Abstract
Antimicrobial peptides are widely believed to exert their effects by nonspecific mechanisms. We assessed the extent to which physicochemical properties can be exploited to promote discriminative activity by manipulating the N-terminal sequence of the 13-mer dermaseptin derivative K4-S4(1-13) (P). Inhibitory activity determined in culture media against 16 strains of bacteria showed that when its hydrophobicity and charge were changed, P became predominantly active against either gram-positive or gram-negative bacteria. Thus, conjugation of various aminoacyl-lysin moieties (e.g., aminohexyl-K-P) led to inactivity against gram-positive bacteria (MIC50 > 50 μM) but potent activity against gram-negative bacteria (MIC50, 6.2 μM). Conversely, conjugation of equivalent acyls to the substituted analog M4-S4(1-13) (e.g., hexyl-M4-P) led to inactivity against gram-negative bacteria (MIC50 > 50 μM) but potent activity against gram-positive bacteria (MIC50, 3.1 μM). Surface plasmon resonance experiments, used to investigate peptides' binding properties to lipopolysaccharide-containing idealized phospholipid membranes, suggest that although the acylated derivatives have increased lipophilic properties with parallel antibacterial behavior, hydrophobic derivatives are prevented from reaching the cytoplasmic membranes of gram-negative bacteria. Moreover, unlike modifications that enhanced the activity against gram-positive bacteria, which also enhanced hemolysis, we found that modifications that enhanced activity against gram-negative bacteria generally reduced hemolysis. Thus, compared with the clinically tested peptides MSI-78 and IB-367, the dermaseptin derivative aminohexyl-K-P performed similarly in terms of potency and bactericidal kinetics but was significantly more selective in terms of discrimination between bacteria and human erythrocytes. Overall, the data suggest that similar strategies maybe useful to derive potent and safe compounds from known antimicrobial peptides.
Peptide-based antimicrobial compounds represent a class of promising agents in fighting the growing bacterial resistance to antibiotics (19, 51). Although their precise mechanism of action is not fully understood, antimicrobial peptides (AMPs) are generally believed to kill target cells by disrupting the cell membrane(s) (23, 47). Such an external site of action and receptor-independent mechanism may prevent or reduce the development of drug resistance. In addition to the membrane target, various potential sites, such as nucleic acids, have been proposed to be targeted by AMPs (15, 19). Still, due to their notorious lipophilic properties, AMPs inevitably interact with the cell membrane on their way to an intracellular target(s). Regardless of the precise mode of action, nonspecific interactions are responsible for AMPs' nonselective activities over a wide range of cell types, including gram-negative and -positive bacteria (17, 30, 52); encapsulated viruses (48, 53); eukaryotic parasites, such as trypanosomes (7), malaria parasites (11, 13), and nematodes (8); and cancer cells (28, 34). Thus, a major challenge in this rapidly growing field of research is to endow antimicrobial peptides with selective activities against specific target cells. To achieve this goal, numerous strategies have been used, including various sequence modification methods that attempt to modify natural peptides by deleting, adding, or replacing one or more residues (27, 29, 33); assembly of chimerical peptides from segments of different natural peptides (3, 10); and combinatorial libraries (5, 6). Although these studies have enhanced the global understanding of the properties governing specific activities between prokaryotic and eukaryotic cells, further research is needed to enable discrimination between prokaryotic cells, i.e., between gram-positive and gram-negative bacteria, for example.
Tree-frog dermaseptins are a large family of linear AMPs (9, 31) whose cytolytic properties are believed to be triggered after interaction of N-terminal residues with the plasma membrane (25, 40). Truncation studies of dermaseptin S4 resulted in various short derivatives, such as the 13-mer K4-S4(1-13), which maintained or improved structural characteristics and rapid cytolytic activity against a variety of pathogens (14, 24, 25). Bacteria were shown to develop resistance to commercial antibiotics but not to the l or d isomers of dermaseptin derivatives (33). The effects of acylated derivatives on malaria-infected red blood cells were investigated, taking advantage of their ability to cross the mammalian cell membrane spontaneously (20). Acylation, in general, was shown to enhance hemolysis (11, 41), antiprotozoan activity (11), and antibacterial activity against gram-positive but not gram-negative bacteria (41). These findings correlated with the peptides' tendency for self-assembly in solution due to increased hydrophobic interactions (41).
Here, we extend this investigation by further exploring mechanisms that might govern peptide specificity between gram-positive and gram-negative bacteria based on the peptides' physicochemical properties. Using the 13-mer derivative K4-S4(1-13) (designated P) as a reference compound, various types of acyl chains were conjugated to its amino terminus in order to alter hydrophobicity, while point mutations were performed to modulate the net charge. The data presented show how hydrophobicity and charge affect the peptides' organization in solution, lipophilic properties, and antimicrobial behavior.
MATERIALS AND METHODS
Peptides.
The peptides were synthesized by the solid-phase method, applying the Fmoc (9-fluorenylmethyloxy carbonyl) active-ester chemistry on an Applied Biosystems model 433A peptide synthesizer. 4-Methylbenzhydrylamine resin (Novabiochem, Darmstadt, Germany) was used to obtain amidated peptides. The acylated analogs were prepared by linking the amino termini of the peptides to lauric or aminolauric acid as described previously (41). The crude peptide was purified to ≥95% chromatographic homogeneity by reverse-phase high-performance liquid chromatography (Alliance-Waters). Purification and refolding of IB-367, which contains cysteine residues, was performed basically according to the procedure described by Harwig et al. (21); repurification was done by high-performance liquid chromatography as described above, and the β-sheet content was confirmed by circular dichroism (CD). The purified peptides were subjected to electrospray mass spectrometry (Micromass ZQ; Waters) to confirm their compositions and stored as lyophilized powders at −20°C. Prior to being tested, fresh solutions were prepared in water (10 mM acetate buffer for IB-367), briefly vortexed, sonicated, centrifuged, and then diluted in the appropriate medium. Buffers were prepared with double-distilled water. Rifampin and piperacillin were obtained from Sigma. All other reagents were analytical grade.
