Abstract
The C-8-methoxy fluoroquinolone moxifloxacin was more lethal against chloramphenicol-treated Mycobacterium tuberculosis than Bay y3114, a C-8-H cognate of moxifloxacin, and two C-8-methoxy fluoroquinolones, gatifloxacin and BMS-433368, which have different C-7 substituents. Thus, an optimal combination of C-7 and C-8 substituents is likely to be important for killing nongrowing M. tuberculosis.
The fluoroquinolones moxifloxacin and gatifloxacin are among the new compounds being considered as antituberculosis agents. Both exhibit good activity against M. tuberculosis in vitro (8, 12) and in animal models (1, 10, 16). Moreover, both show good activity against subpopulations of resistant mutants (3). One way to distinguish among quinolones is to measure lethal activity in the absence of ongoing protein synthesis. Early studies with Escherichia coli led to the proposal that the quinolones kill cells by two modes, one that requires protein synthesis and one that does not (4, 7). The former may involve induction of a “suicide” protein (6, 15), while the latter may arise from a quinolone-mediated destabilization of drug-gyrase-DNA complexes that causes chromosome fragmentation (2). Quinolone structure influences the relative contribution of each pathway to lethal action, with the protein synthesis-independent mode being stimulated by the presence of a methoxy group at the C-8 position (9, 17, 18). Both gatifloxacin and moxifloxacin contain a C-8-methoxy group. Here we compare the lethal activities of these and several other fluoroquinolones against M. tuberculosis in the presence and absence of chloramphenicol.
M. tuberculosis strain H37Rv was grown at 37°C as liquid cultures in Middlebrook 7H9 medium containing 10% albumin and 0.05% Tween 80 or as colonies on 7H10 agar plates (5). Ciprofloxacin, moxifloxacin, and Bay y3114 were obtained from Bayer Corp. (West Haven, CT); BMS-433368 and gatifloxacin were from Bristol-Myers Squibb (Wallingford, CT). Fluoroquinolones were dissolved in 0.1 ml of 1 N NaOH at 1/10 the final volume, and then sterile water was added to obtain a final concentration of 10 mg/ml. The MIC was determined as the minimum concentration required to inhibit growth in quinolone-containing 7H9 medium during a 6-day incubation. To measure lethal action, cells were grown in 7H9 medium to mid-log phase with agitation by rolling. Cultures were divided into 1-ml aliquots, and various concentrations of quinolone were added. After incubation for 6 days, cells were diluted in drug-free 7H9 medium and applied to 7H10 agar plates lacking drug. Agar plates were incubated for 4 to 5 weeks to determine the number of CFU; survival was calculated relative to CFU determined at the time of quinolone addition. For the measurement of killing in the absence of protein synthesis, chloramphenicol was added to 20 μg/ml 1 h prior to the addition of fluoroquinolone. At this concentration, chloramphenicol blocked growth but did not kill cells during the course of the experiment (not shown).
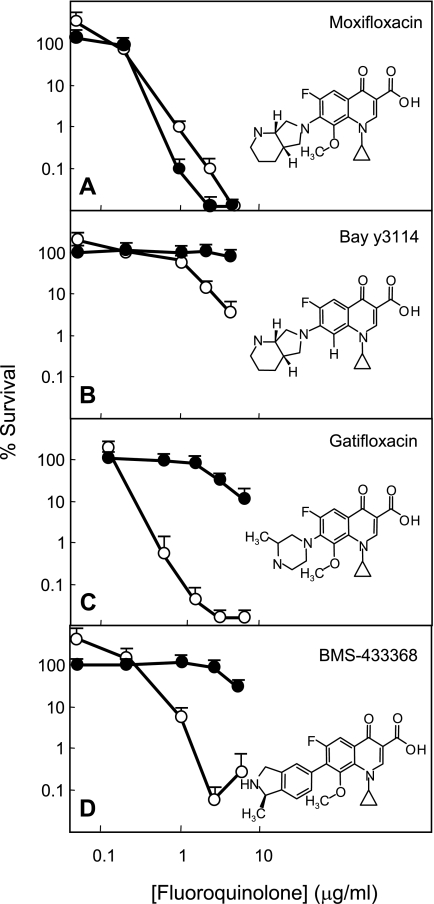
As shown in Table 1, four agents, gatifloxacin, moxifloxacin, Bay y3114, and BMS-433368, exhibited similar bacteriostatic activities (MIC = 0.03 to 0.05 μg/ml) against M. tuberculosis. Ciprofloxacin was less active. When lethal activities were compared in terms of absolute quinolone concentration (the 99% lethal dose [LD99]), moxifloxacin was 10 times more active than Bay y3114, a compound that is identical to moxifloxacin except for the absence of a C-8-methoxy group (Table 1 and Fig. 1A and B). Comparison of three C-8-methoxy compounds (moxifloxacin, gatifloxacin, and BMS-433368), which differed only in C-7 ring structure, showed little difference (Table 1 and Fig. 1). Thus, an 8-methoxy group is important for killing M. tuberculosis growing in vitro, but the C-7 ring structures tested are not. Normalization of LD99 to MIC to reveal lethal activity independent of factors likely to affect ternary complex formation, such as drug uptake and efflux, indicated that Bay y3114, the C-8-H derivative of moxifloxacin, was 10 times less effective than moxifloxacin. The three C-8-methoxy compounds had similar activities.
