Abstract
Immunization with peptide P10, derived from gp43, and chemotherapy were used together in an attempt to improve treatment of paracoccidioidomycosis and prevent relapses. The combined treatment showed an additive protective effect when administered at 48 h or 30 days after intratracheal challenge. Its use is recommended to improve regular chemotherapy and reduce the duration of treatment.
Extended periods of chemotherapy are often necessary to treat paracoccidioidomycosis, with a significant frequency of relapsing disease. In the present work we investigated the combination of a vaccine of peptide P10 (10), derived from the major diagnostic antigen gp43, and chemotherapy in mice infected with a highly virulent human isolate of Paracoccidioides brasiliensis, Pb18 (3). Antifungal drugs were tested in vitro to determine the MIC by a modification of the method of Shadomy et al. (9). MICs were in the range of values previously reported for these fungi (4, 5, 7, 8).
To explore the effects of P10 immunization combined with antifungal drug treatment, we used two different protocols and 14 groups (n = 10 each) of BALB/c mice (male, 6 to 8 weeks old) in each protocol. Controls included a group infected with Pb18 only, another group infected and immunized with P10, and a group infected and treated with Freund's adjuvant. Five groups of mice were infected and treated with a single antifungal drug: fluconazole (Pfizer), ketoconazole (Janssen-Cilag), itraconazole (Janssen-Cilag), amphotericin B (Fungizone; Bristol Mayers Squibb), sulfamethoxazole (Sigma, St. Louis, MO), or trimethoprim-sulfamethoxazole (Bac-sulfitrin; Ducto). An additional five groups were treated with antifungal drugs combined with P10 immunization.
Drug treatment in the first and second protocols was started after 48 h and 30 days, respectively, after intratracheal infection with 3 × 105 yeast cells (10). The treatment was continued for 30 days, during which groups of mice received intraperitoneal doses of itraconazole (10 mg/kg), fluconazole (10 mg/kg), ketoconazole (8 mg/kg), sulfamethoxazole (15 mg/kg), or trimethoprim-sulfamethoxazole (3 and 15 mg/kg, respectively) every 24 h. Amphotericin B (1.5 mg/kg) was administered every 48 h. Immunization was carried out weekly for 4 weeks with 20 μg of P10 in complete Freund's adjuvant (CFA) once and in incomplete Freund's adjuvant (IFA) three times.
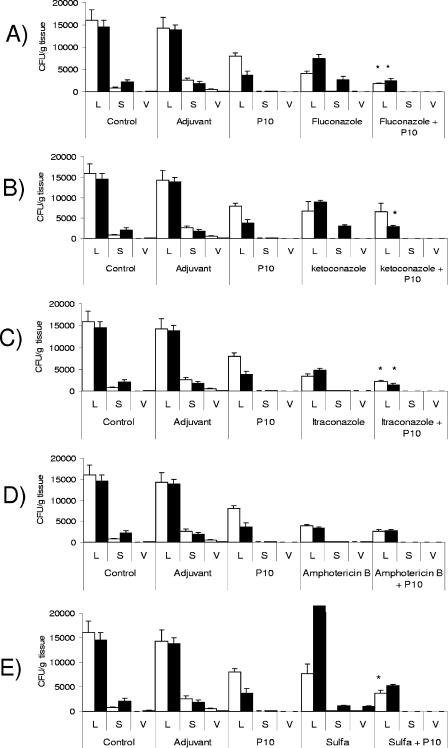
Analysis of CFU in organs was carried out after 30 and 90 days of infection under protocol 1. The pattern of CFU in organs showed a significant reduction of fungi recovered from animals either immunized only or treated with antifungal drugs. An additive protective effect of P10 and antifungal drugs was observed. In the group of mice treated with sulfamethoxazole, an early protection was followed by relapse, although the group with associated sulfamethoxazole and P10 vaccination succeeded in controlling the disease (Fig. 1). The combination of drugs and P10 led to increased protection as reflected in organ histological areas with very few or undetectable yeast cells.
FIG. 1.
