Abstract
AG205 was identified from high-throughput screening as a potent inhibitor of FabK, the enoyl-ACP reductase in Streptococcus pneumoniae. Specific inhibition of lipid biosynthesis in a macromolecular biosynthesis assay and identification of an Ala141Ser substitution in FabK from spontaneous AG205-resistant mutants indicated that AG205 exerts antibacterial activity against S. pneumoniae through the specific inhibition of FabK.
The enzymes of bacterial fatty acid synthesis have significant potential for the development of novel antibacterials with selectivity (4). Enoyl-acyl carrier protein (ACP) reductase, which catalyzes the last step in each cycle of fatty acid elongation, is a promising target because it plays a key role in the regulation of the pathway. The essential requirement of the enzyme activity was demonstrated by analyzing a fabI(Ts) mutant in Escherichia coli (2) and also by the use of specific inhibitors, such as triclosan (1, 5, 15) and isoniazid (12). While most bacteria possess the enoyl-ACP reductase FabI, Streptococcus pneumoniae has an alternative enoyl-ACP reductase, FabK, which displays no significant sequence homology to FabI (3). Although novel FabI inhibitors have been reported by several groups (6, 8, 11, 14), there are very few reports of FabK inhibitors except for a small number of compounds with weak inhibitory activity (11, 13). Prior to this study there was no clear evidence that a FabK inhibitor would prevent the growth of S. pneumoniae.
MICs were determined with the broth microdilution method according to an NCCLS standard (10). For S. pneumoniae strains, brain heart infusion (BHI) broth instead of cation-adjusted Mueller-Hinton broth supplemented with lysed horse blood was used. Thirty clinical isolates of S. pneumoniae isolated in Japan between 2002 and 2003 were used.
The fabK gene amplified by PCR using fabK1 (5′-GGAATTCCATATGAAAACGCGTATTACAGAA-3′) and fabK2 (5′-CCGCTCGAGGTCATTTCTTACAACTCCTGT-3′) was digested with NdeI and XhoI, and cloned into pET-21b(+). The resulting plasmid was transformed into E. coli BL21(DE3). Cells were harvested after the induction of gene expression, and a cell extract was prepared by sonication. His-tagged FabK was purified using a Ni-nitrilotriacetic acid agarose column (QIAGEN). The activity of FabK was assayed using crotonoyl coenzyme A as a substrate and by monitoring the decrease in absorbance at 340 nm (9). The reaction mixture consisted of 100 mM 2-(N-morpholino)ethanesulfonic acid (pH 7.0), 100 mM NH4Cl, 0.2 mM crotonoyl coenzyme A, 0.4 mM NADH, and 2 to 20 μg/ml of purified FabK.
In macromolecular biosynthesis assay, exponentially growing S. pneumoniae IP692 in BHI broth was used. AG205 was added to the cell culture before the addition of [2-14C]thymidine, [U-14C]uridine, l-[4,5-3H]leucine, [2-14C]acetic acid, and N-acetyl-d-[1-14C]glucosamine (Amersham Biosciences Corp., Piscataway, NJ). Sixty minutes after the initiation, part of the cell suspension was transferred to 10% trichloroacetic acid to precipitate macromolecules. The radioactivity of the biomass filtered on a GF/C glass filter (Whatman, Clifton, NJ) was measured using a liquid scintillation counter.
The full-length fabK genes were amplified by PCR with fabKup (5′-CGGGATCCAAGACGCATCAGAAGTAACAC-3′) and fabKdown (5′-CGGGATCCAGACAAACCAGCAACCATATC-3′). Nucleotide sequences were determined using an Applied Biosystems 3730 DNA analyzer (Applied Biosystems, Foster City, CA) with the primers fabKup, fabKdown, fabK1, fabK2, and 5′-GGATAATCGTTATTCCTGTTG-3′.