Bacteria.
Antibacterial activities were assessed against Micrococcus luteus (ATCC 9341), Streptococcus pyogenes group A (ATCC 19615), Staphylococcus aureus (ATCC 25923), clinically isolated methicillin-resistant Staphylococcus aureus, Enterococcus faecalis no. 1 (ATCC 29212), Enterococcus faecalis no. 2 (ATCC 6057), Staphylococcus epidermidis (ATCC 12228), Bacillus cereus (ATCC 11778), Escherichia coli no. 2 (ATCC 25922), Escherichia coli no. 3 (ATCC 35218), clinically isolated Escherichia coli O157 no. 1, Pseudomonas aeruginosa no. 1 (ATCC 9027), Pseudomonas aeruginosa no. 2 (ATCC 27853), environmentally isolated Vibrio cholerae O9, Yersinia kristensenii (ATCC 33639), and Salmonella enterica serovar Typhimurium (ATCC 14028), cultured in LB medium (10 g/liter tryptone, 5 g/liter yeast extract, 5 g/liter NaCl, pH 7.4). Inoculates of 106 bacteria/ml were used. Cell populations were estimated by optical density measurements at 620 nm referred to a calibration curve.
Antibacterial assay.
MICs were determined by microdilution susceptibility testing as described previously (25). Briefly, antibacterial assays were performed using 5 × 105 bacteria/ml in LB medium. The cell populations were estimated by optical density measurements at 620 nm referred to a calibration curve. The bacterial suspension (100 μl) was added to 100 μl of culture medium containing no peptide or various peptide concentrations (serial twofold dilutions, with 50 μM the highest concentration tested) in 96-well plates. Proliferation was determined by optical density measurements after overnight incubation at 37°C.
For coculture experiments, S. aureus and E. coli (105 CFU/ml) were exposed for 1 h to buffer alone or to buffer containing 3 μM of the specified peptide at 37°C and then plated on LB agar (1 liter LB medium, 18.5 g/liter agar) plates for a CFU count.
Hemolysis.
The peptides' membranolytic potentials against human red blood cells (RBC) were determined in terms of the lytic concentration that caused 50% hemolysis (LC50) (Table 1) using 1% hematocrit, as described previously (41). Alternatively (as specified below; see also Fig. 4), a 10% hematocrit was used, and hemolysis was determined as described previously (46).
TABLE 1.
Properties of the parent peptide and its derivatives
| Compound | Designation | Qa | Hb | MIC (μM) for:
|
LC50 (μM) for human RBC | |
|---|---|---|---|---|---|---|
| E. coli | S. aureus | |||||
| K4-S4(1-13)c | P | 5 | 45 | 4.5 ± 1.5 | 9 ± 3 | 50 ± 10 |
| Lysyl-P | K-P | 6 | 43 | 3 ± 0 | 25 ± 0 | 55 ± 10 |
| M4S4(1-13)d | M4-P | 4 | 50 | 25 ± 0 | 6 ± 0 | 20 ± 2 |
| Aminoacetylysyl-P | NC2-K-P | 6 | 46 | 3 ± 0 | >50 | 100 ± 10 |
| Aminobutyrylysyl-P | NC4-K-P | 6 | 46 | 3 ± 0 | >50 | >100 |
| Aminohexylysyl-P | NC6-K-P | 6 | 47 | 3 ± 0 | >50 | >100 |
| Acetyl-M4-P | C2-M4-P | 3 | 53 | >50 | 3 ± 0 | 17 ± 1 |
| Butyryl-M4-P | C4-M4-P | 3 | 55 | >50 | 3 ± 0 | 10 ± 1 |
| Hexanoyl-M4-P | C6-M4-P | 3 | 56 | >50 | 3 ± 0 | 7 ± 1 |
| Cholyl-P | ch-P | 4 | 59 | >50 | 2.2 ± 0.8 | 25 ± 3 |
| Fmoc-P | f-P | 4 | 64 | 18.5 ± 6.5 | 4.5 ± 1.5 | 6 ± 0.5 |
| Succinyl-P | s-P | 3 | 52 | >50 | >50 | 30 ± 2 |
| Succinylauryl-P | sC12-P | 3 | 66 | >50 | >50 | 5 ± 1 |
| Butyrylysyl-P | C4-K-P | 5 | 54 | 6 ± 0 | 12 ± 0 | 70 ± 11 |
| Octylysyl-P | C8-K-P | 5 | 58 | 4.5 ± 1.5 | 4.5 ± 1.5 | 10 ± 1 |
| Aminolaurylysyl-P | NC12-K-P | 6 | 51 | 2.2 ± 0.8 | 1.5 ± 0 | 15 ± 3 |
| Aminolauryl-P | NC12-P | 5 | 53 | 1.5 ± 0 | 0.75 ± 0 | 18 ± 2 |
| Magainin analog | MSI-78 | 10 | 43 | 1.5 ± 0 | 9 ± 3 | 45 ± 5 |
| Protegrin analog | IB-367 | 4 | 42 | 3 ± 0 | 3 ± 0 | 9 ± 3 |
| Rifampin | Rif | NDe | ND | 7.5 ± 0 | 9 ± 3 | ND |
| Piperacillin | Pip | ND | ND | 5.5 ± 0 | 3 ± 0 | ND |
Q, molecular charge at pH 7.
H, indication of molecular hydrophobicity determined by high-performance liquid chromatography (11).
Amino acid sequence: ALWKTLLKKVLKACO-NH2.
Met in position 4.
ND, not determined.
