Abstract
A series of azasterol derivatives, designed as potential inhibitors of the Δ24-sterol methyltransferase enzyme (24-SMT), were synthesized and evaluated for their activities against parasitic protozoa. Values in the nanomolar range were obtained for 50% effective dose against the Trypanosoma brucei subsp. rhodesiense bloodstream form cultured in vitro. In order to investigate the mode of action, Trypanosoma brucei subsp. brucei 24-SMT was cloned and overexpressed and compounds were assayed for inhibitory activity. None of the inhibitors tested appeared to be active against the enzyme. Sterol composition analysis showed that only cholestane type sterols are present in membranes of bloodstream forms while ergosterol is a major component of procyclic sterol extracts. Interestingly, Northern blot analysis showed the presence of 24-SMT mRNA in both the procyclic and the bloodstream forms of the parasite, although levels of mRNA were threefold lower in the latter. Likewise, Western blot analysis and activity determinations evidenced the existence of active enzyme in both forms of the parasite. We conclude that the designed compounds act at sites other than 24-SMT in Trypanosoma brucei.
Diseases caused by parasitic protozoa affect many people in large areas of the world (24). Among diseases caused by the protozoa of the Trypanosomatidae family, Chagas' disease (American trypanosomiasis), sleeping sickness (human African trypanosomiasis), and leishmaniasis are of importance. The cure of these diseases depends largely on chemotherapy (4). Although several antiparasitic drugs are available, toxic side effects and development of drug resistance are often associated with them. Therefore, new, effective drugs have to be found.
The sterol biosynthetic pathway presents opportunities for the design of anti-infective agents (1, 15, 19). Ergosterol and 24-alkylated sterols are major components in the cell membranes of fungi and plants and also of Leishmania spp. and Trypanosoma cruzi. This contrasts to the situation in mammalian cells, where cholesterol is the principle steroid found. In the case of Trypanosoma brucei, the procyclic form of the parasite has been reported to biosynthesize ergosterol and other 24-alkylated sterols (2, 3), while in the bloodstream form (BSF), the parasite is reported to scavenge cholesterol from the host with uptake from LDL receptors. These differences in sterol metabolism between the parasites and mammalian cells are clearly important and could be exploited as an antiparasitic drug target.
Biosynthesis of ergosterol and related sterols requires alkylation of the 24 position of the side chain, a step not found in cholesterol biosynthesis. This step is catalyzed by S-adenosyl-l-methionine:Δ24-sterol methyltransferase (24-SMT), which is a key difference between cholesterol and ergosterol biosynthesis. Azasterols have been shown to cause inhibition of 24-SMT; examples are given in references 10, 11, 13, 16, 20, and 22. The supposed mechanism of action of 24-SMT (18) goes through a carbocationic intermediate which could be mimicked by azasterols protonated at physiological pH (10). However, some azasterols have also been reported to inhibit the sterol 24-reductase, an enzyme involved in biosynthesis of cholesterol. This latter effect leads to a buildup of steroid precursors to toxic concentrations (17).
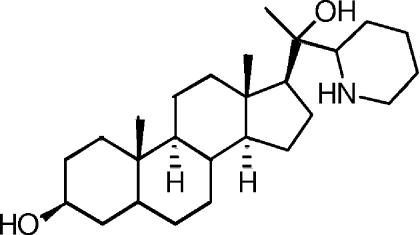
Previously, we (9, 16, 21-23) and others (5) reported the activity of 22,26-azasterol (AZA) (Fig. 1) as an agent against Leishmania and T. cruzi. AZA was found to be active against both promastigote/epimastigote and intracellular amastigote forms of the parasites and has been shown to give parasitological cure in a rodent model of Chagas' disease (21). Encouraged by those findings, we decided to extend the range of azasterols; some of these compounds were active against bloodstream form Trypanosoma brucei subsp. rhodesiense, the causative organism of human African trypanosomiasis (7, 8). In the present paper, we report some new analogues, their biological evaluation, and studies on their mode of action.
FIG. 1.
Structure of 22,26-azasterol (AZA).
MATERIALS AND METHODS
Materials.
Chemicals, reagents, and solvents were purchased from Aldrich, Sigma, Lancaster, Merck, Fisher, or Steraloids and employed without further purification, unless otherwise stated. Genomic DNA from the Trypanosoma brucei subsp. brucei 427 strain was used for 24-SMT cloning. Fetal calf serum was obtained from Gibco. Trypanosoma brucei subsp. rhodesiense STIB900 and Trypanosoma brucei subsp. brucei 427 were used in growth inhibition assays.
Inhibitor synthesis.