High-throughput screening of our compound library resulted in the identification of two chemically related compounds as inhibitors of S. pneumoniae FabK (Fig. 1). AG205 (50% inhibitory concentration [IC50] = 1.5 μM) showed stronger FabK inhibitory activity than AE848 (IC50 = 5.1 μM). Although no antibacterial activity was observed when MIC testing was performed by the reference broth microdilution method (10), AG205 exhibited antibacterial activity against S. pneumoniae strains when the assay medium was changed to BHI. AG205 was found to readily degrade at the amide group in the presence of blood. No growth inhibition was observed against organisms possessing FabI, such as Staphylococcus aureus, E. coli, and Pseudomonas aeruginosa (MICs of >32 μg/ml). We chose the more potent inhibitor of FabK, AG205, for further investigation.
FIG. 1.
Chemical structures of novel FabK inhibitors AG205 (left) and AE848 (right).
MICs of AG205 in BHI broth and amino acid substitutions in FabK for 30 strains are shown in Table 1. AG205 exhibited antibacterial activity at 1 to 8 μg/ml against most of the isolates tested, although 6 out of 30 strains showed reduced susceptibility (MICs of >16 μg/ml). Amino acid substitutions V161I, E276D, and T318A were found among the strains. To our knowledge, this is the first report describing the amino acid substitutions in FabK proteins from numerous clinical isolates. However, no correlation was observed between alterations in FabK and reduced susceptibility to AG205. Indeed, FabK with the three mutations that originated from S. pneumoniae KU197 was shown to be susceptible to AG205 (IC50 = 2.2 μM), as was that from the R6 strain (IC50 = 1.5 μM). Moreover, the crystal structure of FabK reveals that these three residues are located at the surface of the protein (J. Saito, submitted for publication). From these observations, the mutations found in the clinical isolates are not responsible for the decrease in affinity for AG205. Instead, an alternative mechanism, such as overexpression of FabK and/or efflux pumps, is likely to be involved in the isolates with reduced susceptibility to AG205.
TABLE 1.
Deduced amino acid substitutions in FabK and susceptibility to AG205 for clinical isolates of S. pneumoniae
| No. of strains | Amino acid substitutiona at:
|
No. of isolates showing a MIC (μg/ml)b of:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| V161 | E276 | T318 | 1 | 2 | 4 | 8 | 16 | >16 | |
| 1 | 1 | ||||||||
| 3 | A | 1 | 2 | ||||||
| 3 | D | 2 | 1 | ||||||
| 1 | D | A | 1 | ||||||
| 10 | I | D | 1 | 2 | 6 | 1 | |||
| 12 | I | D | A | 1 | 6 | 2 | 3 | ||
FabK sequences were compared to that of the R6 strain (GenBank accession no. AE008418).
MIC testing was performed with brain heart infusion broth.
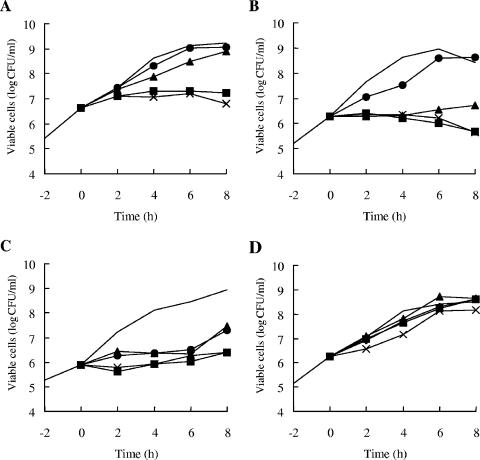
Time-kill studies were performed to confirm the growth-inhibitory effect of AG205 against S. pneumoniae in BHI broth. AG205 at 1 to 4 μg/ml inhibited the growth of S. pneumoniae isolates, including the penicillin-macrolide-resistant strain KU197 (Fig. 2A to C). The growth-inhibitory effect of AG205 against S. pneumoniae was bacteriostatic rather than bactericidal, similar to that of triclosan against E. coli (7).
FIG. 2.
Growth-inhibitory effect of AG205 against S. pneumoniae strains. (A) S. pneumoniae IP692; (B) S. pneumoniae ATCC 49619; (C) S. pneumoniae KU197; (D) S. pneumoniae KU197 mutant with FabK(Ala141Ser). Growth curves for drug-free controls are shown with no symbol. AG205 was used at 1 (•), 2 (▴), 4 (▪), and 8 (×) μg/ml for the clinical isolates (A to C) and at 2 (▴), 4 (▪), 8 (×), and 16 (⧫) μg/ml for the mutant strain (D).
