Abstract
Seven open reading frames, annotated as potential penicillin-binding-protein-encoding genes (lmo0441, lmo0540, lmo1438, lmo1892, lmo2039, lmo2229, and lmo2754), were targeted for insertional mutagenesis in Listeria monocytogenes EGDe. These genes were found to contribute in various degrees to β-lactam resistance, cell morphology, or the virulence potential of this organism.
Listeria monocytogenes is an important intracellular pathogen that is responsible for almost 30% of food-related deaths in the United States every year (19). In general, L. monocytogenes is susceptible to a wide range of antibiotics, including penicillins, aminopenicillins, and carbapenems (5, 11). Clinicians treating patients suspected of having L. monocytogenes infection normally utilize ampicillin or benzylpenicillin, either alone or in combination with an aminoglycoside (30). Although rare, there have been some reports of penicillin-resistant Listeria isolated from foods (35), and ampicillin-resistant and imipenem-resistant clinical isolates have also been reported (24, 26). L. monocytogenes strains also possess an innate natural resistance to monobactams, β-lactams such as methicillin, and the expanded-spectrum cephalosporins, including cefotaxime and ceftazidime (5). The last of these traits is of clinical significance, as the cephalosporin antibiotics are often the first therapy used to treat fever in hospitals when the etiological agent is unknown.
To date there have been a limited number of studies pertaining to the penicillin-binding proteins (PBP) of L. monocytogenes, and, significantly, the majority of these were carried out before sequencing of a L. monocytogenes genome was completed (8, 9, 10, 22, 32, 33). Recent attempts have been made to reevaluate the molecular weights and cell copy numbers of PBPs in L. monocytogenes EGD (15), and the enzymatic properties of PBP4 (encoded by lmo2229) (36) and PBP5 (encoded by lmo2754) (16, 17) have been established. In this study potential PBP-encoding genes of the genome-sequenced EGDe strain were found through in silico analysis, and subsequently their respective roles were revealed by insertional mutagenesis.
Putative PBPs of L. monocytogenes EGDe were identified using the free text function on the genome web server Listilist (http://genolist.pasteur.fr/listilist/). Subsequently sequences were analyzed using various web-based programs (www.ncbi.nlm.nih.gov/structure/cdd/cddshtml, www.ncbi.nlm.nih.gov/BLAST, www.ncbi.nlm.nih.gov/sutlis/blink, www.tigr.org, www.microbesonline.org, www.ebi.ac.uk/fasta33, www.sanger.ac.uk/software/pfam, and www.ebi.ac.uk/Interpro). The possible functions and closely related homologs of the putative PBPs are outlined in Table 1. Five genes encode >50-kDa high-molecular-mass PBPs (lmo0441, lmo1438, lmo2039 [class B] and lmo1892 and lmo2229 [class A]). Lmo0540 and Lmo2754 (PBP5) are annotated as low-molecular-mass PBPs in the EGDe genome. However, BLAST and domain analyses of Lmo0540 indicate that it shows highest similarities to class C β-lactamases. Also, sequence alignments revealed some slight variations in the positioning of the classical PBP motifs (6). Lmo0540 was nonetheless included in this study to determine its role in L. monocytogenes physiology.
TABLE 1.
