Abstract
The pseudopeptide pyrrolidinedione antibiotics, such as moiramide B, have recently been discovered to target the multisubunit acetyl coenzyme A (acetyl-CoA) carboxylases of bacteria. In this paper, we describe synthetic variations of each moiety of the modularly composed pyrrolidinediones, providing insight into structure-activity relationships of biochemical target activity, in vitro potency, and in vivo efficacy. The novel derivatives showed highly improved activities against gram-positive bacteria compared to those of previously reported variants. The compounds exhibited a MIC90 value of 0.1 μg/ml against a broad spectrum of Staphylococcus aureus clinical isolates. No cross-resistance to antibiotics currently used in clinical practice was observed. Resistance mutations induced by pyrrolidinediones are exclusively located in the carboxyltransferase subunits of the bacterial acetyl-CoA carboxylase, indicating the identical mechanisms of action of all derivatives tested. Improvement of the physicochemical profile was achieved by salt formation, leading to aqueous solubilities of up to 5 g/liter. For the first time, the in vitro activity of this compound class was compared with its in vivo efficacy, demonstrating a path from compounds weakly active in vivo to agents with significant efficacy. In a murine model of S. aureus sepsis, the 100% effective dose of the best compound reported was 25 mg/kg of body weight, only fourfold higher than that of the comparator molecule linezolid. The obvious improvements achieved by chemical derivatization reflect the potential of this novel antibiotic compound class for future therapy.
The bacterial acetyl coenzyme A (acetyl-CoA) carboxylase (ACC) is a multisubunit enzyme that catalyzes the first committed step in fatty acid synthesis and is essential for cell growth. The acetyl-CoA carboxylase reaction can be divided into two partial reactions (3). In the first step, biotin is carboxylated via ATP consumption by the biotin carboxylase. In the second step, the carboxyl group is transferred to acetyl-CoA by the carboxyltransferase, yielding malonyl-CoA. Its broad structural conservation among bacteria and clear distinction from the multifunctional eukaryotic enzyme make ACC a promising target for the design of new broad-spectrum antibacterials with a novel mechanism of action. Nevertheless, until recently no antibiotic has been known which is active against bacteria by selective ACC inhibition. For the first time, we discovered a class of antibiotics, i.e., the pseudopeptidic pyrrolidinedione natural products moiramide B and andrimid (5, 11, 12), that selectively inhibit the ACC-carboxyltransferase reaction (6, 7).
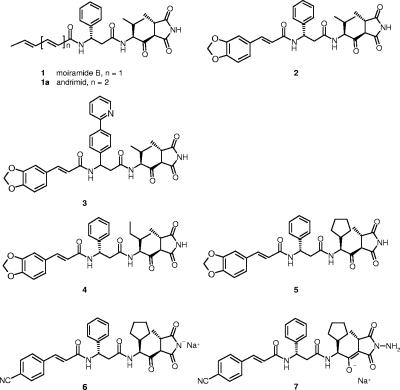
The chemical structures of moiramide B and andrimid (compounds 1 and 1a) (Fig. 1) contain four characteristic subunits, including an unsaturated fatty acid side chain, the β-amino acid (S)-β-phenylalanine, an l-valine-derived β-ketoamide moiety, and the pyrrolidinedione head group. We recently reported the chemical synthesis of moiramide B derivatives based on previously published procedures (13). We presented structure-activity relationship data regarding variations of the two terminal subunits, i.e., the fatty acid side chain and the pyrrolidinedione head group. On the one hand, a high degree of variability was found for the fatty acid side chain, with limited influence on ACC inhibition but a significant impact on MICs, probably due to physicochemical properties of the compounds. On the other hand, only limited variations were tolerated in the pyrrolidinedione head group without losing target as well as antibacterial activity.
FIG. 1.
Chemical structures of the natural products moiramide B and andrimid and of selected synthetic derivatives.
The goal of the study presented here was to evaluate synthetic variations of the two internal parts of the pyrrolidinedione antibiotics that have not been addressed so far regarding their effects on target as well as antibacterial activity. As the next step, we planned to optimize the biological and physicochemical profile by combining ideal variations of all four subunits. We especially wanted to compare the in vitro activities of pyrrolidinediones with their in vivo efficacies in murine models of Staphylococcus aureus sepsis with gradual sensitivity for the first time. All in all, the study aimed to demonstrate a path forward from the weakly active natural products to antibacterial congeners with significant therapeutic in vivo efficacies.