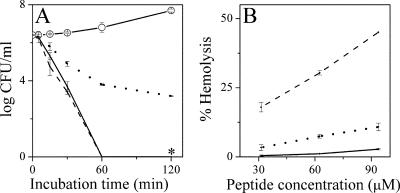
FIG. 4.
Comparative bactericidal kinetics and hemolytic activities. (A) Bactericidal kinetics as determined against E. coli no. 2 at four multiples of the MIC of each peptide: NC6-K-P, solid line; MSI-78, dotted line; IB-367, dashed line; control, line with open circles. (B) Peptide dose-dependent hemoglobin release activity as measured after 1 h of incubation in PBS with washed human erythrocytes (10% hematocrit). The symbols are as in panel A. The asterisks represent negative cultures. The error bars represent standard deviations from the mean determined from two independent experiments performed in duplicate.
Bactericidal kinetics.
Bactericidal kinetics were performed as described previously (50); peptide concentrations were zero or four multiples of the peptides' MICs.
Liposome preparation.
Lipopolysaccharide (LPS)-containing liposomes were prepared as described previously (1). Briefly, a stock solution of E. coli O127:B8 LPS (30 mM; Sigma) in a petroleum ether-chloroform-phenol mixture (8:5:2) was mixed with dried phospholipids (2:1 molar ratio), vacuumed overnight, suspended in phosphate-buffered saline (PBS), heated to 60°C, vortexed, sonicated, and used as a stock solution (30 mM). Large unilamellar vesicles composed of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine-1-palmitoyl-2-oleoyl-sn-glycero-3 phosphoglycerol (POPC-POPG) (3:1 molar ratio) were prepared in PBS by the extrusion method according to the manufacturer's (Avanti Polar) instructions using a LiposoFast-Basic extrusion apparatus (Avestin, Inc.) to give a translucent solution of vesicles with a mean diameter of 100 nm as measured by dynamic light scattering using a BI-200SM Research Goniometer System (Brookhaven Instruments Corp.).
Surface plasmon resonance (SPR).
Peptide binding to phospholipid membranes was determined using an optical biosensor system (BIAcore 2000; BIAcore, Uppsala, Sweden). Experiments, analysis of binding kinetics, and the resulting affinity constants were performed as described previously (16).
CD.
CD spectra were recorded on a model J-810 spectropolarimeter (Jasco, Tokyo, Japan) connected to a Jasco spectrum manager, using a QS Hellma quartz cell of 1 mm path length at 25°C between 190 and 250 nm at a scanning speed of 50 nm/min. The CD spectra were scanned for peptide samples (100 μM) that were dissolved in sodium phosphate buffer in the presence or absence of liposomes with 2 mM POPC-POPG (3:1). Minor contributions of circular differential scattering were eliminated by subtracting the CD spectrum of buffer and liposomes without peptide. The CD data represent average values from three separate recordings.
Peptide assembly in solution.
Peptide self-assembly (aggregation) was investigated by light-scattering measurements as described previously (25).
RESULTS
To evaluate the discriminative effects of hydrophobicity and charge, K4-S4(1-13), whose structural and biological properties are well characterized (11, 14, 25, 41, 43), was selected as a reference peptide. Point substitution and/or addition of hydrophobic and/or charged moieties was performed as detailed in Table 1. Preliminary data on the activities of the resulting derivatives were obtained by evaluating their abilities to inhibit the proliferation of two bacterial strains, S. aureus and E. coli, representatives of gram-positive and gram-negative bacteria, respectively, and to induce hemoglobin leakage from human erythrocytes. Next, representative derivatives were selected to undergo further characterization to establish the scope of selectivity and to evaluate molecular organization and binding properties.
Bacterial growth inhibition and hemolytic activities.
Listed in Table 1 are the physical, antibacterial, and hemolytic properties of the peptides investigated. Nondermaseptin AMPs and antibiotics are also shown for comparison. The properties of the reference peptide were previously established (41) and are presented for comparison: P is twice as efficient against E. coli than against S. aureus (MICs, 4.5 μM and 9 μM, respectively) and displays an LC50 of 50 μM. Note that intravenous injection of P into rats was not toxic at 10 mg peptide per kg of body weight (33).
Increasing the positive charge by adding a lysyl residue at P's N terminus (K-P) resulted in a marginal decrease in hydrophobicity and a marginal decrease in hemolysis. However, this led to enhanced discrimination between bacteria: activity against E. coli was increased (MIC, 3 μM), whereas activity against S. aureus was decreased (MIC, 25 μM). The opposite effect was observed when the molecular charge was reduced and hydrophobicity was enhanced via replacement of lysine in position 4 with methionine (the naturally occurring residue in the parent peptide). This also led to enhanced discrimination between bacteria, but in an inverse manner: activity against E. coli was decreased (MIC, 25 μM), whereas activity against S. aureus was increased (MIC, 6 μM), and hemolysis was substantially increased (LC50, 20 μM).
Additional modifications that further intensified charge/hydrophobicity differences led to further discrimination between bacteria. Thus, addition of aminoacyls (aminoacetyl, aminobutyroyl, or aminohexanoyl) to K-P led to reduced activity against S. aureus (up to the highest concentration tested; MIC > 50 μM) and RBC but maintained activity against E. coli. Conversely, addition of equivalent acyl chains (acetyl, butyroyl, or hexanoyl) to M4-P led to inactivity against E. coli but enhanced activity against S. aureus, as well as against RBC.