Bioinformatic analysis of putative PBPs of L. monocytogenesa
| Gene | Class | No. of amino acids | Predicted mol wt, 103 | Putative function | Homolog(s) | Reference |
|---|---|---|---|---|---|---|
| lmo0441 | B | 678 | 74.6 | Carboxypeptidase | Enterococcus faecalis PBP4 | 21 |
| Transpeptidase | Enterococcus faecium PBP5 | 38 | ||||
| Staphylococcus aureus PBP2′ | 12 | |||||
| lmo0540 | LMWb | 397 | 44.5 | β-Lactamase | Staphylococcus aureus fmtA | 14 |
| lmo1438 | B | 721 | 79.9 | FtsI, cell division | Staphylococcus aureus PBP3 | 23 |
| Transpeptidase | ||||||
| lmo1892 | A | 827 | 90.8 | Carboxypeptidase | Streptococcus pneumoniae PBP1A | 28 |
| Glycosyltransferase | Enterococcus faecalis PBP1A | 4 | ||||
| Transpeptidase | ||||||
| lmo2039 | B | 751 | 81.8 | FtsI cell division | Streptococcus pneumoniae PBP2X | 28 |
| Transpeptidase | Staphylococcus aureus PBP1 | 34 | ||||
| lmo2229c | A | 714 | 77.8 | Carboxypeptidase | Streptococcus pneumoniae PBP2A | 28 |
| Glycosyltransferase | ||||||
| Transpeptidase | ||||||
| lmo2754d | LMW | 445 | 48.08 | Carboxypeptidase | Bacillus subtilis PBP5 | 31 |
| Transpeptidase | Streptococcus pneumoniae PBP3 | 28 |
Gene names, predicted molecular weights and putative functions are based on those assigned by the National Center for Biotechnology Information and by Listilist. Homologs were found by www.ncbi.nih.nlm.gov/blink.
LMW, low molecular weight.
Characteristics of lmo2229 are as outlined previously (36).
Insertional mutagenesis of all seven genes was attempted by the method previously described (3, 18). Primers amplifying central internal fragments of each of the following genes were used for disruption (all sequences are 5′ to 3′): lmo0441 (forward, ACGAATTCGAAATGCGGAC; reverse, TTTCTAGATGTCTTCGGCG), lmo0540 (forward, GATCTAGAACCAGTAGAAG; reverse, GTGAATTCTGGTCGTCCAAC), lmo1438 (forward, CGGAATTCCAATTTGTTGG; reverse, TTTCTAGACCTTCTTTAGC), lmo1892 (forward, AATCTAGAAACGGAAGATGCG; reverse, GCGAATTCCTGTAGTGATAG), lmo2039 (forward, AAGTCGAATTCGGCGCTGCT; reverse, GATCTGCAGGGTTGAAGGA), lmo2229 (forward, AACTGCAGTAGTTTCCATTG; reverse, TGTAGAATTCGCCTTCTGC), and lmo2754 (forward, TCTGAATTCGCTACAAAG; reverse, CGTCTAGACCTTTGCGCC). In each case integration was confirmed by PCR using one primer outside the region of integration and another for the plasmid and was indicated by an Emr Cms phenotype. With the exception of lmo2039, all of the genes were successfully disrupted. lmo2039 is not followed by a termination signal and is located within a putative operon, and thus our inability to isolate an integrant could theoretically be due to a polar impact on this operon. However, as two homologs, PBP2X (36% identity) and PBP1 (37% identity), have been found to be essential for the viability of Streptococcus pneumoniae (13) and Staphylococcus aureus (34), respectively, it is more likely that the consequences of disrupting lmo2039 itself are the reasons why this mutant was not isolated.