MATERIALS AND METHODS
Strains, media, and test compounds.
The bacterial strains used in this study were S. aureus isolate 133 (DSM number 11823; DSMZ, Braunschweig, Germany), Streptococcus pneumoniae isolate G9A, Escherichia coli strain Neumann (DSM number 10650), and E. coli HN818 carrying a deletion of acrA, which encodes an efflux system component (9). In addition, diverse strains of gram-positive bacteria taken from an in-house collection of recent clinical isolates (2) were tested in antibiotic susceptibility tests, including 50 S. aureus strains and 10 S. pneumoniae strains (see Results). The bacteria were grown in Isosensitest broth (Oxoid), in LB medium (Difco), or in the case of S. pneumoniae, in brain heart infusion medium with 10% bovine serum (Oxoid). The agar media used in this study were Columbia agar with 5% sheep blood (BD Biosciences), Isosensitest agar, and LB agar. The compounds which were tested in this study were synthesized analogously to recently described methods (4, 10, 13, 14), using appropriate synthetic precursors (see Results). In addition, linezolid (Zyvox) was used in animal models.
Carboxyltransferase inhibition and antibiotic susceptibility tests.
The carboxyltransferase activities of the ACCs from S. aureus and E. coli were measured as recently described in order to determine the enzyme-inhibitory activities of the test compounds (7). Microdilution MICs were determined against different bacterial strains in 96-well microtiter plates with growth medium containing serial dilutions (twofold) of antibiotics. A starting inoculum of 1.0 × 105 CFU/ml derived from overnight cultures in Isosensitest broth was used. In the case of S. pneumoniae, cells were taken directly from colonies grown overnight on Columbia agar plates with 5% sheep blood (Becton Dickinson) (inoculum, 1.0 × 105 CFU/ml). The strains were incubated aerobically in Isosensitest medium at 37°C, except for S. pneumoniae, which was incubated at 37°C in brain heart infusion broth supplemented with 10% bovine serum in an anaerobic jar with Anaerocult C (Merck, Darmstadt, Germany). The MIC was the lowest concentration of drug that yielded no visible growth after incubation for 18 to 24 h at 37°C. End points were determined by measuring the optical density at 600 nm with an EL312e microtiter plate reader (Bio-Tec Instruments).
Isolation of resistant mutants by serial passages.
LB medium (3 ml) containing serial dilutions (twofold) of each test compound was inoculated with overnight cultures of S. aureus 133 (starting optical density at 600 nm, 0.01). After overnight incubation at 37°C, the culture with the highest antibiotic concentration at which the cells reached the same optical density at 600 nm as the antibiotic-free culture was used for inoculation of fresh growth medium containing a novel series of antibiotic dilutions. This procedure was repeated six times. The resistant cells were then plated on antibiotic-free LB agar plates and incubated overnight at 37°C for isolation of single colonies. The obtained clones were transferred several times on antibiotic-free medium and subsequently tested for antibiotic susceptibility in order to confirm their antibiotic resistance. PCR amplification of the carboxyltransferase genes accA and accD with the primer pairs ACCA1A_SA-ACCA1B_SA and ACCD2A_SA-ACCD2B_SA (7), respectively, was achieved from colonies of the resistant S. aureus strains according to standard protocols. The PCR products were cleaned up by using a QIAquick PCR purification kit (QIAGEN, Hilden, Germany) and sequenced by the QIAGEN Sequencing Service for mutation mapping (sequence analysis was done with Lasergene software 6.0 [DNASTAR, Madison, WI]).
In vivo efficacy tests.
In vivo studies were performed using an S. aureus sepsis model in female CFW1 mice (weight, approximately 20 g; five or six mice per group). The animals were infected with a single intraperitoneal (i.p.) injection of S. aureus 133 (0.25 ml saline containing 5% mucin; 106 CFU/mouse). Thirty minutes after infection, the mice were treated intravenously (i.v.) or i.p. with 0.2 ml of a solution of the test compound in the appropriate solvent (solvent for compound 2, 6.5% dimethyl sulfoxide [DMSO], 60% solutol-ethanol (5:1), 33.5% water; solvent for linezolid and compounds 6 and 7, 0.9% aqueous NaCl solution). The mice were monitored over a 5-day period, and the results are reported as percentages of surviving mice.
