Abstract
(S)-9-[3-Hydroxy-2-(phosphonomethoxy)propyl]adenine [(S)-HPMPA], is an effective broad-spectrum antiviral against many DNA viruses but has been reported to be inactive against human immunodeficiency virus (HIV). We synthesized several alkoxyalkyl esters of (S)-HPMPA and now report that hexadecyloxypropyl-(S)-HPMPA [HDP-(S)-HPMPA] and octadecyloxyethyl-(S)-HPMPA [ODE-(S)-HPMPA]had 50% effective concentrations of 0.4 to 7.0 nanomolar and were nearly fully active against HIV variants having reverse transcriptase mutations M184V and K103N and against a zidovudine-resistant variant with mutations D67N, K70R, T215Y, and K219Q. Resistance to HDP-(S)-HPMPA and ODE-(S)-HPMPA was noted for a mutant with mutation K65R. HDP-(S)-HPMPA is also active against herpes simplex virus type 1, human cytomegalovirus, hepatitis B virus, adenoviruses, and orthopoxviruses and is worthy of further evaluation as a possibly therapy for HIV infection.
9-(S)-[3-Hydroxy-2-(phosphonomethoxy)propyl]adenine [(S)-HPMPA] was first synthesized and evaluated as an antiviral by De Clercq et al. in 1986 (9, 13) and was shown to inhibit replication of many double-stranded DNA viruses, including herpes simplex virus, human cytomegalovirus, varicella-zoster virus, Epstein-Barr virus, and adenoviruses (10) and various orthopoxviruses, including vaccinia, cowpox, monkeypox, and variola viruses (2, 14, 15). However, numerous studies indicate that (S)-HPMPA lacks antiviral activity against RNA viruses and retroviruses, including human immunodeficiency virus type 1 (HIV-1) (3, 8, 13, 17).
Our group reported previously that conversion of acyclic nucleoside phosphonates to alkoxyalkyl or alkyl esters greatly increases their antiviral activity (4, 16), and structure-activity studies have established that the optimal length of the alkoxyalkyl ester is about 20 atoms (20). Alkoxyalkyl esters of cidofovir (CDV) are orally active in lethal infection models with ectromelia (6) and cowpox and vaccinia (18). To evaluate this approach with (S)-HPMPA, we synthesized a series of alkoxyalkyl esters of (S)-HPMPA and tested them against HIV-1 infection in MT-2 cells. We now report that alkoxyalkyl esters of (S)-HPMPA are active against HIV-1 in the low-nanomolar range, while (S)-HPMPA itself has essentially no activity, as reported previously.
(Portions of this paper were presented in abstract form at the International Conference on Antiviral Research, 11 to 14 April 2005, Barcelona, Spain.)
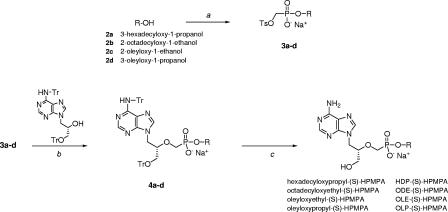
The route of synthesis of the (S)-HPMPA alkoxyalkyl esters is shown in Fig. 1. Details of the synthesis, purification, and purity (>98%) have been reported elsewhere (5). MT-2 cells (AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health) were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (JRH Biosciences, Lenexa, Kans.), 10 mM HEPES buffer, 50 IU of penicillin/ml, and 50 μg of streptomycin/ml. HIV-1LAI and HIV variants M184V, K65R, and K103N were obtained from the AIDS Research and Reference Reagent Program. Zidovudine (AZT)-resistant HIV-1 with four mutations (D67N, K70R, T215Y, and K219Q) in the reverse transcriptase (4×AZT) was kindly provided by Douglas D. Richman of the University of California, San Diego.
FIG. 1.
Synthesis of alkoxyalkyl esters of (S)-HPMPA. Reagents and conditions: step a, diethyl p-toluenesulfonyloxymethylphosphonate and trimethylsilyl bromide followed by oxalyl chloride; step b, NaH and triethylamine, 60°C; step c, 80% aqueous acetic acid.