Before detailed investigations were carried out, the growth rates of the six mutants and the parent strain in tryptic soy broth-yeast extract (TSB-YE) at 37°C were compared (data not shown). While the growth rate of the lmo2754 mutant did differ from that of the parent strain, this difference was not statistically significant as determined by Student's t test (P < 0.05) (data not shown). To determine if the disrupted PBP-encoding genes play a role in the β-lactam resistance of L. monocytogenes, the six mutants were subjected to antibiotic disk assays with a large number of β-lactam disks on tryptone soy agar (Difco) supplemented with 0.6% yeast extract (Merck) (TSA-YE) (data not shown). Following this preliminary assay, specific cephalosporin antibiotics and penicillin G were chosen for MIC determination using the broth dilution (TSB-YE) method outlined by the CLSI (formerly NCCLS) (20) (Table 2). Results were analyzed after 16 to 20 h at 37°C. Both assays demonstrated that interruption of two of the pbp homologs, lmo0441 or lmo2229, increased the sensitivity of these strains to β-lactam antibiotics (Table 2). It is interesting to note that for certain antibiotics the disk assays revealed the presence of single colonies of Listeria on the outer edges of these zones of clearing, possibly indicating spontaneous resistance development. Of the two, the consequences of mutating lmo0441 were greater as evidenced by a 16-fold reduction in the MICs of ceftazidime and cefotaxime (Table 2). Interestingly, Lmo0441 is homologous to the low-affinity PBPs of Enterococcus faecium (PBP5) (44% identity) (38) and S. aureus (PBP2′) (34% identity) (12), which contribute greatly to β-lactam resistance in those genera. Although less dramatic, mutation of lmo2229 results in a decrease in the MICs of some cephalosporins against EGDe (Table 2) and L. monocytogenes LO28 (unpublished data). The contrast between these results and those of Zawadzka-Skomial et al. (36), who did not observe a difference between the MICs for EGD and a 2229 mutant, may be due to strain variation (EGD versus EGDe/LO28) or to the growth of spontaneously resistant cells over the extended duration used for MIC determination (36). PBP2A of S. pneumoniae, a homolog of Lmo2229, has been found to have an important role in the β-lactam resistance of this organism (29, 37). We also investigated the MICs of two other cell-wall-acting antimicrobials, the lantibiotics nisin and lacticin 3147, against the parent and six mutants. Although the MIC of lacticin 3147 was identical in all cases, one mutant, pORI::2229, displayed enhanced nisin sensitivity (1.66-fold decrease in MIC for triplicate experiments; data not shown). This is in agreement with previous findings for mutants of L. monocytogenes 412 (7).
TABLE 2.
MICs of penicillin G and cephalosporin antibiotics against EGDe and mutants
| Antibiotica | MIC (μg/ml) against:
|
||||||
|---|---|---|---|---|---|---|---|
| EGDe | pORI::0441 | pORI::0540 | pORI::1438 | pORI::1892 | pORI::2229 | pORI::2754 | |
| P | 0.125 | 0.06 | 0.125 | 0.125 | 0.125 | 0.06 | 0.125 |
| CAZ | 128 | 8 | 128 | 128 | 128 | 32 | 64 |
| CTX | 8 | 0.5 | 8 | 8 | 8 | 2 | 8 |
| CXM | 4 | 0.5 | 4 | 4 | 4 | 2 | 4 |
P, penicillin G; CAZ, ceftazidime; CTX, cefotaxime; CXM, cefuroxime.
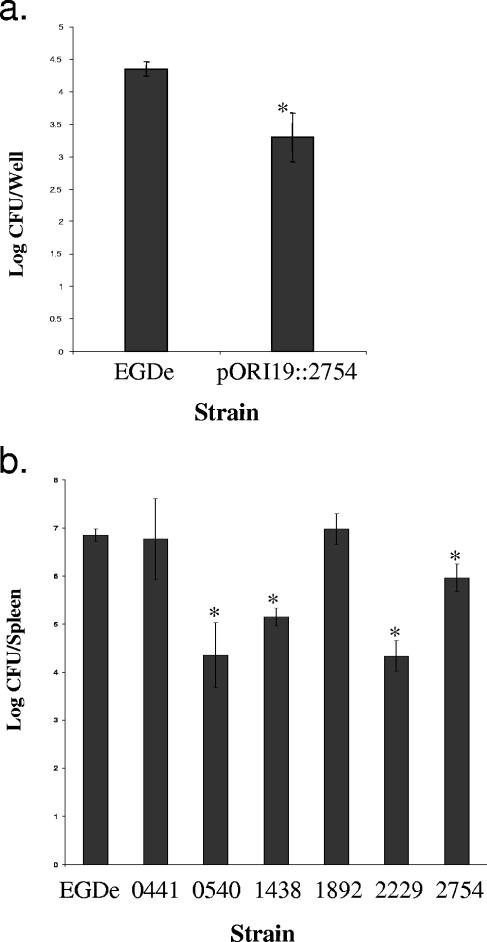
The impact of PBP mutations on the virulence of L. monocytogenes was investigated by both in vitro and in vivo assays. In vitro assays revealed no significant reduction in the ability of the strains to grow intracellularly in J774 macrophages (not shown), although the lmo2754 mutant strain exhibited a reduced ability (almost a 1-log reduction) to invade C2Bbe1 cells (Fig. 1a). Macrophage assays (27) and invasion assays (2) were performed as previously described. In vivo survival was assayed by intraperitoneal infection of 6- to 8-week-old BALB/c mice and was quantified on the basis of the number of Listeria cells in the spleen at 3 days postinfection. Cells were counted on TSA-YE plates incubated overnight at 37°C. A number of mutants displayed dramatic reductions in virulence (Fig. 1b). Here it is demonstrated that in addition to having a role in mouse brain colonization following intravenous inoculation, Lmo0540 also contributes to virulence as determined by colonization of the spleen (1). Disruptions of lmo1438 or lmo2229 also greatly attenuated virulence as demonstrated by almost 2- and 3-log reductions in numbers in the spleen, respectively, while a mutant lacking a functional PBP5 also showed a virulence defect (Fig. 1b).