The murine model of S. aureus sepsis was modified to detect the in vivo efficacies of less potent derivatives with a higher sensitivity. For this purpose, the inoculum of S. aureus 133 was reduced to 104 CFU/mouse and the adjuvant mucin concentration was increased to 20%. Thirty minutes or 30 and 90 min after infection, the mice were treated i.v. with 0.1 ml of a solution of the test compound (compound 2, 4, or 5) in 5% DMSO, 30% solutol, and 65% water (solvent for linezolid, 0.9% aqueous NaCl solution). After 5 h, different organs were removed aseptically and homogenized in a Potter S homogenizer (B. Braun, Melsungen, Germany) in 1 ml of 0.1 M phosphate-buffered saline. Peritoneal bacteria were collected by peritoneal lavage using 2 ml sterile phosphate-buffered saline. Viable bacterial counts in the lungs, livers, peritoneums, and blood of infected mice were determined by plating serial 10-fold dilutions in duplicate on agar.
Determination of solubility.
Each test compound was dissolved in water. The solution was agitated at room temperature for 24 h. After ultracentrifugation at 224,000 × g for 30 min, the supernatant was diluted in DMSO and analyzed by analytical high-pressure liquid chromatography. Quantification was done versus a two-point calibration curve of the test compound in DMSO. High-pressure liquid chromatography was performed using an Agilent 1100 system (Agilent Technologies, Palo Alto, CA) with a Gemini C18 column (5 μm; Phenomenex, Torrance, CA). Elutions were performed at 40°C, using a gradient made of water-phosphoric acid, pH 2 (eluent A), and acetonitrile (eluent B) (flow rate, 0.7 ml/min; 0 to 0.5 min, 85% eluent A and 15% eluent B; 0.5 to 3 min, ramp to 10% A and 90% B; 3 to 3.5 min, 10% A and 90% B; 3.5 to 4 min, ramp to 85% A and 15% B; and 4 to 5 min, 85% A and 15% B).
Pharmacokinetic studies.
Pharmacokinetic analyses were performed with female CFW1 mice and male Wistar rats according to previously reported protocols (8). Plasma drug concentrations were quantified by a liquid chromatography-mass spectrometry method as described previously (8).
RESULTS
Target activities and antibacterial potencies of novel derivatives.
Based on results presented previously (7, 13), we continued the systematic structural variation of the pyrrolidinedione antibiotics. We routinely tested the target activity, i.e., the inhibition of the carboxyltransferase reactions of the ACCs from S. aureus and E. coli, and we determined the MICs against different bacterial strains. Here we report the MIC data for S. aureus, S. pneumoniae, and two E. coli strains, one of which is impaired in drug efflux (see Materials and Methods). Having reported that variations of the terminal subunits of moiramide B lead to a slight improvement in target activity and antibacterial activity (Fig. 1 and Table 1, compounds 1 and 2), we now focused on structural variation of the two internal parts of the molecules, the (S)-β-phenylalanine subunit and the l-valine-derived β-ketoamide moiety.
TABLE 1.
Target inhibition and MIC data for pyrrolidinedione derivatives
| Compound | IC50 (nM) for S. aureus 133a | MIC (μg/ml)
|
IC50 (nM) for E. colia | MIC (μg/ml)
|
||
|---|---|---|---|---|---|---|
| S. aureus 133 | S. pneumoniae G9A | E. coli HN818b | E. coli Neumann | |||
| 1 | 96 | 8 | 32 | 6 | 4 | 32 |
| 2 | 91 | 2 | 16 | 4 | 1 | 16 |
| 3 | 264 | 1 | 32 | 14 | 2 | 64 |
| 4 | 68 | 0.25 | 32 | 7 | 1 | 64 |
| 5 | 48 | 0.06 | 4 | 11 | 1 | 16 |
| 6 | 44 | 0.03 | 1 | 4 | 1 | 32 |
| 7 | 33 | 0.01 | 0.25 | 2 | 0.5 | 16 |
Test results were obtained with the recombinant ACC-carboxyltransferases from E. coli and S. aureus, respectively.
acrA efflux pump deletion strain.
