Abstract
A plasmid-encoded class II transposon element was identified in a carbapenem-resistant Pseudomonas putida isolate. Tn1332, closely related to Tn1331, harbored the metallo-β-lactamase gene blaVIM-2 in addition to four other antibiotic resistance genes, aacA4, aadA1, blaOXA-9, and blaTEM-1, and two novel insertion sequences, ISPpu17 and ISPpu18.
Pseudomonas putida is a gram-negative aerobe rarely involved in human infections and considered, as opposed to Pseudomonas aeruginosa, to be a low-grade pathogen (22). Resistance to carbapenems in P. putida may be due to metallo-β-lactamases (MBLs) such as IMP-1, IMP-12, VIM-1, VIM-2, and VIM-6 (4-7, 9, 14, 16). These MBL-encoding genes are part of gene cassettes located in class 1 integron structures (21).
The blaVIM-2 gene was first identified from a P. aeruginosa isolate recovered in 1996 from Marseilles, France (13). Further studies showed that a single blaVIM-2-positive isolate had disseminated in the hematology unit where it had initially been identified (1). The blaVIM-2 gene cassette was always identified throughout the world as part of class 1 integrons varying in size and structure and is now the most prevalent carbapenemase gene (10, 12, 21).
The aim of our study was to analyze the β-lactamase content of a carbapenem-resistant P. putida strain that was isolated from the same hospitalization unit as that mentioned above and that had carbapenemase activity. P. putida strain 9335 was isolated in January 2004 from bronchial aspirate and several blood cultures of a 59-year-old immunocompromised patient treated with an imipenem-containing regimen for 5 days. P. putida 9335 was identified using the API 32GN system (bioMérieux, Marcy-L'Etoile, France) and confirmed by 16S rRNA gene sequencing. P. putida 9335 was resistant to all β-lactams, including meropenem and imipenem (Table 1). It was also resistant to aminoglycosides, fluoroquinolones, sulfonamides, and chloramphenicol and remained susceptible only to colistin and rifampin.
TABLE 1.
MICs of β-lactams for P. putida 9335, P. putida reference strain CIP104063, E. coli DH10B harboring recombinant plasmid p9335H, and E. coli reference strain DH10B
| β-Lactam | MIC (μg/ml) for:
|
|||
|---|---|---|---|---|
| P. putida 9335 | P. putida CIP104063b | E. coli DH10B(p9335H) | E. coli DH10B | |
| Amoxicillin | >512 | >512 | >512 | 4 |
| Ticarcillin | >512 | >512 | >512 | 4 |
| Piperacillin | 128 | 16 | 512 | 1 |
| Piperacillin + TZBa | 128 | 16 | 512 | 1 |
| Cefuroxime | >512 | >512 | >512 | 2 |
| Ceftazidime | 256 | 2 | 32 | 0.06 |
| Cefotaxime | >512 | >512 | 64 | 0.12 |
| Cefepime | 128 | 2 | 0.25 | 0.06 |
| Cefoxitin | >512 | 512 | 512 | 4 |
| Moxalactam | >512 | >512 | >512 | 0.06 |
| Aztreonam | 256 | 8 | 0.25 | 0.12 |
| Imipenem | 64 | 0.5 | 1 | 0.06 |
| Meropenem | 64 | 1 | 2 | 0.12 |
TZB, tazobactam at a fixed concentration of 4 μg/ml.
Expressing a wild-type β-lactam resistance profile.
Production of an MBL was revealed by using Etest strips with imipenem and EDTA (AB Biodisk, Solna, Sweden) (20). Whole-cell DNA of P. putida 9335 was extracted as described previously (11) and used as a template in PCR, followed by cloning experiments. Cloning of the blaVIM-2 gene into Escherichia coli DH10B was performed by using HindIII-restricted genomic DNA of P. putida 9335 that was subsequently ligated into HindIII-restricted pBK-CMV phagemid (Stratagene, Amsterdam, The Netherlands), and the recombinant plasmid p9335H expressing VIM-2 (Table 1) was selected as described previously (11).