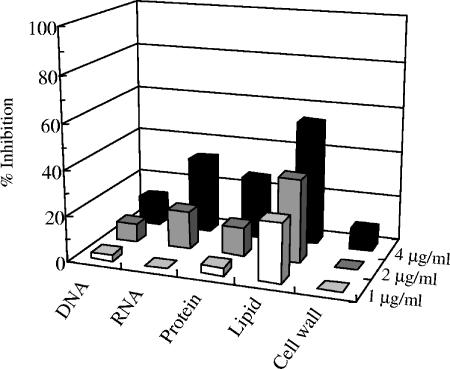
The macromolecular synthesis assay demonstrated that AG205 selectively inhibits the incorporation of acetic acid at 1 μg/ml (Fig. 3), indicating this compound to be a specific inhibitor of lipid biosynthesis. Although AG205 inhibited RNA and protein syntheses at higher doses, the inhibition of lipid synthesis by AG205 was dose dependent. From these results we conclude that the primary target of AG205 is lipid biosynthesis.
FIG. 3.
Effect of AG205 on macromolecular biosynthesis in S. pneumoniae.
To evaluate the target specificity more precisely, we obtained spontaneous AG205-resistant mutants from strain KU197 through three serial passages in BHI broth containing 8 μg/ml of AG205. Six independent mutants (MICs of >32 μg/ml) were selected for sequencing the fabK gene. Five out of six mutants had the same mutation in FabK, in which the alanine residue at position 141 was replaced by serine. This mutant FabK, designated FabK(Ala141Ser), was purified and tested by enzymatic assay. AG205 did not inhibit the enzymatic activity of FabK(Ala141Ser) even at a concentration of 100 μM. This loss of inhibitory activity against FabK(Ala141Ser) is entirely consistent with the observation that AG205 exhibits no growth-inhibitory activity against the mutant possessing FabK(Ala141Ser) even at the highest concentration tested (Fig. 2D). In conclusion, we have identified the first effective FabK-directed antibacterial agent.
Acknowledgments
We thank Hiroshi Nose of Trans Genic Inc. for his encouragement during this study and for providing excellent technical advice.
REFERENCES
- 1.Heath, R. J., J. Li, G. E. Roland, and C. O. Rock. 2000. Inhibition of the Staphylococcus aureus NADPH-dependent enoyl-acyl carrier protein reductase by triclosan and hexachlorophene. J. Biol. Chem. 275:4654-4659. [DOI] [PubMed] [Google Scholar]
- 2.Heath, R. J., and C. O. Rock. 1995. Enoyl-acyl carrier protein reductase (fabI) plays a determinant role in completing cycles of fatty acid elongation in Escherichia coli. J. Biol. Chem. 270:26538-26542. [DOI] [PubMed] [Google Scholar]
- 3.Heath, R. J., and C. O. Rock. 2000. A triclosan-resistant bacterial enzyme. Nature 406:145-146. [DOI] [PubMed] [Google Scholar]
- 4.Heath, R. J., S. W. White, and C. O. Rock. 2001. Lipid biosynthesis as a target for antibacterial agents. Prog. Lipid Res. 40:467-497. [DOI] [PubMed] [Google Scholar]
- 5.Heath, R. J., Y.-T. Yu, M. A. Shapiro, E. Olson, and C. O. Rock. 1998. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J. Biol. Chem. 273:30316-30320. [DOI] [PubMed] [Google Scholar]
- 6.Heerding, D. A., G. Chan, W. E. DeWolf, Jr., A. P. Fosberry, C. A. Janson, D. D. Jaworski, E. McManus, W. H. Miller, T. D. Moore, D. J. Payne, X. Qiu, S. F. Rittenhouse, C. Slater-Radosti, W. Smith, D. T. Takata, K. S. Vaidya, C. C. K. Yuan, and W. F. Huffman. 2001. 1,4-Disubstituted imidazoles are potential antibacterial agents functioning as inhibitors of enoyl acyl carrier protein reductase (FabI). Bioorg. Med. Chem. Lett. 11:2061-2065. [DOI] [PubMed] [Google Scholar]