FIG. 1.
(a) Effect of disruption of lmo2754 on the invasion of the organism on C2Bbe1 cells. Numbers of L. monocytogenes cells invaded were expressed as log CFU/well. Results are representative of triplicate experiments. (b) Effect of pORI19 disruption of PBPs on the survival of EGDe in vivo. Numbers indicate the genes that were disrupted. Mice were injected intraperitoneally, and the number of bacteria recovered from the spleen was determined at 3 days postinfection. Error bars represent the standard deviations from the means (n = 5). *, mean is statistically significant with respect to the wild-type value (P < 0.05).
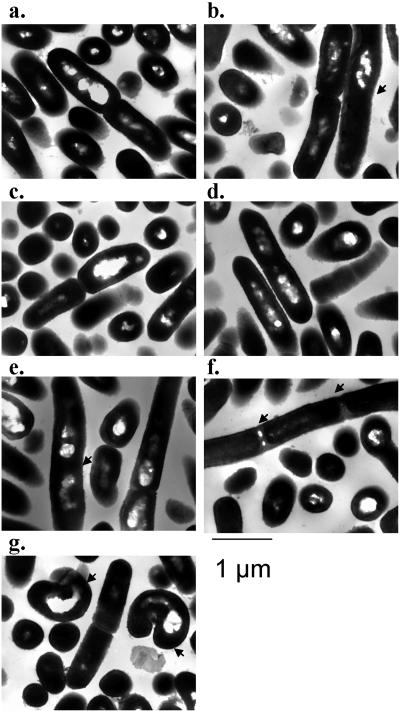
The impact of the loss of PBP function on the morphology of log-phase L. monocytogenes in TSB-YE was initially investigated with a light microscope (magnification of ×1,000). The mutant lacking lmo2229 showed an alteration in morphology, with chains of three or four cells observed (data not shown). As this phenomenon has not been described previously (36), it may indicate that the phenotype is growth phase dependent. Further morphological investigation of all strains was performed by transmission electron microscopy (TEM) on log-phase cells. TEM, in addition to confirming the chaining effect in the lmo2229 mutant, identified a number of additional phenotypes associated with this and other mutants (Fig. 2a to g). The cell lengths were determined for each strain (n = 30 to 40 for each strain), except for those cells lacking PBP5, which displayed an irregular morphology (Fig. 2g). Only 3% of parental cells were greater than 2.0 μm in length. This value increased to 29% for the lmo0441 mutant, 27% for the lmo1892 mutant, and 31.8% for the lmo2229 mutant. As observed previously (17), strains lacking PBP5 showed a thicker cell wall (EGDe, 25.12 ± 2.81 nm; PBP5 mutant, 33.68 ± 3.89 nm). It was also noted that 50% of PBP5-lacking cells had an irregular shape (Fig. 2g). This phenotype is not unexpected, as it has previously been documented that the loss of low-molecular-mass PBPs can result in altered cell shape (25). It was reported that the EGDΔPBP5 strain has an altered ratio of pentapeptides to tripeptides (16). This has been suggested to lead to an imbalance of peptidoglycan, resulting in an altered shape. Finally, slight alterations were found with the disruption of lmo0441 (Fig. 2b) and lmo1892 (Fig. 2e).