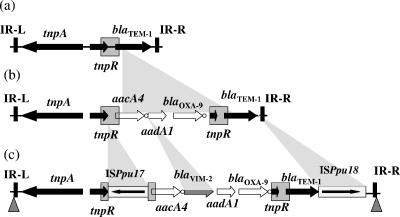
The nucleotide sequence of the ca. 14-kb insert of plasmid p9335H was determined. It contained an 11,172-bp-long transposon termed Tn1332, closely related to Tn1331 and belonging to the Tn3 family, that had been identified previously in a Klebsiella pneumoniae isolate from Argentina (17). Tn1331 is closely related to Tn3, with an additional 3-kb fragment containing several antibiotic resistance genes, namely, aacA4, aadA1, and blaOXA-9, but does not possess any attI1 site (Fig. 1) (3). As reported for Tn1331, the aadA1 and blaOXA-9 gene cassettes are fused as a single gene cassette that may have arisen as a consequence of a recombination event involving two integrons (15). The first five amino acids of the leader peptide of β-lactamase TEM-1 were fused to the AAC(6′)-Ib protein (2), with this fusion likely being the consequence of a 520-bp duplication, including part of tnpR and the 5′ part of the blaTEM-1 gene, during the genesis of Tn1331 (15, 17, 18). Compared to Tn1331, Tn1332 carried the blaVIM-2 gene cassette that had been inserted between the aacA4 and aadA1 aminoglycoside resistance gene cassettes (Fig. 1). In addition, Tn1332 carried two novel insertion sequences, ISPpu17 and ISPpu18 (Fig. 1). ISPpu17, an IS30 family member, is 1,066 bp long, and its transposase (321 amino acids) shares 44% identity with that of ISPlu1 from Photorhabdus luminescens. The inverted repeats (IRs) of ISPpu17 are 22 bp long, and transposition of ISPpu17 generated a 3-bp duplication at its insertion site. ISPpu18, a member of the IS4 family, is 1,192 bp long and encodes a 326-amino-acid transposase which shares 94% protein identity with ISPre2 from Pseudomonas resinovorans and with IS1384 located on a plasmid from P. putida. The IRs of ISPpu18 are 12 bp long, and transposition of ISPpu18 generated a 4-bp duplication.
FIG. 1.
Structure comparison of Tn3 (a), Tn1331 (20) (b), and Tn1332 (c). The arrows indicate the locations of the genes (tnpA, tnpR, aacA4, blaVIM-2, aadA1, blaOXA-9, and blaTEM-1). The white circles indicate 59-be associated with genes (aacA4, blaVIM-2, and blaOXA-9). The left terminal inverted repeat (IR-L) and right terminal inverted repeat (IR-R) sequences of transposon Tn1332 are indicated as black vertical rectangles. The gray boxes represent the 520-bp tnpR repeat. The ISPpu17 and ISPpu18 elements are shown as boxes, with arrows indicating orientations of transcription of their tnpA genes. The target site duplication generated by Tn1332 transposition is indicated by gray triangles.
Immediately upstream and downstream of the 38-bp-long IRs of Tn1332, a 5-bp duplication was identified that was the signature of the transposition process for blaVIM-2 acquisition. The common promoter sequences present in the 5′ conserved region of class 1 integrons and responsible for the expression of gene cassettes were absent (8). Thus, the promoter sequences enhancing blaVIM-2 expression in Tn1332 might be the same as those described for Tn1331 that enhanced blaTEM-1 gene expression, being part of the 520-bp direct repeats upstream of the aacA4 gene (19). However, the presence of ISPpu17 in Tn1332 might also be the source of additional promoter sequences involved in expression of the blaVIM-2 gene (Fig. 1).
Analytical isoelectric focusing was performed with β-lactamase extracts of cultures of P. putida 9335 and E. coli DH10B(p9335H), as described previously (11). Three identical pI values were visualized from both extracts that confirmed that all β-lactamase genes were expressed. Two bands were detected at pI values of 5.4 and 6.9, consistent with the expression of β-lactamases TEM-1 and OXA-9 (2), respectively, whereas a pI value of 5.6 corresponded to expression of VIM-2 (13; data not shown).