Consistent with these findings, increasing P's hydrophobicity through conjugation of bulky hydrophobic moieties (such as cholic acid or Fmoc) led to reduced activity against E. coli and enhanced activity against S. aureus and RBC, whereas increasing P's hydrophobicity combined with reduced charge (through conjugation to negatively charged hydrophobic moieties, such as succinic acid or succinyl-lauric acid) led to reduced activity against both E. coli and S. aureus, but not against RBC. Increasing hydrophobicity while maintaining the charge of K-P derivatives through conjugation of butyroic or octanoic acids led to either marginal or marked differences, depending on their respective hydrophobicity levels. Finally, certain combinations that enhanced hydrophobicity alone or both hydrophobicity and positive charge (e.g., via conjugation of aminolauric acid to either P or K-P) led to enhanced activity against all cell types.
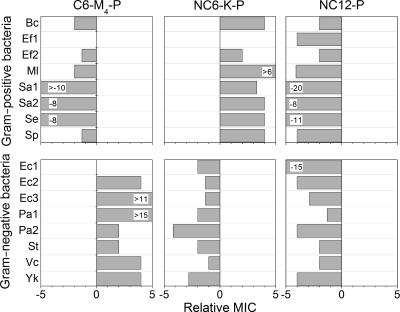
To evaluate the range of the discriminative activities observed with the test bacteria, the MICs of three representative derivatives were determined against a variety of bacterial strains (a total of 14 additional strains) representing either gram-positive or gram-negative bacteria. The derivatives were selected to illustrate either increased hydrophobicity alone (NC12-P), increased hydrophobicity combined with reduced charge (C6-M4-P) or increase of both hydrophobicity and charge (NC6-K-P) relative to P. Figure 1 summarizes the outcomes in terms of relative MICs, defined as the ratio of the average MIC of a derivative to that of P. Derivatives that displayed increased potency (i.e., that had a lower MIC than P) were assigned negative values, and conversely, those that displayed decreased potency were assigned positive values. A MIC identical to that of P was assigned a value of zero for simplicity.
FIG. 1.
Growth-inhibitory activities of representative derivatives against a range of bacterial strains. Relative MIC is a ratio of the average MIC of a derivative to that of P (an identical MIC is assigned a value of zero). Standard deviations for averaged MICs were ≤0.7 μM. Specific values are specified when they were higher or lower than ±5. Bc, Bacillus cereus; Ef, Enterococcus faecalis; Ml, Micrococcus luteus; Sa, Staphylococcus aureus; Se, Staphylococcus epidermidis; Sp, Streptococcus pyogenes; Ec, Escherichia coli; Pa, Pseudomonas aeruginosa; St, Salmonella enterica serovar Typhimurium; Vc, Vibrio cholerae; Yk, Yersinia kristensenii.
With the exception of two strains for which the average MICs remained unchanged, C6-M4-P displayed negative values (up to 10-fold) against gram-positive bacteria but positive values (up to 15-fold) against gram-negative bacteria. Thus, when its hydrophobicity was increased and its positive charge was reduced, P became active predominantly against gram-positive bacteria. Remarkably, a virtually inverse image was obtained with NC6-K-P. NC12-P displayed a nonselective broad-spectrum activity with enhanced potency (up to 20-fold) against all bacteria tested.
Discriminative bactericidal activity in cocultures.
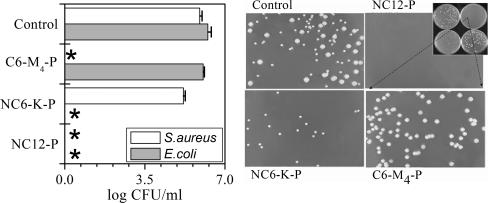
To further evaluate the discriminative abilities of the dermaseptin derivatives, peptides were added to bacterial cultures containing both gram-negative (E. coli) and gram-positive (S. aureus) bacteria. Activity was assessed by counting CFU after 1 hour of exposure to a single concentration (3 μM) of one of the three representative derivatives.
Figure 2 summarizes the outcome of a typical experiment. Plotted on the left are CFU counts obtained from aliquots of cocultures, each treated with the specified compound, while on the right are displayed photographs of the actual petri dishes whose CFU are plotted on the left. The upper left dish displays CFU from the control experiment (culture in the absence of peptide). Bacteria were readily distinguishable (E. coli formed larger white colonies, while S. aureus formed smaller yellowish colonies). In the lower left dish (treated with NC6-K-P), no colonies of E. coli were detected, whereas staphylococcal colonies developed as in the control experiments, indicating discriminative killing of E. coli. Conversely, no colonies of S. aureus were detected in the lower right dish (treated with C6-M4-P), whereas E. coli colonies developed normally. No colonies at all were detected in the upper right dish treated with NC12-P, consistent with a potent but nonselective bactericidal effect.
FIG. 2.
Selective bactericidal activity in coculture. Cocultures of S. aureus and E. coli were exposed to one of the specified peptides (3 μM; 1 h; 37°C) or to buffer (control) and then plated for a CFU count. (Right) A representative set of the resulting plates (inset) and an enlargement of each plate. (Left) Plot of the CFU counts from the plates shown on the right. The asterisks indicate negative cultures. The error bars represent standard deviations of the mean determined from two independent experiments performed in duplicate.
Binding properties.
To investigate the basis for discrimination, the binding properties of P and its derivatives to model membranes were compared. We first determined binding to a negatively charged idealized membrane (POPC-POPG) that mimics bacterial plasma membranes. Next, we assessed how incorporation of LPS in POPC-POPG might affect the binding properties, since the major superficial difference between gram-positive and gram-negative bacteria lies in the external membrane.
The data were unambiguous as to interpretation: the binding constants are summarized in Table 2. Among the acylated derivatives, the binding properties of C6-M4-P were particularly discernible. Despite its reduced charge (−2), its adhesion affinity to the POPC-POPG membrane increased twofold and its insertion affinity increased by an order of magnitude (overall 20-fold-higher apparent affinity) compared with P. This indicated that after the initial adhesion, C6-M4-P has a higher propensity to be incorporated within the bilayer. Conversely, despite its increased charge (+1), NC6-K-P displayed somewhat reduced adhesion affinity. However, although it also had somewhat increased insertion affinity, NC6-K-P displayed an apparent affinity similar to that of P, and despite having an identical charge, NC12-P displayed binding parameters that were significantly higher than those of P. Thus, the binding properties to the negatively charged membrane did not correlate with the peptide's positive charge but with its hydrophobicity.