Protocol 1 (treatment started 48 h after infection). CFU in organs from mice infected intratracheally with 3 × 105 yeast cells and subjected to antifungal treatment combined or not with P10 immunization were determined. Mice were sacrificed after 30 (□) and 90 (▪) days of infection. Control mice were inoculated with phosphate-buffered saline, adjuvant-treated control mice were inoculated with CFA or IFA, and P10-treated mice were immunized with the peptide. All groups of mice were infected with the same number of yeast cells. Experiments were carried out in triplicate. Each bar represents the average counts and standard deviations in organs from 5 to 10 animals in each group. L, lung; S, spleen; V, liver. The whole experiment was repeated twice with similar results. *, significant difference between the combined treatment and both P10 vaccine alone and drug treatment alone (P < 0.05).
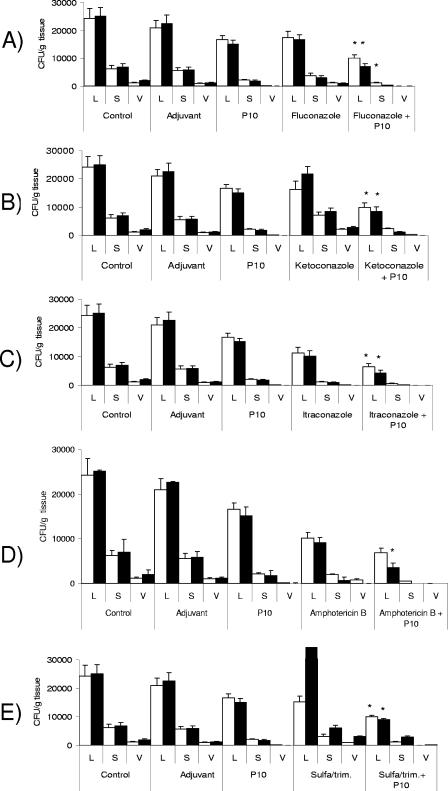
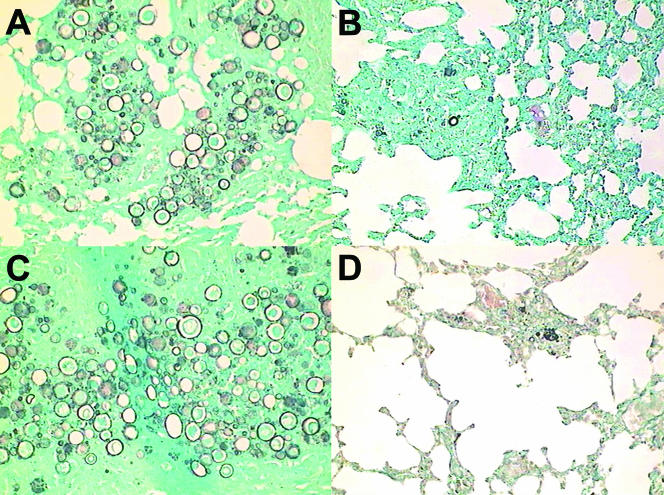
With protocol 2, we aimed at reproducing the situation of established infection. The fungal burden was examined after 60 and 120 days of infection. The pattern of CFU in organs showed an additive effect of P10 immunization and drug treatment at 60 and 120 days after infection, with 60 to 80% reduction of lung CFU compared with controls (untreated infected animals or those that received only CFA). P10 immunization or chemotherapy separately achieved 40 to 60% reduction of CFU; administration of P10 together with the drug fully controlled the “relapsing” effect (Fig. 2). Histopathology showed large preserved areas in the lung and very few yeast cells. In Fig. 3 (protocol 2), we show the lung histopathology of a mouse treated with sulfa-derived drugs alone and combined with P10 vaccination. Clearly, P10 vaccination was the essential adjuvant therapy to contain the infection.
FIG. 2.
Protocol 2 (treatment started 30 days after infection). CFU in organs from mice infected intratracheally with 3 × 105 yeast cells and subjected to antifungal treatment combined or not with P10 immunization were determined. Mice were sacrificed after 60 (□) and 120 (▪) days of infection. Control mice were inoculated with phosphate-buffered saline, adjuvant-treated control mice were inoculated with CFA or IFA, and P10-treated mice were immunized with peptide. All groups of mice were infected with the same number of yeast cells. Experiments were carried out in triplicate. Each bar represents the average counts and standard deviations in organs from 5 to 10 animals in each group. L, lung; S, spleen; V, liver. *, significant differences between the combined treatment and both P10 vaccine alone and drug treatment alone (P < 0.05).