First, we observed that replacement of (S)-β-phenylalanine by nonaromatic β-amino acids led to a loss of antibacterial activity (data not shown), while the introduction of lipophilic substituents on the phenyl ring was tolerated. For instance, compound 3, which carries a phenyl-pyridyl side chain, showed a twofold lower MIC against S. aureus and only two- to fourfold elevated MICs against the other reported species than compound 2, which carries a phenyl group (Fig. 1; Table 1). Remarkably, the S. aureus target activity was reduced by a factor of 3, suggesting improved permeation of the derivative into S. aureus.
Second, changes at the isopropyl side chain of the l-valine-derived β-keto moiety provided derivatives with retained or slightly improved target activity and with significantly raised potencies against gram-positive bacteria. For instance, a 2-butyl side chain (compound 4) led to an eightfold MIC decrease against S. aureus compared to the MIC of compound 2, and a cyclopentyl side chain even lowered the MIC by two additional dilution steps (compound 5) (Fig. 1; Table 1). Moreover, the moderate anti-S. pneumoniae activity was also strongly improved by the introduction of the cyclopentyl side chain. In order to estimate the MIC variation of that highly potent pyrrolidinedione derivative across numerous strains of gram-positive bacteria, including clinical isolates, we tested compound 5 against 50 S. aureus strains (19 of which were methicillin resistant) and 10 S. pneumoniae strains (5 of which were penicillin resistant). Narrow MIC ranges and no a priori resistant strains were identified for S. aureus (MIC90 = 0.1 μg/ml; MIC range, 0.01 to 0.2 μg/ml) and S. pneumoniae (MIC90 = 4 μg/ml; MIC range, 2 to 4 μg/ml). In contrast to this significant improvement of activity against gram-positive bacteria, activity against the gram-negative representative, E. coli, was not improved. The 16- to 64-fold higher MICs against the E. coli wild-type strain (Neumann) than those against the efflux pump deletion strain (HN818) suggested that each derivative tested in this study represents a good substrate for the E. coli multidrug AcrAB efflux system.
Finally, we combined the cyclopentylglycine-derived subunit with improved variations of both terminal parts of the pyrrolidinediones. The potency was further increased by replacing the methylene-dioxo group in the terminal cinnamic acid by a nitrile group (compound 6) and by varying the pyrrolidinedione head group (hydrazide derivative [compound 7]) (Fig. 1; Table 1). The pyrrolidinediones generally exhibited mediocre water solubilities (e.g., for compound 2, the solubility was 15 mg/liter, and for compound 5, the solubility was 12 mg/liter). In order to allow intravenous application of aqueous solutions of the pyrrolidinediones for in vivo experiments, the solubilities of these compounds clearly needed to be increased. By preparation of sodium salts of the most potent compounds, we did indeed reach high solubility levels in water of up to 5 g/liter (compounds 6 and 7).
Confirmation of the cellular mechanism of action of novel derivatives.
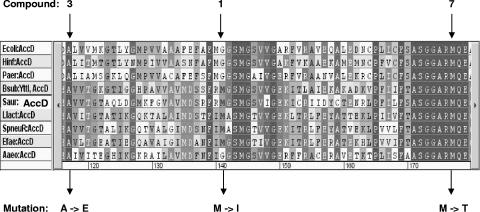
We have previously shown that bacteria resistant to compound 2 carry a mutation in a conserved region of the carboxyltransferase subunit AccA or AccD (7). Transfer of the mutation to a nonresistant Bacillus subtilis strain made this bacterium less susceptible to pyrrolidinediones. Thereby, we proved that the ACC-carboxyltransferase represents the cellular target of the pyrrolidinediones. The derivatives described in this study all exhibit potent target activities; however, the improved whole-cell activities cannot be fully explained by improved target activities alone. In order to confirm that the different pyrrolidinedione variants still acted via ACC inhibition, we tried to generate S. aureus mutants resistant to selected derivatives and to map the resistance mutations. Since we were not able to isolate spontaneously resistant mutants among 109 bacteria with pyrrolidinediones, as reported previously (7), we selected resistant mutants by transferring overnight cultures several times at sublethal concentrations of compounds 1, 3, and 7. Using compound 1 (the natural product moiramide B), we were able to isolate a mutant with a 4-fold increased MIC (from a MIC of 8 μg/ml for the corresponding wild-type strain to a mutant MIC of 32 μg/ml), while repeated exposure of S. aureus 133 to compounds 3 and 7 enabled us to isolate mutants with 16- and 256-fold increased MICs (from 1 μg/ml to 16 μg/ml and from 0.01 μg/ml to 4 μg/ml, respectively). The mutants generated with one of the pyrrolidinediones exhibited corresponding cross-resistance to each of the other derivatives. Remarkably, in each of the resistant S. aureus mutants, we identified a mutation leading to a single amino acid exchange in the ACC-carboxyltransferase β subunit, with each of the mutants carrying a different mutation in a conserved region of that subunit (Fig. 2). In contrast, the recently generated S. aureus 133 mutant, which was at least 64-fold less susceptible to compound 2 (MIC, >64 μg/ml) than the corresponding wild-type strain (MIC, 2 μg/ml), contained another mutation located in the α subunit of the ACC-carboxyltransferase (7). The identification of mutations in one of the carboxyltransferase genes of each mutant clearly indicates that the ACC-carboxyltransferase represents the primary target of each of the different pyrrolidinedione derivatives.
