and R. Cantón. 2005. CTX-M-10 linked to a phage-related element is widely disseminated among Enterobacteriaceae in a Spanish hospital. Antimicrob. Agents Chemother. 49:1567-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborn, M., S. Bron, N. Firth, S. Holsappel, A. Huddleston, R. Kiewiet, W. Meijer, J. Segers, R. Skurray, P. Terpstra, C. M. Thomas, P. Thorsted, E. Tietze, and S. L. Turner. 2000. The evolution of bacterial plasmids, p. 301-361. In C. M. Thomas (ed.), The horizontal gene pool: bacterial plasmids and gene spread. Hardwood Academic Publishers, Amsterdam, The Netherlands.
- 30.Partridge, S., and R. M. Hall. 2003. In34, a complex In5 family class 1 integron containing orf513 and dfrA10. Antimicrob. Agents Chemother. 47:342-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Partridge, S., G. Recchia, H. W. Stokes, and R. M. Hall. 2001. Family of class 1 integrons related to In4 from Tn1696. Antimicrob. Agents Chemother. 45:3014-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Partridge, S. R., and R. M. Hall. 2004. Complex multiple antibiotic and mercury resistance region derived from the r-det of NR1 (R100). Antimicrob. Agents Chemother. 48:4250-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Partridge, S. R., H. J. Brown, H. W. Stokes, and R. M. Hall. 2001. Transposons Tn1696 and Tn21 and their integrons In4 and In2 have independent origins. Antimicrob. Agents Chemother. 45:1263-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabaté, M., F. Navarro, E. Miro, S. Campoy, B. Mirelis, J. Barbe, and G. Prats. 2002. Novel complex sul1-type integron in Escherichia coli carrying blaCTX-M-9. Antimicrob. Agents Chemother. 46:2656-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Schmidt, F. 1984. The role of insertions, deletions, and substitutions in the evolution of R6 related plasmids encoding aminoglycoside transferase ANT-(2"). Mol. Gen. Genet. 194:248-259. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt, F., and I. Klopfer-Kaul. 1984. Evolutionary relationship between Tn21-like elements and pBP201, a plasmid from Klebsiella pneumoniae mediating resistance to gentamicin and eight other drugs. Mol. Gen. Genet. 197:109-119. [DOI] [PubMed] [Google Scholar]
- 38.Szczepanowski, R., S. Braun, V. Riedel, S. Schneiker, I. Krahn, A. Puhler, and A. Schluter. 2005. The 120,592 bp IncF plasmid pRSB107 isolated from a sewage-treatment plant encodes nine different antibiotic-resistance determinants, two iron-acquisition systems and other putative virulence-associated functions. Microbiology 151:1095-1111. [DOI] [PubMed] [Google Scholar]
- 39.Tennstedt, T., R. Szczepanowski, I. Krahn, A. Puhler, and A. Schluter. 2005. Sequence of the 68,869 bp IncP-1a plasmid pBT11 from a waste water treatment plant reveals a highly conversed backbone, a Tn402-like integron and other transposable elements. Plasmid 53:218-238. [DOI] [PubMed] [Google Scholar]
- 40.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Threlfall, E. J., and N. Woodford. 1995. Plasmid profile typing and plasmid fingerprinting. Methods Mol. Biol. 46:225-236. [DOI] [PubMed] [Google Scholar]
- 42.Valverde, A., R. Cantón, J. C. Galán, P. Nordmann, F. Baquero, and T. M. Coque. 2005. In117, an unusual In0-like class 1 integron containing CR1 and blaCTX-M-2 and associated with a Tn21-like element. Antimicrob. Agents Chemother. 50:799-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villa, L., P. Visca, F. Tosini, C. Pezzella, and A. Carattoli. 2002. Composite integron array generated by insertion of an ORF341-type integron within a Tn21-like element. Microb. Drug Resist. 8:1-8. [DOI] [PubMed] [Google Scholar]
- 44.Walsh, T. R., M. A. Toleman, L. Poirel, and P. Nordmann. 2005. Metallo-beta-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, M., J. H. Tran, G. A. Jacoby, Y. Zhang, F. Wang, and D. C. Hooper. 2003. Plasmid-mediated quinolone resistance in clinical isolates of Escherichia coli from Shanghai, China. Antimicrob. Agents Chemother. 47:2242-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkins, B. M. 1995. Gene transfer by bacterial conjugation: diversity of systems and functional specializations, p. 59-89. In S. Baumberg, P. W. Young, E. M. H. Wellington, and J. R. Saunders (ed.), Population genetics of bacteria. Cambridge University Press, Cambridge, United Kingdom.
- 47.Yurieva, O., G. Kholodii, L. Minakhin, Z. Gorlenko, E. Kalyaeva, E. Mindlin, and V. Nikiforov. 1997. Intercontinental spread of promiscuous mercury-resistance transposons in environmental bacteria. Mol. Microbiol. 24:321-329. [DOI] [PubMed] [Google Scholar]