The antiviral activity of each compound was determined by inoculating MT-2 cells with HIV-1LAI at a multiplicity of infection of 0.001 50% tissue culture infective dose/cell, followed by incubation in the presence of threefold serial drug dilutions (three wells per dilution) as previously described (11). Four days after infection, culture supernatants were harvested, lysed with 0.5% Triton X-100, and assayed for p24 antigen concentration by using a commercial enzyme-linked immunosorbent assay (Perkin-Elmer Life Sciences, Boston, MA). The antiviral activity of each compound is expressed as the 50% effective concentration (EC50), which is the concentration required to inhibit p24 antigen production by 50%. To assess cytotoxicity, MT-2 cells were incubated with the drug for 72 h and harvested. Flow count beads (Beckman Coulter, Miami, FL) were added to the cell suspension, followed by propidium iodide staining and analysis using an Epics Elite flow cytometer (Beckman Coulter). The 50% cytotoxic concentration (CC50) was calculated from the cell counts and viability.
The effects of hexadecyloxypropyl-HPMPA (HDP-HPMPA), octadecyloxyethyl-HPMPA (ODE-HPMPA), oleyloxyethyl-HPMPA (OLE-HPMPA), and oleyloxypropyl-HPMPA (OLP-HPMPA) on HIV replication in infected MT-2 cells were assessed by p24 reduction (Table 1). Unmodified (S)-HPMPA was essentially inactive against HIV-1, as reported previously (3, 8, 13, 17), with an EC50 of 77 μM. Contrary to expectation, however, the alkoxyalkyl esters of (S)-HPMPA were active against HIV-1, and all exhibited EC50s in the low-nanomolar range. The most active compound was ODE-(S)-HPMPA, with an EC50 of 0.4 nM and a selectivity index of 75. HDP-(S)-HPMPA was less active, with an EC50 of 7 nM, but more selective, with a selectivity index of 143. Alkoxyalkyl esters of (S)-HPMPA were more cytotoxic than unmodified (S)-HPMPA. This is consistent with the results obtained previously with alkoxyalkyl esters of CDV, which also exhibited greater cytotoxicity and greater selectivity than CDV (4, 16, 20).
TABLE 1.
Antiviral activities and selectivities of alkoxyalkyl esters of (S)-HPMPA against HIVLAI
| Compound | Efficacy (EC50, μM)a | Cytotoxicity (CC50, μM)a | Selectivity indexb |
|---|---|---|---|
| (S)-HPMPA | 77.5 ± 17.1 (4) | >100 (3) | >1.29 |
| HDP-(S)-HPMPA | 0.007 ± 0.007 (4) | 1.0 ± 0.91 (3) | 143 |
| ODE-(S)-HPMPA | 0.0004 ± 0.0004 (4) | 0.03 ± 0.02 (3) | 75 |
| OLE-(S)-HPMPA | 0.008 ± 0.01 (3) | 0.20 ± 0.07 (3) | 25 |
| OLP-(S)-HPMPA | 0.107 ± 0.1 (3) | 7.0 ± 5.3 (3) | 65 |
Data are expressed as means ± standard deviations. The numbers in parentheses represent the number of independent determinations.
Selectivity index, CC50/EC50.
We also evaluated the activities of HDP-(S)-HPMPA and ODE-(S)-HPMPA against a panel of four drug-resistant HIV variants, including viruses resistant to AZT (4×AZT), lamivudine (M184V), tenofovir (K65R), and nevirapine (K103N). HDP-(S)-HPMPA and ODE-(S)-HPMPA retained nearly full antiviral activity against 4×AZT, M184V, and K103N. Significant resistance was noted with K65R, but the EC50s for HDP- and ODE-(S)-HPMPA were 0.16 and 0.003 μM, respectively, suggesting that the compounds could be clinically useful, since plasma levels of 1 to 2 μM are attainable in animal studies (J. Trahan and K. Y. Hostetler, unpublished data) (Table 2).
TABLE 2.
Antiviral activities of HDP-(S)-HPMPA and ODE-(S)-HPMPA against HIVLAI and drug-resistant HIV variants
| Compound | EC50 (nM) againsta:
|
||||
|---|---|---|---|---|---|
| HIV-1LAI | 4×AZT | M184V | K65R | K103N | |
| HDP-(S)-HPMPA | 7.0 | 5.0 | 30 | 160 | 20 |
| ODE-(S)-HPMPA | 0.40 | 0.01 | 0.037 | 3.0 | 0.35 |
4×AZT is an AZT-resistant variant having HIV reverse transcriptase mutations D67N, K70R, T215Y, and K219Q. Data are the averages of two to four determinations.