FIG. 2.
Electron microscopy of EGDe (a) pORI19::0441 (b), pORI19::0540 (c), pORI19::1438 (d), pORI19::1892 (e), pORI19::2229 (f), and pORI19::2754 (g). Arrows in panels b and e indicate irregular curving of cells and increased cell length. Arrows in panel f indicate filamenting of cells. Arrows in panel g indicate irregular shapes of cells.
In this study, we have completed a postgenomic analysis of loci in the EGDe genome that show homology to PBP-encoding genes and have investigated the roles of the individual gene products by insertional mutagenesis. This is the first study that characterizes the PBPs of L. monocytogenes at a genetic level and examines their role in the biology of the organism. Other genes, not studied in this paper, including lmo1855 and lmo2812 (both annotated as dd-carboxypeptidases) and lmo1916 (annotated as a peptidase), with molecular masses of approximately 31, 29, and 38 kDa, respectively, may also merit study. Importantly, this study has identified three new potential therapeutic targets for L. monocytogenes. First, MIC determinations suggest that Lmo0441 is central to the β-lactam resistance of this organism, a role which is consistent with its homology to low-affinity PBPs. Second, Lmo2229 contributes to β-lactam resistance, virulence potential, and morphogenesis of L. monocytogenes. Being a class A protein, this protein has particular potential as a target due to the presence of a transglycosylase domain. Finally, lmo2039 (or at least the operon in which it resides) is essential to the survival of the organism.
Although L. monocytogenes is highly sensitive to a number of antibiotics and at present is treatable with penicillin/ampicillin, the exact mechanism of cell death remains unknown. Also, due to the rapidity with which antibiotic-resistant mutants of other gram-positive bacteria have emerged, it is possible that future alternative therapeutics may be required for the treatment of listeriosis. The characterization of the PBPs of this organism is fundamental to the scientific community's ongoing efforts to limit the mortality associated with this pathogen.
Acknowledgments
We acknowledge the funding received from the Irish Government under the National Development Plan 2000-2006 and through funding of the Alimentary Pharmabiotic Centre by the Science Foundation of Ireland Centres for Science Engineering and Technology (CSET) scheme.
We thank Cormac Gahan and Pat Casey for help with the virulence studies, Elaine Lawton for technical assistance, and Don O'Leary for the TEM photographs presented in this paper.
REFERENCES
- 1.Autret, N., I. Dubail, P. Trieu-Cuot, P. Berche, and A. Charbit. 2001. Identification of new genes involved in the virulence of Listeria monocytogenes by signature-tagged transposon mutagenesis. Infect. Immun. 69:2054-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bron, P. A., I. R. Monk, S. C. Corr, C. Hill, and C. G. Gahan. 2006. Novel luciferase reporter system for in vitro and organ-specific monitoring of differential gene expression in Listeria monocytogenes. Appl. Environ. Microbiol. 72:2876-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotter, P. D., C. G. Gahan, and C. Hill. 2000. Analysis of the role of the Listeria monocytogenes F0F1-ATPase operon in the acid tolerance response. Int. J. Food Microbiol. 60:137-146. [DOI] [PubMed] [Google Scholar]
- 4.Duez, C., S. Hallut, N. Rhazi, S. Hubert, A. Amoroso, F. Bouillenne, A. Piette, and J. Coyette. 2004. The ponA gene of Enterococcus faecalis JH2-2 codes for a low-affinity class A penicillin-binding protein. J. Bacteriol. 186:4412-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espaze, E. P., and A. E. Reynaud. 1988. Antibiotic susceptibilities of Listeria: in vitro studies. Infect. Suppl. 2:S160-S164. [DOI] [PubMed] [Google Scholar]