FIG. 2.
Part of the multiple amino acid sequence alignment of carboxyltransferase β-subunits (AccD) of bacterial acetyl-CoA carboxylases. The amino acid exchanges caused by mutations obtained by serial passaging of S. aureus 133 in the presence of the pyrrolidinedione variants 1, 3, and 7 are indicated below the alignment. The accD mutations are G400-A (compound 1), C332-A (compound 3), and T509-C (compound 7). The mutation isolated with compound 1 (the natural product moiramide B) was found in a strain with a fourfold increased MIC compared to that of the corresponding wild type. The mutation isolated with compound 3 was associated with a 16-fold elevated MIC, while the mutation isolated with compound 7 was identified in a mutant with a 256-fold increased MIC (see Results). Sequences of the following bacteria are included: Ecoli, E. coli; Hinf, Haemophilus influenzae; Paer, Pseudomonas aeruginosa; Bsub, B. subtilis (YttI is a synonym for AccD); Saur, S. aureus; Llact, Lactobacillus lactis; Spneu, S. pneumoniae; Efae, Enterococcus faecalis; and Aaeo, Aquifex aeolicus.
Correlation of in vitro activity and in vivo efficacy for moderately active derivatives.
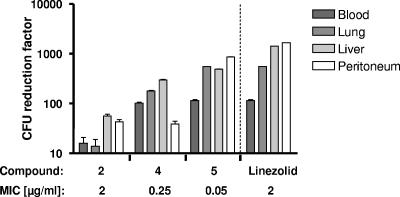
When testing the in vivo efficacy of the initial lead structure 2, we observed that this compound was only weakly efficacious in our standard S. aureus sepsis model (106 CFU/mouse). Compound 2 gave 66% survival at two doses of 100 mg/kg administered i.p. Therefore, we decided to use a modified infection model to measure the efficacies of weakly potent derivatives with a higher sensitivity. In comparison to the initial model, we injected a lower dose of S. aureus (104 CFU/mouse) intraperitoneally, together with an increased amount of the adjuvant mucin (see Materials and Methods), and counted the CFU in different organs in order to correlate in vitro activity with in vivo efficacy for compounds 2, 4, and 5. Indeed, we found that the compounds exhibited better in vitro activities, which also led to increased CFU reductions in lungs, livers, and blood (Fig. 3). Two doses of 10 mg/kg of compound 5 given i.v. (at 30 and 90 min postinfection) were nearly as efficacious as 10 mg/kg of linezolid given once i.v. (at 30 min postinfection). However, this observed in vivo efficacy was relatively low considering the good in vitro activities of pyrrolidinediones 2, 4, and 5 (MICs of 2 to 0.05 μg/ml). Pharmacokinetic evaluation in mice revealed that the compounds had short half-lives (t1/2) of the elimination phase (≤0.2 h). Valid data for plasma clearance could not be calculated due to the high extrapolated area under the concentration-time curve between the time point of compound administration (t0) and the time point of the first sampling (t1) (very rapid decrease of plasma concentrations between 2 and 5 min after administration of the compounds). However, studies with rats enabled us to more precisely determine the pharmacokinetic parameters: after i.v. administration of 1 mg/kg, compound 5 exhibited a plasma clearance rate of 0.74 liter/h · kg and a volume of distribution of 0.14 liter/kg (t1/2 = 0.48 h).
FIG. 3.