TABLE 2.
Affinity constants for P and its derivatives determined by SPRa
| Compound | POPC-POPG (3:1)
|
[POPC-POPG]-LPS (1:2)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Kadhesion (104 M−1) | Kinsertion | Kapparent (105 M−1) | χ2 | Kadhesion (104 M−1) | Kinsertion | Kapparent (105 M−1) | χ2 | |
| P | 10.2 ± 0.3 | 1.5 ± 0.2 | 1.5 ± 0.6 | 16.8 | 24.1 ± 0.2 | 2.6 ± 0.2 | 6.4 ± 0.3 | 13 |
| C6-M4-P | 21.0 ± 0.4 | 17 ± 2 | 35.7 ± 0.4 | 40 | 10.2 ± 0.3 | 0.3 ± 0.3 | 0.3 ± 0.3 | 37 |
| NC6-K-P | 5.7 ± 0.4 | 2.1 ± 0.1 | 1.2 ± 0.3 | 17 | 52.0 ± 0.4 | 8.9 ± 0.1 | 47 ± 0.2 | 46 |
| NC12-P | 27.8 ± 0.2 | 4.5 ± 0.3 | 13.0 ± 0.4 | 21.6 | 49.3 ± 0.6 | 7.2 ± 0.1 | 35 ± 0.5 | 45 |
Affinity constants of the interactions as analyzed by local fitting using a two-stage binding model as detailed previously (16). Kadhesion, affinity for adhering to the membrane surface; Kinsertion, insertion affinity (tendency to insert in the membrane [or efficacy of bilayer penetration by the peptide]); Kapparent, apparent affinity constant for the global interaction (Kapparent = Kadhension × Kinsertion). χ2 < 10% of the maximal resonance units.
In the presence of LPS, the findings were reversed in that NC6-K-P and NC12-P displayed enhanced binding parameters but C6-M4-P showed 20-fold-lower apparent affinity than P.
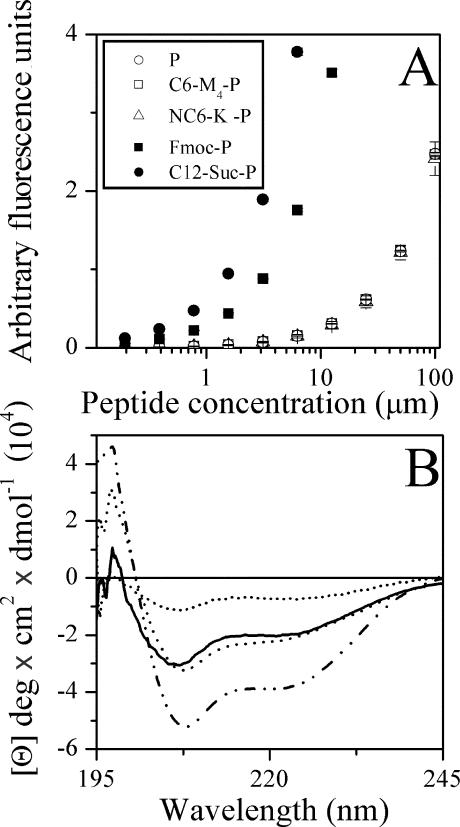
As peptide aggregation in solution—known to occur with hydrophobic peptides—leads to biased binding constants, the peptides' aggregation states were verified by light scattering. Figure 3A depicts the dose-dependent light scattering of P and various derivatives. Highly hydrophobic derivatives, such as C12-Suc-P and Fmoc-P, were found to undergo self-assembly at concentrations estimated at <1 μM (incidentally, the high aggregation state of C12-Suc-P might explain its inactivity toward bacteria and yet potent hemolytic activity, as noted previously [41]), whereas C6-M4-P and NC6-K-P behaved similarly to P, assumed to be monomeric like NC12-P (41).
FIG. 3.
Effects of acylation on peptide organization. (A) Peptide self-assembly in PBS as investigated by light-scattering measurements. The values represent the means from two independent experiments performed in duplicate. The error bars represent standard deviations of the mean. (B) Effects of acylation on peptide secondary structure in the presence of 2 mM POPC-POPG (3:1) liposomes. Shown are far-UV CD spectra of P (dotted line), C6-M4-P (dashed line), NC6-K-P (solid line), and NC12-P (dashed and dotted line). Spectra were scanned for peptide samples of 100 μM in sodium phosphate buffer and expressed as mean residue molar ellipticity (Θ). The plots represent average values from three separate recordings.
Figure 3B shows that binding of P, C6-M4-P, NC6-K-P, and NC12-P to a phospholipid membrane induced a helical conformation, which was enhanced to various extents in acylated derivatives, as observed for other acyls (41), as well.
NC6-K-P is as potent as MSI-78 or IB-367 but not as hemolytic.
The performance of NC6-K-P was compared with those of two intensively studied antimicrobial peptides which were recently evaluated in human clinical trials (2): the alpha-helical 22-residue magainin derivative MSI-78 and the hairpin β-sheet 17-residue protegrin derivative IB-367. The three peptides were compared under identical conditions with respect to their bactericidal kinetics (at four multiples of their respective MICs, as listed in Table 1), as well as their dose-dependent hemoglobin release activities. The results are shown in Fig. 4.