- 6.Goffin, C., and J. M. Ghuysen. 1998. Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs. Microbiol. Mol. Biol Rev. 62:1079-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gravesen, A., B. Kallipolitis, K. Holstrom, P. E. Hoiby, M. Ramnath, and S. Knochel. 2004. pbp2229-mediated nisin resistance mechanism in Listeria monocytogenes confers cross-protection to class IIa bacteriocins and affects virulence gene expression. Appl. Environ. Microbiol. 70:1669-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutkind, G. O., M. E. Mollerach, and R. A. De Torres. 1989. Penicillin-binding proteins in Listeria monocytogenes. APMIS 97:1013-1017. [DOI] [PubMed] [Google Scholar]
- 9.Gutkind, G. O., S. B. Ogueta, A. C. de Urtiaga, M. E. Mollerach, and R. A. de Torres. 1990. Participation of PBP 3 in the acquisition of dicloxacillin resistance in Listeria monocytogenes. J. Antimicrob. Chemother. 25:751-758. [DOI] [PubMed] [Google Scholar]
- 10.Hakenbeck, R., and H. Hof. 1991. Relatedness of penicillin-binding proteins from various Listeria species. FEMS Microbiol. Lett. 68:191-195. [DOI] [PubMed] [Google Scholar]
- 11.Heger, W., M. P. Dierich, and F. Allerberger. 1997. In vitro susceptibility of Listeria monocytogenes: comparison of the E test with the agar dilution test. Chemotherapy 43:303-310. [DOI] [PubMed] [Google Scholar]
- 12.Ito, T., Y. Katayama, K. Asada, N. Mori, K. Tsutsumimoto, C. Tiensasitorn, and K. Hiramatsu. 2001. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1323-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kell, C. M., U. K. Sharma, C. G. Dowson, C. Town, T. S. Balganesh, and B. G. Spratt. 1993. Deletion analysis of the essentiality of penicillin-binding proteins 1A, 2B and 2X of Streptococcus pneumoniae. FEMS Microbiol. Lett. 106:171-175. [DOI] [PubMed] [Google Scholar]
- 14.Komatsuzawa, H., K. Ohta, H. Labischinski, M. Sugai, and H. Suginaka. 1999. Characterization of fmtA, a gene that modulates the expression of methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 43:2121-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korsak, D., J. J. Zawadzka, M. E. Siwinska, and Z. Markiewicz. 2002. Penicillin-binding proteins of Listeria monocytogenes—a re-evaluation. Acta Microbiol. Pol. 51:5-12. [PubMed] [Google Scholar]
- 16.Korsak, D., M. Popowska, and Z. Markiewicz. 2005. Analysis of the murein of a Listeria monocytogenes EGD mutant lacking functional penicillin binding protein 5 (PBP5). Pol. J. Microbiol. 54:339-342. [PubMed] [Google Scholar]
- 17.Korsak, D., W. Vollmer, and Z. Markiewicz. 2005. Listeria monocytogenes EGD lacking penicillin-binding protein 5 (PBP5) produces a thicker cell wall. FEMS Microbiol. Lett. 251:281-288. [DOI] [PubMed] [Google Scholar]
- 18.Law, J., G. Buist, A. Haandrikman, J. Kok, G. Venema, and K. Leenhouts. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J. Bacteriol. 177:7011-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A6, 6th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 21.Ono, S., T. Muratani, and T. Matsumoto. 2005. Mechanisms of resistance to imipenem and ampicillin in Enterococcus faecalis. Antimicrob. Agents Chemother. 49:2954-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierre, J., A. Boisivon, and L. Gutmann. 1990. Alteration of PBP 3 entails resistance to imipenem in Listeria monocytogenes. Antimicrob. Agents Chemother. 34:1695-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinho, M. G., H. de Lencastre, and A. Tomasz. 2000. Cloning, characterization, and inactivation of the gene pbpC, encoding penicillin-binding protein 3 of Staphylococcus aureus. J. Bacteriol. 182:1074-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollock, S. S., T. M. Pollock, and M. J. Harrison. 1986. Ampicillin-resistant Listeria monocytogenes meningitis. Arch. Neurol. 43:106. [DOI] [PubMed] [Google Scholar]