In vitro-in vivo efficacy correlation for variants of pyrrolidinedione antibiotics in a murine sepsis model with a reduced S. aureus inoculum. Compounds 2, 4, and 5 were dosed at 10 mg/kg i.v. at 30 and 90 min postinfection, and linezolid was given only at 30 min postinfection. The reductions of CFU in the blood, lungs, liver, and peritoneum determined 5 h after infection were plotted for the different derivatives (see Materials and Methods). Compound 5 (given twice) approximately reached the efficacy of linezolid (administered once). The in vivo efficacy ranking of pyrrolidinediones reflects the improvements of in vitro MIC data, as shown below the diagram.
In vivo efficacy of highly potent cyclopentylglycine derivatives.
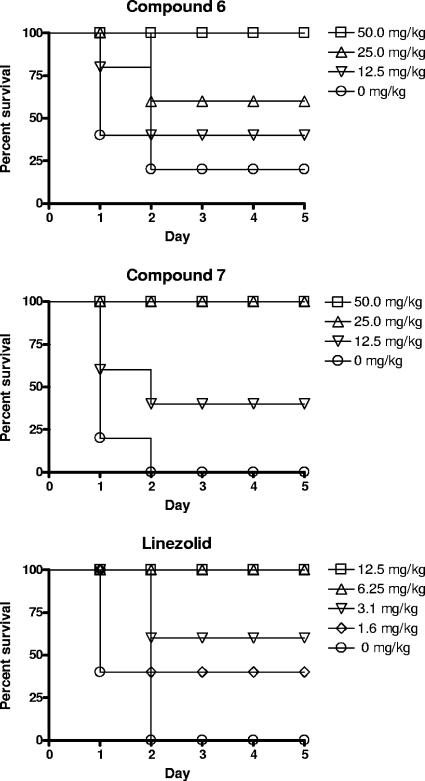
Highly soluble salts of the optimized cyclopentylglycine congeners 6 and 7 dissolved in 0.9% aqueous NaCl solution were tested in the standard S. aureus sepsis model (106 CFU/mouse). We obtained significant in vivo efficacies after i.v. treatment, with 100% effective dose values of 50 and 25 mg/kg, respectively (Fig. 4), while the reference compound linezolid gave 100% survival at a dose of 6.25 mg/kg in the same infection model.
FIG. 4.
Therapeutic effects of pyrrolidinedione derivatives 6 and 7 (see Fig. 1) and of the reference antibiotic linezolid in a murine model of S. aureus sepsis. The percentage of surviving mice among five animals per group was monitored over 5 days (see Materials and Methods).
These efficacy studies were accompanied by pharmacokinetic evaluation. The pyrrolidinediones possessed low to medium plasma clearance rates and volumes of distribution in rats (e.g., compound 6 had a plasma clearance rate of 1.07 liters/h · kg and a volume of distribution of 0.28 liter/kg, with a t1/2 of 0.5 h), and the unbound fraction in mouse and rat sera was low (fu, ∼1%).
DISCUSSION
The natural product moiramide B, a pseudopeptidic pyrrolidinedione, represented an attractive starting point for a medicinal chemistry program. First, this low-molecular-weight compound possessed antibiotic activity against a broad spectrum of bacterial species and was amenable for chemical synthesis and derivatization. Second, moiramide B and its congeners constitute an antibiotic class with a unique mechanism of action. The pyrrolidinediones stop bacterial growth by inhibition of the bacterial ACC without targeting the counterpart enzyme in the human host. Conceptually, this antibiotic class should not cause target mechanism-based toxicity and should break any bacterial resistance against antibiotics currently applied in medical practice. Indeed, our MIC data for derivative 5 obtained with numerous pathogenic strains of gram-positive bacteria confirmed the potential of this compound class to provide resistance-breaking antibiotics.
The modular structure of moiramide B allowed synthetic variations of each part of the molecule. We already reported variations of the terminal fatty acid side chain and described the high degree of variability acceptable for biochemical target inhibition, although certain variations had significant impacts on antibacterial activity (7, 13). Substituted cinnamic acids represented favorable side chains, improving the antibacterial activity. Compound 2 represented the lead structure for our derivatization program (Table 1 and Fig. 1). On the other hand, only limited variations were tolerated in the pyrrolidinedione head group. Only a few selected substituents at the pyrrolidinedione nitrogen atom were not detrimental for the inhibitory potency toward the ACC and, consequently, for the antibacterial activity. For instance, N-methylation of the head group led to only a slight decrease in enzyme inhibitory activity (13), and the corresponding N-amino derivative was at least equipotent to moiramide B (e.g., compound 7) (Table 1 and Fig. 1).