We reported previously that the CDV analog hexadecyloxypropyl-CDV (HDP-CDV) showed >100-fold increases in antiviral activity versus the unmodified CDV against cells infected with orthopoxviruses, cowpox virus, and vaccinia virus (4, 16, 19). The cellular uptake and anabolic metabolism of 14C-labeled CDV and HDP-CDV was evaluated in fibroblasts. HDP-CDV was taken up rapidly by MRC-5 human lung fibroblasts in vitro, but CDV uptake was much slower. Analysis of cellular metabolites showed that levels of CDV diphosphate, the active antiviral compound, were >100 times greater with HDP-CDV than with CDV (1). However, the degree of enhancement of antiviral activity does not always correlate with the increase in cellular uptake and conversion of HDP-CDV to CDV diphosphate. In various viruses of the herpesvirus group, increases in antiviral activity with HDP-CDV varied from 40- to >52,000-fold (21).
HDP-CDV seems to circumvent poor cellular uptake by rapid association with cellular membrane phospholipids, whereas CDV uptake proceeds via the slow process of fluid phase endocytosis (7). This may be, at least in part, the explanation for the enhanced activity of HDP-(S)-HPMPA in HIV-infected MT-2 cells While tenofovir and adefovir are chain terminators, the mechanism of action of HPMPA diphosphate against HIV reverse transcriptase or any other viral polymerase is not presently known.
In summary, esterification of (S)-HPMPA with long-chain alkoxyalkanols resulted in a 3- to 5-log increase in antiviral activity against HIV-1 infection in MT-2 cells versus unmodified HPMPA. HDP-(S)-HPMPA and ODE-(S)-HPMPA are also active against HIV variants which are resistant to AZT, lamivudine, nonnucleoside reverse transcriptase inhibitors, and tenofovir. Enhanced antiviral activity of HDP-(S)-HPMPA and ODE-(S)-HPMPA against human cytomegalovirus, murine cytomegalovirus, vaccinia virus, cowpox virus, and adenovirus (5, 12) and hepatitis B virus (B. Korba and K. Y. Hostetler, unpublished data) was also shown previously. Preliminary studies indicate that HDP-(S)-HPMPA and ODE-(S)-HPMPA are orally active against lethal vaccinia and cowpox challenge in mice (19) and are worthy of further evaluation as a possible broad-spectrum antiviral therapy for HIV-1 infection in humans.
Acknowledgments
This work was funded in part by grants from the National Institute of Allergy and Infectious Diseases (AI-29164 and AI-66499) and the U.S. Army, DAMD (17-01-2-0071).
K. Y. Hostetler has an equity interest in and serves as a consultant to Chimerix Inc. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict-of-interest policies.
REFERENCES
- 1.Aldern, K. A., S. L. Ciesla, K. L. Winegarden, and K. Y. Hostetler. 2003. The increased antiviral activity of 1-O-hexadecyloxypropyl-cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol. Pharmacol. 63:678-681. [DOI] [PubMed] [Google Scholar]
- 2.Baker, R. O., M. Bray, and J. W. Huggins. 2003. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res. 57:13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balzarini, J., A. Holy, J. Jindrich, L. Naesens, R. Snoeck, D. Schols, and E. De Clercq. 1993. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethyoxypropyl)-2,6-diaminopurine. Antimicrob Agents Chemother. 37:332-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beadle, J. R., C. Hartline, K. A. Aldern, N. Rodriguez, E. Harden, E. R. Kern, and K. Y. Hostetler. 2002. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob. Agents Chemother. 46:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beadle, J. R., W. B. Wan, S. L. Ciesla, K. A. Keith, C. Hartline, E. R. Kern, and K. Y. Hostetler. 2006. Synthesis and antiviral evaluation of alkoxyalkyl derivatives of 9-(S)-(3-hydroxy-2-phosphonomethoxypropyl)adenine (HPMPA) against cytomegalovirus and orthopoxviridiae. J. Med. Chem. 49:2010-2015. [DOI] [PubMed] [Google Scholar]