- 7.Li, Q., J. Y. Lee, R. Castillo, M. S. Hixon, C. Pujol, V. R. Doppalapudi, H. M. Shepard, G. M. Wahl, T. J. Lobl, and M. F. Chan. 2002. NB2001, a novel antibacterial agent with broad-spectrum activity and enhanced potency against β-lactamase-producing strains. Antimicrob. Agents Chemother. 46:1262-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling, L. L., J. Xian, S. Ali, B. Geng, J. Fan, D. M. Mills, A. C. Arvanites, H. Orgueira, M. A. Ashwell, G. Carmel, Y. Xiang, and D. T. Moir. 2004. Identification and characterization of inhibitors of bacterial enoyl-acyl carrier protein reductase. Antimicrob. Agents Chemother. 48:1541-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrakchi, H., W. E. DeWolf, Jr., C. Quinn, J. West, B. J. Polizzi, C. Y. So, D. J. Holmes, S. L. Reed, R. J. Heath, D. J. Payne, C. O. Rock, and N. G. Wallis. 2003. Characterization of Streptococcus pneumoniae enoyl-(acyl-carrier protein) reductase (FabK). Biochem. J. 370:1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.Payne, D. J., W. H. Miller, V. Berry, J. Brosky, W. J. Burgess, E. Chen, W. E. DeWolf, Jr., A. P. Fosberry, R. Greenwood, M. S. Head, D. A. Heerding, C. A. Janson, D. D. Jaworski, P. M. Keller, P. J. Manley, T. D. Moore, K. A. Newlander, S. Pearson, B. J. Polizzi, X. Qiu, S. F. Rittenhouse, C. Slater-Radosti, K. L. Salyers, M. A. Seefeld, M. G. Smyth, D. T. Takata, I. N. Uzinskas, K. Vaidya, N. G. Wallis, S. B. Winram, C. C. K. Yuan, and W. F. Huffman. 2002. Discovery of a novel and potent class of FabI-directed antibacterial agents. Antimicrob. Agents Chemother. 46:3118-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quemard, A., J. C. Sacchettini, A. Dessen, C. Vilcheze, R. Bittman, W. R. Jacobs, Jr., and J. S. Blanchard. 1995. Enzymatic characterization of the target for isoniazid in Mycobacterium tuberculosis. Biochemistry 34:8235-8241. [DOI] [PubMed] [Google Scholar]
- 13.Seefeld, M. A., W. H. Miller, K. A. Newlander, W. J. Burgess, W. E. DeWolf, Jr., P. A. Elkins, M. S. Head, D. R. Jakas, C. A. Janson, P. M. Keller, P. J. Manley, T. D. Moore, D. J. Payne, S. Pearson, B. J. Polizzi, X. Qiu, S. F. Rittenhouse, I. N. Uzinskas, N. G. Wallis, and W. F. Huffman. 2003. Indole naphthyridinones as inhibitors of bacterial enoyl-ACP reductases FabI and FabK. J. Med. Chem. 46:1627-1635. [DOI] [PubMed] [Google Scholar]
- 14.Seefeld, M. A., W. H. Miller, K. A. Newlander, W. J. Burgess, D. J. Payne, S. F. Rittenhouse, T. D. Moore, W. E. DeWolf, Jr., P. M. Keller, X. Qiu, C. A. Janson, K. Vaidya, A. P. Fosberry, M. G. Smyth, D. D. Jaworski, C. Slater-Radosti, and W. F. Huffman. 2001. Inhibitors of bacterial enoyl acyl carrier protein reductase (FabI): 2,9-disubstituted 1,2,3,4-tetrahydropyrido[3,4-b]indoles as potential antibacterial agents. Bioorg. Med. Chem. Lett. 11:2241-2244. [DOI] [PubMed] [Google Scholar]
- 15.Slater-Radosti, C., G. Van Aller, R. Greenwood, R. Nicholas, P. M. Keller, W. E. DeWolf, Jr., F. Fan, D. J. Payne, and D. D. Jaworski. 2001. Biochemical and genetic characterization of the action of triclosan on Staphylococcus aureus. J. Antimicrob. Chemother. 48:1-6. [DOI] [PubMed] [Google Scholar]