The time-kill curves (Fig. 4A) reveal that IB-367 induced CFU reduction by 6 log units within 60 min of incubation, whereas MSI-78 displayed slower kinetics, reducing CFU counts by 3 log units (even though it demonstrated the highest potency in terms of MIC). Both MIC and kinetic results of the magainin and protegrin derivatives obtained in this study are essentially consistent with those published in the literature (18, 32). The dermaseptin derivative performed similarly to IB-367. Note that a certain variability in the kinetics of all three peptides was observed when different bacterial strains were used, with NC6-K-P often displaying the most rapid kinetics (data not shown). As shown in Fig. 4B, hemolysis of human RBC was most pronounced with IB-367; MSI-78 was considerably less hemolytic, and NC6-K-P was the least hemolytic. Thus, at a concentration corresponding to 10 times the MIC, for example, NC6-K-P displayed 0.4% hemolysis.
DISCUSSION
Unlike conventional drug design, which basically attempts to optimize the fit between a “lock” and its “key,” the design of specific AMPs suffers from a major handicap, as they notoriously use a nonspecific mechanism(s) of action. Since AMP activity is believed to be governed by physicochemical properties (i.e., peptide charge, hydrophobic and amphipathic characteristics, and membrane properties of target cells, e.g., charge distribution and fluidity), this study essentially dealt with the question of to what extent the peptides' properties could be exploited to bestow specificity. A wide range of experimental evidence scattered throughout the literature hints that it might be a considerable extent (4, 11, 12, 21, 22, 26, 29, 36, 44, 49). To our knowledge, this is the first study that considers the characteristics important for specificity between bacterial cells rather than for selectivity between bacterial and mammalian cells. The data show that through changes in its hydrophobicity and charge, P became active predominantly against either gram-positive or gram-negative bacteria. Such specificity was achieved through enhanced potency against one class of bacteria, but also through reduced potency against the other class. Inspection of the results shown in Table 1 reveals two clear trends: modifications that increased the positive charge (while maintaining hydrophobicity) led to enhanced activity against gram-negative bacteria and concomitantly reduced activity against gram-positive bacteria, whereas modifications that enhanced hydrophobicity and concurrently decreased positive charge led to the opposite effect. Various other analogs of P support this view (41).
This study also provides a possible explanation for the observed selective antibacterial activities. CD data indicated a correlation between active acylated peptides and secondary structure. Helical conformation is known to stabilize amphipathic organization, which is critical for the activities of dermaseptins (25, 42) and of many AMPs (35, 45). Acyl-based structure stabilization is plausibly mediated by interaction of the acyl chain with at least the N-terminal residues, thereby affecting amphipathic organization (43). SPR data show certain manipulations of the physicochemical properties of P that lead to enhanced binding affinities. The binding parameters of the reference peptide and NC12-P were previously established and validated by isothermal titration calorimetry experiments (43). These data showed that the binding affinity of P was of an order of magnitude comparable to that of other antimicrobial peptides, while acylation was responsible for increasing the binding affinity. In this respect, the present data suggest that although the new acylated derivatives have increased lipophilic properties, along with increased antibacterial activity, hydrophobic peptides (such as C6-M4-P) do not reach the cytoplasmic membranes of LPS-containing bacteria, being repelled by the hydrophilic nature of the external membrane. In addition, the mechanism by which the modifications that produced NC6-K-P resulted in loss of activity against gram-positive bacteria could be linked to the occurrence of positively charged phospholipids in the cytoplasmic membranes of gram-positive bacteria (37-39), which is likely to reduce the binding affinities of highly cationic AMPs. This is consistent with the observed reduced efficacy of the highly cationic NC6-K-P as opposed to the enhanced efficacy of the less cationic analog, C6-M4-P, against gram-positive bacteria.
In conclusion, the data provided can be useful in developing specific antimicrobial drugs. Compared with optimized AMPs (MSI-78 and IB-367), some dermaseptin derivatives performed at least similarly but were less hemolytic. This study revealed a short derivative (NC6-K-P) that was at least as potent but more selective, both in terms of discrimination between gram-negative and gram-positive bacteria and in terms of discrimination between bacteria and human erythrocytes. Similar studies may reveal potent and safe antimicrobial compounds (peptide or peptidomimetic) for various antimicrobial applications and possibly suitable for systemic administration.
Acknowledgments
This research was supported by the Israel Science Foundation (grant 387/03).