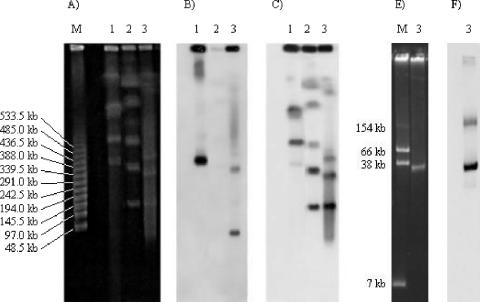
A pulsed-field gel electrophoresis analysis was performed as described previously (1), followed by an I-CeuI digestion and a Southern blot hybridization (13) analysis for determination of the precise genetic location of the blaVIM-2 gene in P. putida 9335. Chromosomal DNAs from VIM-2-positive P. aeruginosa isolate COL-1 (13) and from the P. putida CIP104063 reference strain were included. The membrane was successively hybridized with probes for blaVIM-2 and 16S and 23S rRNA genes, as described previously (13). A strong hybridization signal was detected with the blaVIM-2-specific probe that did not cohybridize with the RNA-specific probe (Fig. 2). Plasmid content of the P. putida 9335 isolate identified a ca. 30-kb plasmid by a positive signal with the blaVIM-2 probe after Southern blot hybridization (Fig. 2). However, attempts to transfer the β-lactam resistance marker into E. coli DH10B and P. aeruginosa PU21 by electroporation failed (13).
FIG. 2.
(A) Pulsed-field gel electrophoresis profiles of I-CeuI digestion of whole-cell DNAs of Pseudomonas strains. Lane M, molecular size marker (band sizes are in kilobase pairs); lane 1, P. aeruginosa COL-1 (blaVIM-2 positive) (13); lane 2, P. putida reference strain CIP104063 (blaVIM-2 negative); lane 3, P. putida 9335 (blaVIM-2 positive) (this work). Southern hybridization was performed with a specific internal probe for the blaVIM-2 gene (B) and a probe for the 16S-23S rRNA gene (C). (E) Plasmid analysis. Lane M, E. coli 50192 (band sizes are in kb); lane 3, P. putida 9335. (F) Results of a Southern hybridization performed with probe for blaVIM-2.
We report here another transposon structure likely at the origin of blaVIM-2 acquisition that did not directly involve an integron structure. Surprisingly, whereas transfer of an identical blaVIM-2-carrying genetic structure from P. aeruginosa to P. putida could have been expected, it was not the case here. These findings underline the important genetic plasticity at the origin of acquisition and dissemination of MBL genes.
Nucleotide sequence accession number.
The entire nucleotide sequence of Tn1332 reported in this work has been assigned to the GenBank and EMBL databases under the accession number DQ174113. The two insertion sequences identified, ISPpu17 and ISPpu18, have been registered in the IS database (http://www-is.biotoul.fr/).
Acknowledgments
This work was funded by a grant from the Ministère de l'Education Nationale et de la Recherche (UPRES-EA3539), Université Paris XI, France, and mostly by the European Community 6th PCRD, LSHM-CT-2005-018705. L.P. is a researcher from the INSERM, Paris, France.
REFERENCES
- 1.Aubron, C., L. Poirel, N. Fortineau, L. Collet, and P. Nordmann. 2005. Nosocomial spread of Pseudomonas aeruginosa isolates expressing the metallo-β-lactamase VIM-2 in an haematology unit of a French hospital. Microb. Drug Resist. 11:254-259. [DOI] [PubMed] [Google Scholar]
- 2.Bojorquez, D., M. Belei, S. F. Delira, S. Sholly, J. Mead, and M. E. Tolmasky. 1998. Characterization of OXA-9, a β-lactamase encoded by the multiresistance transposon Tn1331. Cell. Mol. Biol. (Noisy-Le-grand) 44:483-491. [PubMed] [Google Scholar]
- 3.Dery, K. J., B. Soballe, M. S. Witherspoon, D. Bui, R. Koch, D. J. Sherratt, and M. E. Tolmasky. 2003. The aminoglycoside 6′-N-acetyltransferase type Ib encoded by Tn1331 is evenly distributed within the cell's cytoplasm. Antimicrob. Agents Chemother. 47:2897-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Docquier, J. D., M. L. Riccio, C. Mugnaioli, F. Luzzaro, A. Endimiani, A. Toniolo, G. Amicosante, and G. M. Rossolini. 2003. IMP-12, a new plasmid-encoded metallo-β-lactamase from a Pseudomonas putida clinical isolate. Antimicrob. Agents Chemother. 47:1522-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiett, J., A. Baraniak, A. Mrówka, M. Fleischer, Z. Drulis-Kawa, L. Naumiuk, A. Samet, W. Hryniewicz, and M. Gniadkowski. 2006. Molecular epidemiology of acquired-metallo-β-lactamase-producing bacteria in Poland. Antimicrob. Agents Chemother. 50:880-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh, T. H., G. C. Wang, and L.