TABLE 1.
Susceptibility of M. tuberculosis to quinolones
| Compound | MIC (μg/ml)a | LD99 (μg/ml)a | LD99/MIC |
|---|---|---|---|
| Moxifloxacin | 0.04 | 0.8 | 20 |
| Bay y3114b | 0.05 | 10c | 200c |
| BMS-433368 | 0.05 | 1.5 | 30 |
| Gatifloxacin | 0.03 | 0.5 | 15 |
| Ciprofloxacin | 0.15 | 5 | 34 |
Similar values were obtained in replicate experiments. Concentration increments were 20% for determination of MIC.
Bay y3114 is a C-8-H cognate of moxifloxacin (Fig. 1).
LD99 was determined by extrapolation.
FIG. 1.
Lethal activity of fluoroquinolones in the presence and absence of chloramphenicol. Exponentially growing cultures of M. tuberculosis H37Rv were incubated with the indicated concentrations of fluoroquinolone for 6 days in the presence (filled circles) or absence (open circles) of chloramphenicol. Aliquots were then removed, diluted, and plated on drug-free 7H10 agar for determination of CFU. Names and structures of compounds tested are indicated in each panel. Data shown are averages, with error bars indicating ranges of values.
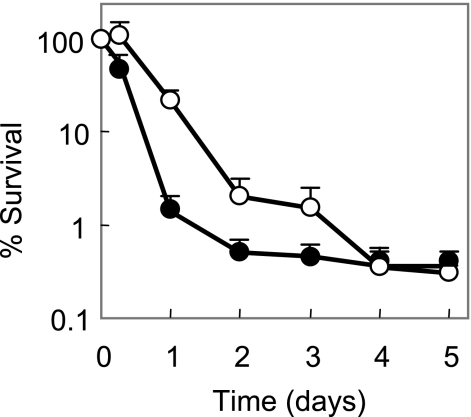
The ability of moxifloxacin to kill M. tuberculosis was unaffected by the presence of chloramphenicol, an inhibitor of protein synthesis (Fig. 1A). Lethal activity of two other C-8-methoxy compounds, gatifloxacin and BMS-433368, was dramatically reduced by chloramphenicol (Fig. 1C and D). Thus, the C-8-methoxy group does not by itself account for the ability of moxifloxacin to kill M. tuberculosis in the absence of protein synthesis, nor does the C-7 ring system, since chloramphenicol blocked the lethal activity of Bay y3114 (Fig. 1B). If moxifloxacin does not require the production of new protein to kill cells while other quinolones do, it should exhibit faster killing. As shown in Fig. 2, moxifloxacin killed M. tuberculosis about twice as fast as gatifloxacin.
FIG. 2.
Rate of killing by moxifloxacin and gatifloxacin. Exponentially growing cultures of M. tuberculosis H37Rv were incubated for the indicated times with 20 times the MIC of gatifloxacin (open circles) or moxifloxacin (filled circles). Aliquots were then removed, diluted, and plated on drug-free 7H10 agar for determination of CFU. Data shown are averages, with error bars indicating ranges of values.
The results described above, which agree well with an earlier study with Mycobacterium smegmatis (9), emphasize the unusual ability of moxifloxacin to kill mycobacteria in the absence of ongoing protein synthesis. With M. tuberculosis, comparisons between moxifloxacin and several related compounds (Fig. 1) indicate that the presence of a C-8-methoxy group and the diazabicyclo C-7 ring system are both important for the activity.
Lethal action probably arises through two steps, formation of bacteriostatic quinolone-gyrase-DNA complexes followed by chromosome fragmentation. Support for the two-step hypothesis was seen in the present work, as the quinolones (moxifloxacin and Bay y3114) exhibited similar MICs but very different abilities to kill cells (Table 1). The two-step phenomenon is also seen with quinolone treatment of E. coli (2, 18). With this organism, we recently found that under anaerobic conditions, all quinolones tested blocked growth, but only the fluoroquinolones killed cells (M. Malik, S. Hussain, and K. Drlica, unpublished observations). Thus, a low MIC, the traditional measure of quinolone activity, does not necessarily mean that a quinolone will be highly lethal.
