Antifungal prophylaxis for patients at high risk for aspergillosis is appealing due to the high morbidity and mortality of aspergillosis despite antifungal therapy (1-3). While voriconazole is a logical option, reports of breakthrough mucormycosis are a cause for concern (5). To identify alternative prophylactic strategies, we utilized our novel, inhalational model of pulmonary Aspergillus fumigatus infection in neutropenic BALB/c mice (4).
Initially liposomal amphotericin B (LAmB) at 5 mg/kg of body weight in 5% dextrose in water (D5W) was given intravenously at either 6 or 24 h (one dose) or 6 and 24 h (two doses) prior to infection. Negative control mice received the D5W carrier. Two doses of LAmB significantly improved survival compared to either single-dose regimen (P < 0.05 for both comparisons by log rank test). The efficacy of a single 15-mg/kg dose of LAmB was then compared to that of two doses of 7.5 mg/kg given every other day, with the last dose administered 24, 48, or 72 h prior to infection. Dual 7.5-mg/kg doses of LAmB ending at 24 or 48 h prior to infection significantly improved survival (100% survival for both versus 50% survival of placebo; P < 0.05 by log rank test) and significantly reduced the A. fumigatus lung burden compared to results with the placebo (P = 0.008 and 0.03, respectively, by Mann Whitney U test). Single-dose LAmB at 15 mg/kg 24 or 48 h prior to infection resulted in trends (P = 0.09 for both) to improved survival versus results with the placebo. In contrast, when infection occurred 72 h after the last dose of LAmB, no survival advantage was seen, and the single dose of LAmB at 15 mg/kg caused a statistically significant worsening of survival compared to results with the placebo (P = 0.042, by log rank test).
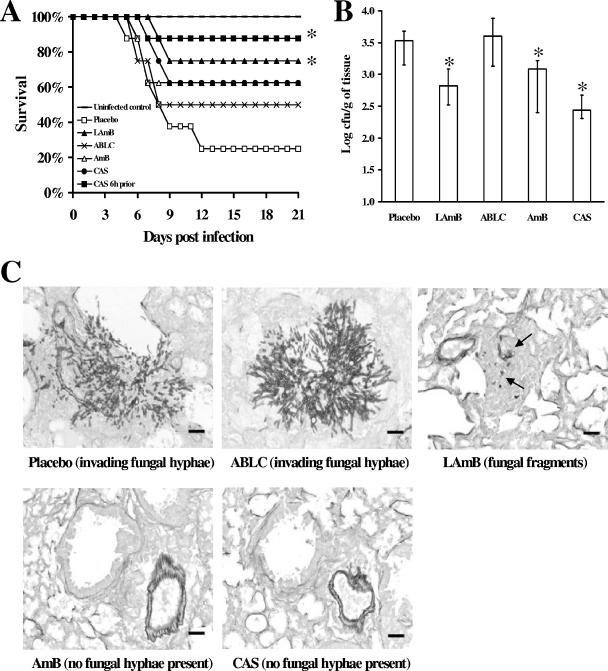
Next we compared LAmB at 7.5 mg/kg with amphotericin B lipid complex (ABLC) at 7.5 mg/kg, amphotericin B deoxycholate (AmB) at 1 mg/kg, and caspofungin (CAS) at 1 mg/kg. Two doses of polyene or CAS were given intravenously on days −4 and −2 prior to infection. In a separate arm, CAS was also administered as a single dose 6 h prior to infection. Only LAmB and single-dose CAS significantly improved survival compared to the placebo (75% and 88% survival versus 25% survival with the placebo; P < 0.04) (Fig. 1A).
FIG. 1.
Prophylactic treatment of neutropenic mice with LAmB or CAS protected mice from infection with A. fumigatus. (A) Survival of mice treated intravenously with two doses of polyenes (LAmB or ABLC at 7.5 mg/kg and AmB at 1.0 mg/kg) or CAS (1 mg/kg) on days −4 and −2 prior to infection or with a single dose of CAS (1 mg/kg) 6 h prior to infection (n = 8 mice per group; *, P < 0.04 versus placebo results). (B) Lung fungal burden was significantly reduced by prophylactic administration of LAmB, AmB (P = 0.03), or CAS (given 6 h prior to infection) but not ABLC (n = 13 mice per group; P ≤ 0.03 versus placebo results). Data in B are the median ± interquartile ranges. In all experiments mice were infected via aerosolization of 1.2 × 1010 conidia. (C) Invasive necrotizing fungal pneumonia with abundant intralesional elongated fungal hyphae and vascular thrombosis are seen in the placebo- and ABLC-treated mice. In contrast, lungs of mice prophylactically treated with LAmB, AmB, or CAS had only pulmonary edema, hemorrhage, alveolar histiocytosis, and rare focal inflammation centered on degenerate fungal hyphal fragments (arrows) with no evidence of active infection. Lungs were harvested 96 h postinfection, fixed in 10% zinc-buffered formalin, embedded in paraffin, and sectioned and Gridley stained. Magnification, ×200. Bar = 30 μm.
Pulmonary fungal burden was significantly reduced by prophylactic administration of LAmB (P = 0.005), AmB (P = 0.03), or CAS (given 6 h prior to infection; P = 0.0002) (Fig. 1B). ABLC did not decrease lung fungal burden. Lungs from mice receiving a placebo or ABLC but not LAmB, CAS, or AmB contained invasive necrotizing fungal pneumonia with abundant elongated fungal hyphae and vascular thrombosis (Fig. 1C).
These data indicate that LAmB administered every other day is a promising candidate for prophylaxis against pulmonary aspergillosis in neutropenic hosts, but high doses of LAmB (i.e., 15 mg/kg) appear to be toxic. While CAS also offers protection as prophylaxis, it must be dosed more often than LAmB for efficacy. In contrast, with the dosing regimens tested, ABLC and AmB were inferior to LAmB and CAS for the prevention of experimental inhalational pulmonary aspergillosis.
Acknowledgments
This study was supported by a research and educational grant from Gilead Sciences Inc. Research described here was conducted in part at the research facilities of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
REFERENCES
- 1.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23:608-615. [DOI] [PubMed] [Google Scholar]
- 2.Denning, D. W., A. Marinus, J. Cohen, D. Spence, R. Herbrecht, L. Pagano, C. Kibbler, V. Kcrmery, F. Offner, C. Cordonnier, U. Jehn, M. Ellis, L. Collette, R. Sylvester, et al. 1998. An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. J. Infect. 37:173-180. [DOI] [PubMed] [Google Scholar]
- 3.Graybill, J. R., R. Bocanegra, L. K. Najvar, M. F. Luther, and D. Loebenberg. 1998. SCH56592 treatment of murine invasive aspergillosis. J. Antimicrob. Chemother. 42:539-542. [DOI] [PubMed] [Google Scholar]
- 4.Sheppard, D. C., G. Rieg, L. Y. Chiang, S. G. Filler, J. E. Edwards, Jr., and A. S. Ibrahim. 2004. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 48:1908-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spellberg, B., J. Edwards, Jr., and A. Ibrahim. 2005. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 18:556-569. [DOI] [PMC free article] [PubMed] [Google Scholar]