FIG. 3.
Additive effect of P10 immunization and trimethoprim-sulfamethoxazole treatment, after 120 days, in mice intratracheally infected under protocol 2 (treatment after 30 days of infection). (A) Lung section from untreated mouse; (B) lung section from mouse immunized with P10; (C) lung section from mouse treated with trimethoprim-sulfamethoxazole; (D) apparently resolved granulomas, with very few fungal cells, in lung section of mouse subjected to P10 immunization and drug treatment. Three hundred microscopic fields for all four groups were analyzed, with the following results: group A, granulomas with yeasts, 24%, and noninflamed areas, 27%; group B, granulomas with yeasts, 15%, and noninflamed areas, 46%; group C, granulomas with yeasts, 32%, and noninflamed areas, 26%; group D, granulomas with yeasts, 12%, and noninflamed areas, 55%. Statistically significant differences (P < 0.05) were observed for P10-treated groups. Gomori silver staining was used; magnification, ×25.
P10 activates a Th-1 immune response with a known pattern of cytokine production in vitro and in vivo (10). First, we studied the cytokine levels in the lungs of mice after 48 h of infection. Even though a few results were not statistically significant, a pattern was clearly defined. At 30 days no changes in cytokine levels were observed in drug-treated groups of mice in combination or not with P10. After 90 days, however, there was a consistent decrease of interleukin-4 (IL-4) and IL-10 and an increase of gamma interferon (IFN-γ) in drug-treated animals also immunized with P10 in relation to mice treated with drugs only (Table 1). In relation to controls, no differences were seen between sham infection and the group that received only adjuvant. Animals that were only infected or only immunized with P10 showed increased levels of both type 1 and type 2 cytokines (Table 1).
TABLE 1.
Cytokine levels in lungs of mice infected and subjected to chemotherapy and/or P10 vaccination after 48 h of infection
| Treatment group | Cytokine level (pg/ml) at the indicated time (days) of infectiona
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IL-4
|
IL-10
|
IL-12
|
IFN-γ
|
|||||
| 30 | 90 | 30 | 90 | 30 | 90 | 30 | 90 | |
| Sham | 952 ± 62 | 962 ± 62.4 | 5,680 ± 461 | 5,680 ± 461 | 16,950 ± 1,744 | 18,950 ± 2,744 | 826 ± 171 | 836 ± 71 |
| Adjuvant | 988 ± 111 | 854 ± 70.7 | 4,997 ± 488 | 4,221 ± 726 | 15,021 ± 1,012 | 10,121 ± 1,014 | 911 ± 111 | 914 ± 102 |
| Untreated | 2,616 ± 271 | 1,771 ± 170.7 | 15,652 ± 2,416 | 12,850 ± 2,853 | 36,033 ± 2,251 | 35,800 ± 2,848 | 2,906 ± 327 | 3,170 ± 14 |
| P10 | 1,640 ± 250 | 1,065 ± 163.3 | 12,525 ± 1,611 | 8,500 ± 628.3 | 46,233 ± 3,767 | 31,600 ± 2,707 | 4,396 ± 352 | 4,515 ± 77 |
| Fluconazole | 2,900 ± 903 | 4,480 ± 728# | 23,600 ± 2,608# | 22,650 ± 2,089# | 37,566 ± 4,564 | 95,100 ± 5,433 | 5,446 ± 333 | 4,400 ± 669 |