- 6.Buller, R. M., G. Owens, J. Schriewer, L. Melman, J. R. Beadle, and K. Y. Hostetler. 2004. Efficacy of oral active ether lipid analogs of cidofovir in a lethal mousepox model. Virology 318:474-481. [DOI] [PubMed] [Google Scholar]
- 7.Connelly, M. C., B. L. Robbins, and A. Fridland. 1993. Mechanism of uptake of the phosphonate analog (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) in Vero cells. Biochem. Pharmacol. 46:1053-1057. [DOI] [PubMed] [Google Scholar]
- 8.De Clercq, E. 1991. Chemotherapy of the acquired immune deficiency syndrome (AIDS): acyclic nucleoside phosphonate analogs. Int. J. Immunopharmacol. 13(Suppl. 1):91-98. [DOI] [PubMed] [Google Scholar]
- 9.De Clercq, E., A. Holy, I. Rosenberg, T. Sakuma, J. Balzarini, and P. C. Maudgal. 1986. A novel selective broad-spectrum anti-DNA virus agent. Nature 323:464-467. [DOI] [PubMed] [Google Scholar]
- 10.De Clercq, E., T. Sakuma, M. Baba, R. Pauwels, J. Balzarini, I. Rosenberg, et al. 1987. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res. 8:261-272. [DOI] [PubMed] [Google Scholar]
- 11.Hammond, J. L., D. Koontz, H. Z. Bazmi, J. R. Beadle, S. E. Hostetler, G. D. Kini, K. A. Aldern, D. D. Richman, and K. Y. Hostetler. 2001. Alkylglycerol prodrugs of phosphonoformate are potent in vitro inhibitors of nucleoside resistant human immunodeficiency virus type 1 and select for resistance mutations that reverse zidovudine resistance. Antimicrob. Agents Chemother. 45:1621-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartline, C. B., K. M. Gustin, W. B. Wan, S. L. Ciesla, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2005. Activity of ether lipid ester prodrugs of acyclic nucleoside phosphonates against adenovirus replication in vitro. J. Infect. Dis. 191:396-399. [DOI] [PubMed] [Google Scholar]
- 13.Holy, A. 2003. Phosphonomethoxyalkyl analogs of nucleotides. Curr. Pharmaceut. Design 9:2567-2592. [DOI] [PubMed] [Google Scholar]
- 14.Holy, A., I. Rosenberg, and K. Slama. 1987. Isomeric O-phosphonylmethyl derivatives of enantiomeric and racemic 9-(2,3-dihydroxypropyl)adenine. U.S. patent 4,659,825.
- 15.Keith, K. A., M. J. M. Hitchcock, W. A. Lee, A. Holy, and E. R. Kern. 2003. Evaluation of nucleoside phosphonates and their analogs and prodrugs for inhibition of orthopoxvirus replication. Antimicrob. Agents Chemother. 47:2193-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kern, E. R., C. Hartline, E. Harden, K. Keith, N. Rodriguez, J. R. Beadle, and K. Y. Hostetler. 2002. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 46:991-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naesens, L., J. Balzarini, and E. De Clercq. 1995. The potential of acyclic nucleoside phosphonates as broad-spectrum antiviral agents. Postepy Biochemii 41:347-351. [PubMed] [Google Scholar]
- 18.Quenelle, D. C., D. J. Collins, K. Y. Hostetler, J. R. Beadle, W. B. Wan, and E. R. Kern. 2004. Oral treatment of cowpox and vaccinia infections in mice with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 48:404-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quenelle, D. C., D. J. Collins, K. A. Keith, J. Trahan, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2005. Effect of oral treatment with HDP-(S)-HPMPA or ODE-(S)-HPMPA on cowpox or vaccinia virus infections in mice. Antiviral Res. 65:76, A81. [Google Scholar]
- 20.Wan, W. B., J. R. Beadle, C. Hartline, E. R. Kern, S. L. Ciesla, N. Valiaeva, and K. Y. Hostetler. 2005. Comparison of the antiviral activities of alkoxyalkyl and alkyl esters of cidofovir against human and murine cytomegalovirus replication in vitro. Antimicrob. Agents Chemother. 49:656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams-Aziz, S. L., C. B. Hartline, E. A. Harden, S. M. Daily, M. N. Prichard, N. L. Kushner, J. R. Beadle, W. B. Wan, K. Y. Hostetler, and E. R. Kern. 2005. Comparative activity of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro. Antimicrob. Agents Chemother. 49:3724-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]