-H. Sng. 2004. IMP-1 and a novel metallo-β-lactamase, VIM-6, in fluorescent pseudomonads isolated in Singapore. Antimicrob. Agents Chemother. 48:2334-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, K., J. B. Lim, J. H. Yum, D. Yong, Y. Chong, J. M. Kim, and D. M. Livermore. 2002. blaVIM-2 cassette-containing novel integrons in metallo-β-lactamase-producing Pseudomonas aeruginosa and Pseudomonas putida isolates disseminated in a Korean hospital. Antimicrob. Agents Chemother. 46:1053-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lévesque, C., L. Piche, C. Larose, and P. H. Roy. 1995. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 39:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lombardi, G., F. Luzzaro, J. D. Docquier, M. L. Riccio, M. Perilli, A. Coli, G. Amicosante, G. M. Rossolini, and A. Toniolo. 2002. Nosocomial infections caused by multidrug-resistant isolates of Pseudomonas putida producing VIM-1 metallo-β-lactamase. J. Clin. Microbiol. 40:4051-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pallecchi, L., M. L. Riccio, J. D. Docquier, R. Fontana, and G. M. Rossolini. 2001. Molecular heterogeneity of blaVIM-2-containing integrons from Pseudomonas aeruginosa plasmids encoding the VIM-2 metallo-β-lactamase. FEMS Microbiol. Lett. 195:145-150. [DOI] [PubMed] [Google Scholar]
- 11.Poirel, L., M. Guibert, D. Girlich, T. Naas, and P. Nordmann. 1999. Cloning, sequence analyses, expression, and distribution of ampC-ampR from Morganella morganii clinical isolates. Antimicrob. Agents Chemother. 43:769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poirel, L., T. Lambert, S. Türkoglü, E. Ronco, J.-L. Gaillard, and P. Nordmann. 2001. Characterization of class 1 integrons from Pseudomonas aeruginosa that contain the blaVIM-2 carbapenem-hydrolyzing β-lactamase gene and of two novel aminoglycoside resistance gene cassettes. Antimicrob. Agents Chemother. 45:546-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poirel, L., T. Naas, D. Nicolas, L. Collet, S. Bellais, J. D. Cavallo, and P. Nordmann. 2000. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 44:891-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riccio, M. L., L. Pallecchi, J. D. Docquier, S. Cresti, M. R. Catania, L. Pagani, C. Lagatolla, G. Cornaglia, R. Fontana, and G. M. Rossolini. 2005. Clonal relatedness and conserved integron structures in epidemiologically unrelated Pseudomonas aeruginosa strains producing the VIM-1 metallo-β-lactamase from different Italian hospitals. Antimicrob. Agents Chemother. 49:104-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarno, R., G. McGillivary, D. J. Sherratt, L. A. Actis, and M. E. Tolmasky. 2002. Complete nucleotide sequence of Klebsiella pneumoniae multiresistance plasmid pJHCMW1. Antimicrob. Agents Chemother. 46:3422-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibata, N., Y. Doi, K. Yamane, T. Yagi, H. Kurokawa, K. Shibayama, H. Kato, K. Kai, and Y. Arakawa. 2003. PCR typing of genetic determinants for metallo-β-lactamases and integrases carried by gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J. Clin. Microbiol. 41:5407-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolmasky, M. E. 1990. Sequencing and expression of aadA, bla, and tnpR from the multiresistance transposon Tn1331. Plasmid 24:218-226. [DOI] [PubMed] [Google Scholar]
- 18.Tolmasky, M. E. 2000. Bacterial resistance to aminoglycosides and β-lactams: the Tn1331 transposon paradigm. Front. Biosci. 5:D20-D29. [DOI] [PubMed] [Google Scholar]
- 19.Tolmasky, M. E., and J. H. Crosa. 1993. Genetic organization of antibiotic resistance genes (aac(6′)-Ib, aadA, and oxa9) in the multiresistance transposon Tn1331. Plasmid 29:31-40. [DOI] [PubMed] [Google Scholar]
- 20.Walsh, T. R., A. Bolmström, A. Qwarnström, and A. Gales. 2002. Evaluation of a new Etest for detecting metallo-β-lactamases in routine clinical testing. J. Clin. Microbiol. 40:2755-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh, T. R., M. A. Toleman, L. Poirel, and P. Nordmann. 2005. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang, C. H., T. Young, M. Y. Peng, and M. C. Weng. 1996. Clinical spectrum of Pseudomonas putida infection. J. Formos. Med. Assoc. 95:754-761. [PubMed] [Google Scholar]