| Fluconazole + P10 | 2,850 ± 534 | 2,530 ± 314 | 16,633 ± 2,609 | 14,400 ± 2,565 | 56,600 ± 3,786* | 94,500 ± 4,534 | 6,970 ± 405* | 6,280 ± 854* |
| Ketoconazole | 5,293 ± 913# | 2,055 ± 249 | 25,500 ± 2,500# | 10,035 ± 1,987# | 47,566 ± 4,429 | 43,950 ± 2,767 | 5,610 ± 355 | 2,885 ± 390 |
| Ketoconazole + P10 | 2,936 ± 258 | 1,845 ± 237 | 21,666 ± 2,159 | 7,750 ± 353 | 64,200 ± 4,333* | 52,100 ± 1,989* | 6,990 ± 493* | 3,955 ± 577* |
| Itraconazole | 2,893 ± 273 | 2,170 ± 328# | 20,700 ± 2,577# | 11,950 ± 2,433 | 58,000 ± 2,873 | 48,765 ± 2,888 | 2,643 ± 399 | 1,750 ± 295 |
| Itraconazole + P10 | 3,486 ± 705 | 1,130 ± 284 | 15,613 ± 608 | 13,050 ± 2,453 | 49,500 ± 3,786 | 48,600 ± 4,121 | 4,313 ± 256* | 2,790 ± 454* |
| Amphotericin B | 1,546 ± 274 | 1,345 ± 106 | 16,100 ± 2,458# | 13,500 ± 707 | 35,533 ± 3,132 | 42,900 ± 2,565 | 4,240 ± 117 | 3,490 ± 697 |
| Amphotericin B + P10 | 2,486 ± 405 | 2,295 ± 389# | 13,500 ± 1,707 | 12,700 ± 424 | 61,266 ± 4,034* | 46,300 ± 2,765* | 3,390 ± 140 | 4,630 ± 899* |
| Sulfamethoxazole | 4,597 ± 697# | 3,145 ± 863# | 21,366 ± 3,450 | 22,450 ± 2,353# | 52,200 ± 2,878 | 33,700 ± 2,555 | 3,603 ± 361 | 2,505 ± 548 |
| Sulfamethoxazole + P10 | 3,026 ± 587 | 1,265 ± 345 | 19,333 ± 2,577 | 13,000 ± 1,221 | 51,427 ± 3,777 | 61,266 ± 4,324 | 4,560 ± 311* | 8,520 ± 513 |
Boldface indicates a predominat Th-1 or Th-2-like response according to the cytokines produced (IL-12/IFN-γ or IL-4/IL-10, respectively). *, statistically significant difference (P < 0.05) relative to mice treated only with the drug; #, statistically significant difference (P < 0.05) relative to mice immunized with P10 and treated with the drug. Values are means ± standard deviations of measurements from 5 to 10 animals in each group. The whole experiment was repeated twice with reproducible results.
In animals with established infection, sham- and adjuvant-treated groups of mice did not show differences, whereas the group infected with P. brasiliensis but untreated showed increased production of IL-4, IL-10, IL-12, and IFN-γ. P10 immunization promoted even higher levels of IL-12 and IFN-γ but decreased concentrations of IL-4 and IL-10. Drug treatment generally stimulated IL-4 and IL-10 cytokines compared to the case for the untreated infected group. The combination with P10 immunization showed, in general, increased production of IL-12 and IFN-γ and reduction of IL-4 and IL-10, with little exception (Table 2). Thus, although both Th-1 and Th-2 cellular immune responses were present in all cases, drug treatment either had little influence or caused an increase in the Th-2 response, possibly correlated with increased destruction of fungal cells. The least effective chemotherapy, with trimethoprim-sulfamethoxazole, which allowed a late relapsing infection, doubled the production of IL-4 and IL-10 and had little effect on IL-12 and IFN-γ. Association with P10 greatly stimulated a Th-1 response with increased production of IL-12 and IFN-γ, clearly leading to protection.
TABLE 2.