The two internal parts of the pyrrolidinediones have not been addressed previously. The variations we report here for the β-amino acid subunit located adjacent to the terminal fatty acid side chain revealed a structure-activity relationship similar to that of the terminal side chain. The introduction of lipophilic substituents on the phenyl ring of the (S)-β-phenylalanine was tolerated. These observations indicate that the fatty acid/(S)-β-phenylalanine part of the molecule is less important for interaction with the target ACC than the pyrrolidinedione head group. Nevertheless, increased lipophilicity at the fatty acid/(S)-β-phenylalanine part improves the antibacterial activity, presumably by improved penetration into bacteria. The second internal part of the pyrrolidinediones, the l-valine-derived β-keto moiety, represents a site at which derivatization enabled us to achieve a significant improvement of potency against gram-positive bacteria. Compounds deriving from cyclopentylglycine instead of l-valine showed strong antibacterial in vitro activity, especially against S. aureus.
However, such improvements in antibacterial activity with less significantly increased target inhibition often raise speculations that additional antibiotic mechanisms might be involved. For that reason, we isolated resistant bacterial mutants with the following three different pyrrolidinedione derivatives: compound 1, the natural product moiramide B; compound 3, carrying a variation in the (S)-β-phenylalanine part; and compound 7, carrying a cyclopentylglycine instead of l-valine and a modified head group. For each compound, we were able to map a mutation in one of the carboxyltransferase subunits of the ACC. Remarkably, the resistant S. aureus mutants carried mutations located at three different sites of the ACC-carboxyltransferase β-subunit AccD. This finding might reflect the different levels of pyrrolidinedione resistance of the mutants, with 4-, 16-, and 256-fold elevated MICs. The previously described mutation generated with compound 2 was located in the second carboxyltransferase subunit, AccA, of S. aureus, whereas the same compound led to a mutation in a homologous region of AccD in E. coli (7). All in all, these data might indicate the relevant interaction sites of the pyrrolidinediones with both subunits of bacterial ACCs. In addition, the results strongly suggest that the newly synthesized pyrrolidinedione variants, such as compounds 3 and 7, keep acting as antibacterials via ACC inhibition, as previously shown for compound 2. Just recently, the three-dimensional structures of the ACC-carboxyltransferase complexes from S. aureus and E. coli have been reported (1). Based on these data, modeling and cocrystallization of the pyrrolidinediones might provide deeper insight into the target-inhibitor interaction.
By structural variation of the different parts of the pyrrolidinedione antibiotics, we reached a clear improvement in the antibacterial activity of this compound class, especially against gram-positive bacteria. In addition, an important advancement was achieved by significantly increasing the aqueous solubility via salt formulation. Thus, i.v. treatment with aqueous solutions became feasible. The early derivatives needed to be tested in a mouse model with a reduced S. aureus inoculum and with CFU reductions in different organs as the readout in order to quantify in vivo efficacy and to assess the correlation between in vitro activity and in vivo potency. The in vivo efficacy of the optimized cyclopentylglycine derivative 7 could be tested in a standard murine model of S. aureus sepsis. Remarkably, the efficacy was only fourfold inferior to that of the reference compound linezolid after i.v. treatment. However, to ultimately turn the high in vitro activities of the pyrrolidinediones into adequate in vivo efficacies, further optimization of the pharmacokinetic properties and the physicochemical profile seems to be necessary. Improvements of the plasma half-life, the volume of distribution, and the unbound fraction might allow these antibiotics to reach in vivo efficacies equivalent to those of antibiotics in current medical use.
In conclusion, we demonstrated that systematic structural variation of the natural product moiramide B, a pseudopeptidic pyrrolidinedione antibiotic with insufficient solubility and moderate antibacterial potency, enabled us to identify highly soluble analogues with strong in vitro activities against gram-positive bacteria and significant in vivo efficacies in a murine model of S. aureus sepsis. Continuation of the systematic exploration of structure-activity and structure-property relationships will help to improve in vivo potencies in order to evaluate the full potential of this attractive compound class for future antibacterial therapy.
Acknowledgments
We thank S. Doerlemann, M. Haas, U. Dohnau, and U. Schick for technical assistance and especially J. Keldenich for solubility measurements.