- 25.Popham, D. L., and K. D. Young. 2003. Role of penicillin-binding proteins in bacterial cell morphogenesis. Curr. Opin. Microbiol. 6:594-599. [DOI] [PubMed] [Google Scholar]
- 26.Rapp, M. F., H. A. Pershadsingh, J. W. Long, Jr., and J. M. Pickens. 1984. Ampicillin-resistant Listeria monocytogenes meningitis in a previously healthy 14-year-old athlete. Arch. Neurol. 41:1304. [DOI] [PubMed] [Google Scholar]
- 27.Rea, R., C. Hill, and C. G. Gahan. 2005. Listeria monocytogenes PerR mutants display a small-colony phenotype, increased sensitivity to hydrogen peroxide, and significantly reduced murine virulence. Appl. Environ. Microbiol. 71:8314-8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanbongi, Y., T. Ida, M. Ishikawa, Y. Osaki, H. Kataoka, T. Suzuki, K. Kondo, F. Ohsawa, and M. Yonezawa. 2004. Complete sequences of six penicillin-binding protein genes from 40 Streptococcus pneumoniae clinical isolates collected in Japan. Antimicrob. Agents Chemother. 48:2244-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith, A. M., C. Feldman, O. Massidda, K. McCarthy, D. Ndiweni, and K. P. Klugman. 2005. Altered PBP 2A and its role in the development of penicillin, cefotaxime, and ceftriaxone resistance in a clinical isolate of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 49:2002-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Temple, M. E., and M. C. Nahata. 2000. Treatment of listeriosis. Ann. Pharmacother. 34:656-661. [DOI] [PubMed] [Google Scholar]
- 31.Todd, J. A., A. N. Roberts, K. Johnstone, P. J. Piggot, G. Winter, and D. J. Ellar. 1986. Reduced heat resistance of mutant spores after cloning and mutagenesis of the Bacillus subtilis gene encoding penicillin-binding protein 5. J. Bacteriol. 167:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vicente, M. F., J. C. Perez-Daz, F. Baquero, M. Angel de Pedro, and J. Berenguer. 1990. Penicillin-binding protein 3 of Listeria monocytogenes as the primary lethal target for beta-lactams. Antimicrob. Agents Chemother. 34:539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vicente, M. F., J. Berenguer, M. A. de Pedro, J. C. Perez-Diaz, and F. Baquero. 1990. Penicillin binding proteins in Listeria monocytogenes. Acta Microbiol. Hung. 37:227-231. [PubMed] [Google Scholar]
- 34.Wada, A., and H. Watanabe. 1998. Penicillin-binding protein 1 of Staphylococcus aureus is essential for growth. J. Bacteriol. 180:2759-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh, D., J. J. Sheridan, G. Duffy, I. S. Blair, D. A. McDowell, and D. Harrington. 2001. Thermal resistance of wild-type and antibiotic-resistant Listeria monocytogenes in meat and potato substrates. J. Appl. Microbiol. 90:555-560. [DOI] [PubMed] [Google Scholar]
- 36.Zawadzka-Skomial, J., Markiewicz, Z., Nguyen-Disteche, M., Devreese, B., Frere, J. M., and M. Terrak. 2006. Characterization of the bifunctional glycosyltransferase/acyltransferase penicillin-binding protein 4 of Listeria monocytogenes. J. Bacteriol. 188:1875-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao, G., T. I. Meier, J. Hoskins, and K. A. 2000. Identification and characterization of the penicillin-binding protein 2a of Streptococcus pneumoniae and its possible role in resistance to beta-lactam antibiotics. Antimicrob. Agents Chemother. 44:1745-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zorzi, W., X. Y. Zhou, O. Dardenne, J. Lamotte, D. Raze, J. Pierre, L. Gutmann, and J. Coyette. 1996. Structure of the low-affinity penicillin-binding protein 5 PBP5fm in wild-type and highly penicillin-resistant strains of Enterococcus faecium. J. Bacteriol. 178:4948-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]