Cytokine levels in lungs of mice infected and subjected to chemotherapy and/or P10 vaccination after 30 days of infection
| Treatment group | Cytokine level (pg/ml) at the indicated time (days) of infectiona
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IL-4
|
IL-10
|
IL-12
|
IFN-γ
|
|||||
| 60 | 120 | 60 | 120 | 60 | 120 | 60 | 120 | |
| Sham | 752 ± 78 | 845 ± 85 | 4,680 ± 511 | 3,852 ± 665 | 13,211 ± 958 | 11,789 ± 1,214 | 712 ± 222 | 648 ± 65 |
| Adjuvant | 988 ± 111 | 854 ± 70 | 4,997 ± 488 | 4,221 ± 326 | 15,021 ± 1,012 | 10,121 ± 1,014 | 911 ± 111 | 914 ± 102 |
| Untreated | 2,654 ± 112 | 2,312 ± 522 | 16,021 ± 612 | 10,520 ± 1,584 | 38,021 ± 1,562 | 29,522 ± 2,011 | 2,615 ± 214 | 3,852 ± 545 |
| P10 | 1,510 ± 88 | 1,121 ± 232 | 11,115 ± 522 | 6,250 ± 1,128 | 78,521 ± 2,123 | 63,160 ± 3,602 | 5,741 ± 612 | 6,412 ± 1,369 |
| Fluconazole | 3,120 ± 166# | 3,321 ± 665# | 24,021 ± 1,021# | 27,321 ± 2,358# | 59,564 ± 1,855 | 63,555 ± 4,312 | 2,985 ± 251 | 3,621 ± 511 |
| Fluconazole + P10 | 1,712 ± 98 | 2,011 ± 501 | 14,333 ± 633 | 22,619 ± 1,545 | 77,512 ± 2,213* | 88,888 ± 3,101* | 6,021 ± 452* | 9,021 ± 741* |
| Ketoconazole | 5,312 ± 615# | 3,842 ± 852# | 26,313 ± 1,245# | 14,222 ± 2,354# | 45,326 ± 2,651 | 52,321 ± 2,988 | 3,011 ± 365 | 2,014 ± 636 |
| Ketoconazole + P10 | 3,021 ± 188 | 2,011 ± 654 | 20,231 ± 999 | 11,050 ± 1,655 | 76,582 ± 2,365* | 56,500 ± 4,021* | 7,112 ± 522* | 8,844 ± 766* |
| Itraconazole | 3,011 ± 318 | 3,015 ± 925# | 19,744 ± 823# | 17,455 ± 2,011# | 59,621 ± 1,025 | 54,114 ± 3,022 | 2,744 ± 329 | 2,113 ± 527 |
| Itraconazole + P10 | 2,633 ± 215 | 1,855 ± 357 | 13,212 ± 655 | 11,456 ± 1,045 | 79,625 ± 1,653* | 44,112 ± 2,958 | 6,623 ± 444* | 9,571 ± 1,712* |
| Amphotericin B | 1,546 ± 274 | 1,879 ± 603 | 17,100 ± 555# | 14,101 ± 1,789 | 33,265 ± 2,012 | 44,101 ± 3,012 | 3,111 ± 363 | 4,511 ± 951 |
| Amphotericin B + P10 | 1,115 ± 63 | 2,011 ± 518 | 11,011 ± 456 | 13,011 ± 1,412 | 68,222 ± 2,322* | 64,111 ± 5,114* | 7,751 ± 454* | 6,255 ± 1,874* |
| Trimethoprim-sulfamethoxazole | 5,311 ± 512# | 5,845 ± 1,232# | 23,121 ± 1,066# | 21,456 ± 1,654# | 33,665 ± 1,988 | 38,258 ± 2,145 | 2,655 ± 388 | 2,487 ± 358 |
| Trimethoprim-sulfamethoxazole + P10 | 2,955 ± 311 | 3,695 ± 989 | 15,321 ± 633 | 14,258 ± 1,658 | 69,852 ± 2,011* | 60,115 ± 2,532* | 5,111 ± 522* | 10,421 ± 654* |
Boldface indicates a predominat Th-1 or Th-2-like response according to the cytokines produced (IL-12/IFN-γ or IL-4/IL-10, respectively). *, statistically significant difference (P < 0.05) relative to mice treated only with the drug; #, statistically significant difference (P < 0.05) relative to mice immunized with P10 and treated with the drug. Values are means ± standard deviations of measurements from 5 to 10 animals in each group. The whole experiment was repeated twice with reproducible results.