REFERENCES
- 1.Allende, D., and T. J. McIntosh. 2003. Lipopolysaccharides in bacterial membranes act like cholesterol in eukaryotic plasma membranes in providing protection against melittin-induced bilayer lysis. Biochemistry 42:1101-1108. [DOI] [PubMed] [Google Scholar]
- 2.Andres, E., and J. L. Dimarcq. 2005. Clinical development of antimicrobial peptides. Int. J. Antimicrob. Agents 25:448-449. [DOI] [PubMed] [Google Scholar]
- 3.Balaban, N., Y. Gov, A. Giacometti, O. Cirioni, R. Ghiselli, F. Mocchegiani, F. Orlando, G. D'Amato, V. Saba, G. Scalise, S. Bernes, and A. Mor. 2004. A chimeric peptide composed of a dermaseptin derivative and an RNA III-inhibiting peptide prevents graft-associated infections by antibiotic-resistant staphylococci. Antimicrob. Agents Chemother. 48:2544-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blondelle, S. E., and R. A. Houghten. 1991. Hemolytic and antimicrobial activities of the twenty-four individual omission analogues of melittin. Biochemistry 30:4671-4678. [DOI] [PubMed] [Google Scholar]
- 5.Blondelle, S. E., and K. Lohner. 2000. Combinatorial libraries: a tool to design antimicrobial and antifungal peptide analogues having lytic specificities for structure-activity relationship studies. Biopolymers 55:74-87. [DOI] [PubMed] [Google Scholar]
- 6.Blondelle, S. E., E. Takahashi, R. A. Houghten, and E. Perez-Paya. 1996. Rapid identification of compounds with enhanced antimicrobial activity by using conformationally defined combinatorial libraries. Biochem. J. 313:141-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand, G. D., J. R. Leite, L. P. Silva, S. Albuquerque, M. V. Prates, R. B. Azevedo, V. Carregaro, J. S. Silva, V. C. Sa, R. A. Brandao, and C. Bloch, Jr. 2002. Dermaseptins from Phyllomedusa oreades and Phyllomedusa distincta. Anti-Trypanosoma cruzi activity without cytotoxicity to mammalian cells. J. Biol. Chem. 277:49332-49340. [DOI] [PubMed] [Google Scholar]
- 8.Chalk, R., H. Townson, and P. J. Ham. 1995. Brugia pahangi: the effects of cecropins on microfilariae in vitro and in Aedes aegypti. Exp. Parasitol. 80:401-406. [DOI] [PubMed] [Google Scholar]
- 9.Chen, T., L. Tang, and C. Shaw. 2003. Identification of three novel Phyllomedusa sauvagei dermaseptins (sVI-sVIII) by cloning from a skin secretion-derived cDNA library. Regul. Pept. 116:139-146. [DOI] [PubMed] [Google Scholar]
- 10.Chicharro, C., C. Granata, R. Lozano, D. Andreu, and L. Rivas. 2001. N-terminal fatty acid substitution increases the leishmanicidal activity of CA(1-7)M(2-9), a cecropin-melittin hybrid peptide. Antimicrob. Agents Chemother. 45:2441-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagan, A., L. Efron, L. Gaidukov, A. Mor, and H. Ginsburg. 2002. In vitro antiplasmodium effects of dermaseptin S4 derivatives. Antimicrob. Agents Chemother. 46:1059-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dathe, M., T. Wieprecht, H. Nikolenko, L. Handel, W. L. Maloy, D. L. MacDonald, M. Beyermann, and M. Bienert. 1997. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett. 403:208-212. [DOI] [PubMed] [Google Scholar]
- 13.Efron, L., A. Dagan, L. Gaidukov, H. Ginsburg, and A. Mor. 2002. Direct interaction of dermaseptin S4 aminoheptanoyl derivative with intraerythrocytic malaria parasite leading to increased specific antiparasitic activity in culture. J. Biol. Chem. 277:24067-24072. [DOI] [PubMed] [Google Scholar]
- 14.Feder, R., A. Dagan, and A. Mor. 2000. Structure-activity relationship study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity. J. Biol. Chem. 275:4230-4238. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich, C. L., D. Moyles, T. J. Beveridge, and R. E. Hancock. 2000. Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria. Antimicrob. Agents Chemother. 44:2086-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaidukov, L., A. Fish, and A. Mor. 2003. Analysis of membrane-binding properties of dermaseptin analogues: relationships between binding and cytotoxicity. Biochemistry 42:12866-12874. [DOI] [PubMed] [Google Scholar]
- 17.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 18.Ge, Y., D. L. MacDonald, K. J. Holroyd, C. Thornsberry, H. Wexler, and M. Zasloff. 1999. In vitro antibacterial properties of pexiganan, an analog of magainin. Antimicrob. Agents Chemother. 43:782-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock, R. E. 2001. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 1:156-164. [DOI] [PubMed] [Google Scholar]
- 20.Hariton-Gazal, E., R. Feder, A. Mor, A. Graessmann, R. Brack-Werner, D. Jans, C. Gilon, and A. Loyter. 2002. Targeting of nonkaryophilic cell-permeable peptides into the nuclei of intact cells by covalently attached nuclear localization signals. Biochemistry 41:9208-9214. [DOI] [PubMed] [Google Scholar]
- 21.Harwig, S. S., A. Waring, H. J. Yang, Y. Cho, L. Tan, and R. I. Lehrer. 1996. Intramolecular disulfide bonds enhance the antimicrobial and lytic activities of protegrins at physiological sodium chloride concentrations. Eur. J. Biochem. 240:352-357. [DOI] [PubMed] [Google Scholar]
- 22.Houston, M. E., Jr., L. H. Kondejewski, D. N. Karunaratne, M. Gough, S. Fidai, R. S. Hodges, and R. E. Hancock. 1998. Influence of preformed alpha-helix and alpha-helix induction on the activity of cationic antimicrobial peptides. J. Pept. Res. 52:81-88. [DOI] [PubMed] [Google Scholar]
- 23.Huang, H. W. 2000. Action of antimicrobial peptides: two-state model. Biochemistry 39:8347-8352. [DOI] [PubMed] [Google Scholar]
- 24.Krugliak, M., R. Feder, V. Y. Zolotarev, L. Gaidukov, A. Dagan, H. Ginsburg, and A. Mor. 2000. Antimalarial activities of dermaseptin S4 derivatives. Antimicrob. Agents Chemother. 44:2442-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kustanovich, I., D. E. Shalev, M. Mikhlin, L. Gaidukov, and A. Mor. 2002. Structural requirements for potent versus selective cytotoxicity for antimicrobial dermaseptin S4 derivatives. J. Biol. Chem. 277:16941-16951. [DOI] [PubMed] [Google Scholar]