REFERENCES
- 1.Bilder, P., S. Lightle, G. Bainbridge, J. Ohren, B. Finzel, F. Sun, S. Holley, L. Al-Kassim, C. Spessard, M. Melnick, M. Newcomer, and G. L. Waldrop. 2006. The structure of the carboxyltransferase component of acetyl-CoA carboxylase reveals a zinc-binding motif unique to the bacterial enzyme. Biochemistry 45:1712-1722. [DOI] [PubMed] [Google Scholar]
- 2.Broetz-Oesterhelt, H., D. Beyer, H. P. Kroll, R. Endermann, C. Ladel, W. Schroeder, B. Hinzen, S. Raddatz, H. Paulsen, K. Henninger, J. E. Bandow, H. G. Sahl, and H. Labischinski. 2005. Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat. Med. 11:1082-1087. [DOI] [PubMed] [Google Scholar]
- 3.Cronan, J. E., Jr., and G. L. Waldrop. 2002. Multi-subunit acetyl-CoA carboxylases. Prog. Lipid Res. 41:407-435. [DOI] [PubMed] [Google Scholar]
- 4.Davies, S. G., and D. J. Dixon. 1998. Asymmetric syntheses of moiramide B and andrimid. J. Chem. Soc. Perkin 1 17:2635-2644. [Google Scholar]
- 5.Fredenhagen, A., S. Y. Tamura, P. T. M. Kenny, H. Komura, Y. Naya, K. Nakanishi, K. Nishiyama, M. Sugiura, and H. Kita. 1987. Andrimid, a new peptide antibiotic produced by an intracellular bacterial symbiont isolated from a brown planthopper. J. Am. Chem. Soc. 109:4409-4411. [Google Scholar]
- 6.Freiberg, C., H. P. Fischer, and N. A. Brunner. 2005. Discovering the mechanism of action of novel antibacterial agents through transcriptional profiling of conditional mutants. Antimicrob. Agents Chemother. 49:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freiberg, C., G. Schiffer, N. Brunner, T. Lampe, J. Pohlmann, M. Brands, D. Haebich, and K. Ziegelbauer. 2004. Identification and characterization of the first class of potent bacterial acetyl-CoA carboxylase inhibitors with antibacterial activity. J. Biol. Chem. 279:26066-26073. [DOI] [PubMed] [Google Scholar]
- 8.Kuhl, A., N. Svenstrup, C. Ladel, M. Otteneder, A. Binas, G. Schiffer, M. Brands, T. Lampe, K. Ziegelbauer, H. Rubsamen-Waigmann, D. Haebich, and K. Ehlert. 2005. Biological characterization of novel inhibitors of the gram-positive DNA polymerase IIIC enzyme. Antimicrob. Agents Chemother. 49:987-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 16:45-55. [DOI] [PubMed] [Google Scholar]
- 10.McWorther, W., A. Fredenhagen, K. Nakanishi, and H. Komura. 1989. Stereocontrolled synthesis of andrimid and a structural requirement for the activity. J. Chem. Soc. Chem. Commun. 5:299-301. [Google Scholar]
- 11.Needham, J., M. T. Kelly, M. Ishige, and R. J. Andersen. 1994. Andrimid and moiramides A-C, metabolites produced in culture by a marine isolate of the bacterium Pseudomonas fluorescens: structure eludication and biosynthesis. J. Org. Chem. 59:2058-2063. [Google Scholar]
- 12.Oclarit, J. M., H. Okada, S. Ohta, K. Kaminura, Y. Yamaoka, T. Iizuka, S. Miyashiro, and S. Ikegami. 1994. Anti-bacillus substance in the marine sponge, Hyatella species, produced by an associated Vibrio species bacterium. Microbios 78:7-16. [PubMed] [Google Scholar]
- 13.Pohlmann, J., T. Lampe, M. Shimada, P. G. Nell, J. Pernersdorfer, N. Svenstrup, N. Brunner, G. Schiffer, and C. Freiberg. 2005. Pyrrolidinedione derivatives as antibacterial agents with a novel mode of action. Bioorg. Med. Chem. Lett. 15:1189-1192. [DOI] [PubMed] [Google Scholar]
- 14.Rao, A. V. R., A. K. Singh, and C. V. N. S. Varaprasad. 1991. A convenient diastereoselective total synthesis of andrimid. Tetrahedron Lett. 32:4393-4396. [Google Scholar]