Several features have been examined for validation of P10 as a candidate vaccine, including (i) the presentation of P10 by different mouse haplotypes (10); (ii) conservation in nature, determined by examining gp43 molecules from different isolates (1); (iii) immunogenicity in constructions that do not require complete Freund's adjuvant (6, 11); (iv) presentation by a large number of human HLA-DR molecules, more than that for other promiscuous peptides derived from gp43 (2); and (v) its role in the immunoprotection of mice infected with P. brasiliensis and subjected to chemotherapy, which was the focus of the present work. The data obtained strongly suggest that a P10-based vaccine should be used to improve chemotherapy, reduce treatment time, and prevent relapsing disease.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (Fapesp) grant 02/09192-5. C.P.T. and L.R.T. are research fellows of the CNPq.
REFERENCES
- 1.Carvalho, K. C., L. Ganiko, W. L. Batista, F. V. Morais, E. R. Marques, G. H. Goldman, M. F. Franco, and R. Puccia. 2005. Virulence of Paracoccidioides brasiliensis and gp43 expression in isolates bearing known PbGP43 genotype. Microbes Infect. 7:55-65. [DOI] [PubMed] [Google Scholar]
- 2.Iwai, L. K., M. Yoshida, J. Sidney, M. A. Shikanai-Yasuda, A. C. Goldberg, M. A. Juliano, J. Hammer, L. Juliano, A. Sette, J. Kalil, L. R. Travassos, and E. Cunha-Neto. 2003. In silico prediction of peptides binding to multiple HLA-DR molecules accurately identifies immunodominant epitopes from gp43 of Paracoccidioides brasiliensis frequently recognized in primary peripheral blood mononuclear cell responses from sensitized individuals. Mol. Med. 9:209-219. [PMC free article] [PubMed] [Google Scholar]
- 3.Kashino, S. S., V. L. Calich, E. Burger, and L. M. Singer-Vermes. 1985. In vivo and in vitro characteristics of six Paracoccidioides brasiliensis strains. Mycopathologia 92:173-178. [DOI] [PubMed] [Google Scholar]
- 4.Lacaz, C. S., C. M. Ulson, and S. A. De Prado Sampaio. 1959. In vitro action of amphotericin B on Paracoccidioides brasiliensis. Rev. Paul. Med. 54:357-360. [PubMed] [Google Scholar]
- 5.McGinnis, M. R., L. Pasarell, D. A. Sutton, A. W. Fothergill, C. R. Cooper, Jr., and M. G. Rinaldi. 1997. In vitro evaluation of voriconazole against some clinically important fungi. Antimicrob. Agents Chemother. 41:1832-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinto, A. R., R. Puccia, S. N. Diniz, M. F. Franco, and L. R. Travassos. 2000. DNA-based vaccination against murine paracoccidioidomycosis using the gp43 gene from Paracoccidioides brasiliensis. Vaccine 18:3050-3058. [DOI] [PubMed] [Google Scholar]
- 7.Restrepo, A., and B. Tabares Cde. 1984. In vitro susceptibility of Paracoccidioides brasiliensis yeast form to antifungal agents. Rev. Inst. Med. Trop. Sao Paulo 26:322-328. [DOI] [PubMed] [Google Scholar]
- 8.San-Blas, G., A. M. Calcagno, and F. San-Blas. 1993. A preliminary study of in vitro antibiotic activity of saperconazole and other azoles on Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 31:169-174. [PubMed] [Google Scholar]
- 9.Shadomy, H. J., S. Wood-Helie, S. Shadomy, W. E. Dismukes, and R. Y. Chau. 1987. Biochemical serogrouping of clinical isolates of Cryptococcus neoformans. Diagn. Microbiol. Infect. Dis. 6:131-138. [DOI] [PubMed] [Google Scholar]
- 10.Taborda, C. P., M. A. Juliano, R. Puccia, M. Franco, and L. R. Travassos. 1998. Mapping of the T-cell epitope in the major 43-kilodalton glycoprotein of Paracoccidioides brasiliensis which induces a Th-1 response protective against fungal infection in BALB/c mice. Infect. Immun. 66:786-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taborda, C. P., C. R. Nakaie, E. M. Cilli, E. G. Rodrigues, L. S. Silva, M. F. Franco, and L. R. Travassos. 2004. Synthesis and immunological activity of a branched peptide carrying the T-cell epitope of gp43, the major exocellular antigen of Paracoccidioides brasiliensis. Scand. J. Immunol. 59:58-65. [DOI] [PubMed] [Google Scholar]