- 26.Larrick, J. W., M. Hirata, Y. Shimomoura, M. Yoshida, H. Zheng, J. Zhong, and S. C. Wright. 1993. Antimicrobial activity of rabbit CAP18-derived peptides. Antimicrob. Agents Chemother. 37:2534-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lockwood, N. A., J. R. Haseman, M. V. Tirrell, and K. H. Mayo. 2004. Acylation of SC4 dodecapeptide increases bactericidal potency against Gram-positive bacteria, including drug-resistant strains. Biochem. J. 378:93-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mai, J. C., Z. Mi, S. H. Kim, B. Ng, and P. D. Robbins. 2001. A proapoptotic peptide for the treatment of solid tumors. Cancer Res. 61:7709-7712. [PubMed] [Google Scholar]
- 29.Maloy, W. L., and U. P. Kari. 1995. Structure-activity studies on magainins and other host defense peptides. Biopolymers 37:105-122. [DOI] [PubMed] [Google Scholar]
- 30.Merrifield, R. B., P. Juvvadi, D. Andreu, J. Ubach, A. Boman, and H. G. Boman. 1995. Retro and retroenantio analogs of cecropin-melittin hybrids. Proc. Natl. Acad. Sci. USA 92:3449-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mor, A., and P. Nicolas. 1994. Isolation and structure of novel defensive peptides from frog skin. Eur. J. Biochem. 219:145-154. [DOI] [PubMed] [Google Scholar]
- 32.Mosca, D. A., M. A. Hurst, W. So, B. S. C. Viajar, C. A. Fujii, and T. J. Falla. 2000. IB-367, a protegrin peptide with in vitro and in vivo activities against the microflora associated with oral mucositis. Antimicrob. Agents Chemother. 44:1803-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navon-Venezia, S., R. Feder, L. Gaidukov, Y. Carmeli, and A. Mor. 2002. Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob. Agents Chemother. 46:689-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papo, N., A. Braunstein, Z. Eshhar, and Y. Shai. 2004. Suppression of human prostate tumor growth in mice by a cytolytic d-, l-amino acid peptide: membrane lysis, increased necrosis, and inhibition of prostate-specific antigen secretion. Cancer Res. 64:5779-5786. [DOI] [PubMed] [Google Scholar]
- 35.Park, C. B., K. S. Yi, K. Matsuzaki, M. S. Kim, and S. C. Kim. 2000. Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc. Natl. Acad. Sci. USA 97:8245-8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peck-Miller, K. A., J. Blake, W. L. Cosand, R. P. Darveau, and H. P. Fell. 1994. Structure-activity analysis of the antitumor and hemolytic properties of the amphiphilic alpha-helical peptide, C18G. Int. J. Pept. Protein Res. 44:143-151. [DOI] [PubMed] [Google Scholar]
- 37.Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10:179-186. [DOI] [PubMed] [Google Scholar]
- 38.Peschel, A., R. W. Jack, M. Otto, L. V. Collins, P. Staubitz, G. Nicholson, H. Kalbacher, W. F. Nieuwenhuizen, G. Jung, A. Tarkowski, K. P. van Kessel, and J. A. van Strijp. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peschel, A., M. Otto, R. W. Jack, H. Kalbacher, G. Jung, and F. Gotz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 40.Pouny, Y., D. Rapaport, A. Mor, P. Nicolas, and Y. Shai. 1992. Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues with phospholipid membranes. Biochemistry 31:12416-12423. [DOI] [PubMed] [Google Scholar]
- 41.Radzishevsky, I. S., S. Rotem, F. Zaknoon, L. Gaidukov, A. Dagan, and A. Mor. 2005. Effects of acyl versus aminoacyl conjugation on the properties of antimicrobial peptides. Antimicrob. Agents Chemother. 49:2412-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shalev, D. E., A. Mor, and I. Kustanovich. 2002. Structural consequences of carboxyamidation of dermaseptin S3. Biochemistry 41:7312-7317. [DOI] [PubMed] [Google Scholar]
- 43.Shalev, D. E., S. Rotem, and A. Mor. 2006. Consequences of N-acylation on structure and membrane binding properties of dermaseptin derivative K4S4(1-13). J. Biol. Chem. 281:9432-9438. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu, M., Y. Shigeri, Y. Tatsu, S. Yoshikawa, and N. Yumoto. 1998. Enhancement of antimicrobial activity of neuropeptide Y by N-terminal truncation. Antimicrob. Agents Chemother. 42:2745-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tossi, A., L. Sandri, and A. Giangaspero. 2000. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4-30. [DOI] [PubMed] [Google Scholar]
- 46.Tossi, A., M. Scocchi, M. Zanetti, R. Gennaro, P. Storici, and D. Romeo. 1997. An approach combining rapid cDNA amplification and chemical synthesis for the identification of novel, cathelicidin-derived, antimicrobial peptides. Methods Mol. Biol. 78:133-150. [DOI] [PubMed] [Google Scholar]
- 47.Wu, M., E. Maier, R. Benz, and R. E. Hancock. 1999. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 38:7235-7242. [DOI] [PubMed] [Google Scholar]
- 48.Wu, Z., F. Cocchi, D. Gentles, B. Ericksen, J. Lubkowski, A. Devico, R. I. Lehrer, and W. Lu. 2005. Human neutrophil alpha-defensin 4 inhibits HIV-1 infection in vitro. FEBS Lett. 579:162-166. [DOI] [PubMed] [Google Scholar]
- 49.Yan, H., S. Li, X. Sun, H. Mi, and B. He. 2003. Individual substitution analogs of Mel(12-26), melittin's C-terminal 15-residue peptide: their antimicrobial and hemolytic actions. FEBS Lett. 554:100-104. [DOI] [PubMed] [Google Scholar]
- 50.Yaron, S., T. Rydlo, D. Shachar, and A. Mor. 2003. Activity of dermaseptin K-4-S4 against foodborne pathogens. Peptides 24:1815-1821. [DOI] [PubMed] [Google Scholar]
- 51.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]
- 52.Zasloff, M., B. Martin, and H. C. Chen. 1988. Antimicrobial activity of synthetic magainin peptides and several analogues. Proc. Natl. Acad. Sci. USA 85:910-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, L., and R. E. Hancock. 2000. Peptide antibiotics, p. 209-232. In D. Hughes and D. I. Andersson (ed.), Antibiotic resistance and antibiotic development. Harwood Academic, Amsterdam, The Netherlands.