A central feature of tuberculosis is the tendency of M. tuberculosis to enter a dormant state in which the bacterium is likely to exhibit low susceptibility to chemotherapeutic agents (13, 14). The exceptional ability of moxifloxacin to kill M. tuberculosis when protein synthesis is blocked (Fig. 1) encourages additional testing with more relevant models of growth arrest. For example, with low-dose respiratory infection of mice, M. tuberculosis grows exponentially during the first 3 weeks postinfection and then enters a nongrowing, chronic state (11). Thus, growing and nongrowing bacteria can be tested in the same system. Comparison of the fluoroquinolones described above in the murine system might support the use of moxifloxacin as a lead compound for finding quinolones that are even more active for treatment of tuberculosis.
Acknowledgments
We thank the following people for critical comments on the manuscript: Marila Gennaro, Richard Pine, and Xilin Zhao.
This work was supported by NIH grants AI35257 and AI063431.
REFERENCES
- 1.Alvirez-Freites, E., J. Carter, and M. Cynamon. 2002. In vitro and in vivo activities of gatifloxacin against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 46:1022-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, C.-R., M. Malik, M. Snyder, and K. Drlica. 1996. DNA gyrase and topoisomerase IV on the bacterial chromosome: quinolone-induced DNA cleavage. J. Mol. Biol. 258:627-637. [DOI] [PubMed] [Google Scholar]
- 3.Dong, Y., X. Zhao, B. Kreiswirth, and K. Drlica. 2000. Mutant prevention concentration as a measure of antibiotic potency: studies with clinical isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 44:2581-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard, B. M., R. J. Pinney, and J. T. Smith. 1993. 4-Quinolone bactericidal mechanisms. Arzneimittelforschung 43:1125-1129. [PubMed] [Google Scholar]
- 5.Jacobs, W. R., G. V. Kalpana, J. D. Cirillo, L. Pascopella, S. B. Snapper, R. A. Udani, W. Jones, R. G. Barletta, and B. R. Bloom. 1991. Genetic systems in mycobacteria. Methods Enzymol. 204:537-555. [DOI] [PubMed] [Google Scholar]
- 6.Lewin, C., I. Morrissey, and J. Smith. 1991. The mode of action of quinolones: the paradox in activity of low and high concentrations and activity in the anaerobic environment. Eur. J. Clin. Microbiol. Infect. Dis. 10:240-248. [DOI] [PubMed] [Google Scholar]
- 7.Lewin, C., and J. Smith. 1988. Bactericidal mechanisms of ofloxacin. J. Antimicrob. Chemother. 22(Suppl. C):1-8. [DOI] [PubMed] [Google Scholar]
- 8.Lu, T., and K. Drlica. 2003. In vitro activity of C-8-methoxy fluoroquinolones against mycobacteria when combined with anti-tuberculosis agents. J. Antimicrob. Chemother. 52:1025-1028. [DOI] [PubMed] [Google Scholar]
- 9.Malik, M., T. Lu, X. Zhao, A. Singh, C. Hattan, J. Domagala, R. Kerns, and K. Drlica. 2005. Quinolone lethality in the presence and absence of chloramphenicol: studies with Mycobacterium smegmatis. Antimicrob. Agents Chemother. 49:2008-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyazaki, E., M. Miyazaki, J. Chen, R. Chaisson, and W. Bishai. 1999. Moxifloxacin (Bay12-8039), a new 9-methoxyquinolone, is active in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 43:85-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mogues, B. T., M. Goodrich, L. Ryan, R. LaCourse, and R. North. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez, H., M. Ruiz, M. Lopez, and G. Royo. 2002. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin, and linezolid against Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 20:464-467. [DOI] [PubMed] [Google Scholar]
- 13.Wayne, L. G. 1994. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur. J. Clin. Microbiol. Infect. Dis. 13:908-914. [DOI] [PubMed] [Google Scholar]
- 14.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfson, J., and D. Hooper. 1989. Bacterial resistance to quinolones: mechanisms and clinical importance. Rev. Infect. Dis. 11(Suppl. 5):S960-S968. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimatsu, T., E. Nuermberger, S. Tyagi, R. Chaisson, W. Bishai, and J. Grosset. 2002. Bactericidal activity of increasing daily and weekly doses of moxifloxacin in murine tuberculosis. Antimicrob. Agents Chemother. 46:1875-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao, X., J.-Y. Wang, C. Xu, Y. Dong, J. Zhou, J. Domagala, and K. Drlica. 1998. Killing of Staphylococcus aureus by C-8-methoxy fluoroquinolones. Antimicrob. Agents Chemother. 42:956-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao, X., C. Xu, J. Domagala, and K. Drlica. 1997. DNA topoisomerase targets of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc. Natl. Acad. Sci. USA 94:13991-13996. [DOI] [PMC free article] [PubMed] [Google Scholar]