Nuclear magnetic resonance spectra were obtained with a Bruker Avance DPX 300-MHz spectrometer at 300 MHz for 1H and 75 MHz for 13C. Mass spectra and exact mass measurements were performed on a Waters ZQ4000 and a Finnigan MAT 95XP, respectively. Precoated Merck silica gel F254 plates were used for thin-layer chromatography, and spots were examined with phosphomolybdic acid (0.5% in ethanol) solution. Column chromatography was performed on silica gel 60 (0.035 to 0.070 mm). The 1H and 13C nuclear magnetic resonance spectra allowed the characterization of all purified intermediates in the synthesis and final products. The full synthetic details are described elsewhere (4a).
Growth inhibition of Trypanosoma brucei subsp. rhodesiense and Trypanosoma brucei subsp. brucei.
Trypanosoma brucei subsp. rhodesiense STIB900 BSF trypomastigotes were maintained in HMI-18 medium (6) with 15% heat-inactivated fetal calf serum (Harlan-SeraLab, United Kingdom) at 37°C in a 5% CO2-95% air mixture. Trypomastigotes were washed and resuspended in fresh medium at a concentration of 2 × 105/ml. The top concentration for the test compounds was 30 μg/ml. Five different concentrations of drug were tested in triplicate. The 50% effective dose (ED50) for pentamidine was usually between 1.0 and 0.1 ng/ml. Plates were incubated for 72 h at 37°C in a 5% CO2-95% air mixture. At 72 h, the plates were assessed microscopically before alamarBlue was added (14). Plates were read after 5 to 6 h on a Gemini Fluorescent plate reader (Softmax Pro. 3.1.1, Molecular Devices, United Kingdom) at an excitation/emission of 530/585 nm, with a filter cutoff at 550 nm. ED50 values were calculated with Msxlfit (IDBS, United Kingdom). For studies with Trypanosoma brucei subsp. brucei bloodstream forms, trypomastigotes were maintained in HMI-9 medium with 10% heat-inactivated fetal calf serum (Gibco) at 37°C in a 5% CO2-95% air mixture. The HMI-9 medium was supplemented with 1 μg/ml of ergosterol, which was dissolved in dimethyl sulfoxide. Procyclic forms were grown in SDM-79 with 10% heat-inactivated fetal calf serum at 27°C.
Cytotoxicity.
Plates were seeded with 100 μl human epidermal nasopharyngeal carcinoma KB cells at 4 × 104/ml and RPMI 1640 plus 10% heat-inactivated fetal calf serum and incubated at 37°C in 5% CO2-95% air for 24 h. The overlay was removed and replaced by the drugs to be tested in fresh medium at 300, 30, 3, and 0.3 μg/ml in triplicate at each concentration. The positive-control drug was podophyllotoxin (Sigma, United Kingdom). Plates were incubated for a further 72 h, at 37°C in 5% CO2-95% air. The wells were microscopically assessed for cell growth. The overlay was removed and wells washed three times with phosphate-buffered saline (PBS; pH 7.0). Then, 100 μl PBS plus 10 μl alamarBlue was added per well and plates incubated for 2 to 4 h (37°C, 5% CO2-95% air) before reading at an excitation/emission of 530/585 nm (cutoff, 550 nm) in a Gemini plate reader. ED50 values were calculated compared to blanks and untreated controls.
Bacterial strains and growth conditions.
Escherichia coli BL21(DE3) bacteria were grown in Luria-Bertani (LB) medium supplemented with the following antibiotics, when needed, at the indicated concentrations: ampicillin, 100 μg/ml; chloramphenicol, 34 μg/ml; and kanamycin, 30 μg/ml. Plasmid preparation, agarose gel electrophoresis DNA ligation, transformation, and other cloning procedures were done by standard methods.
T. brucei subsp. brucei 24-SMT cloning and overexpression.
The Trypanosoma brucei subsp. brucei 24-SMT gene (GenBank accession number DQ126002) was cloned by PCR using genomic DNA as a template. The oligonucleotide primers used for PCR amplification were synthesized by the Technical Services department of the Instituto de Parasitologia y Biomedicina López-Neyra. Restriction sites (NdeI and BamHI) were introduced at the 5′ and 3′ ends for convenient cloning. The primers used were the N-terminal primer GGAATTCCATATGTCGGCCGGATCTCGT and the C-terminal primer CGGGATCCTTAGCACGACAGCTCTTCCCC. The entire coding sequence was cloned in pET28a(+) to give pET28TbSMT, and the resulting construct was used to transform E. coli BL21(DE3) cells, which were plated on LB agar plates containing 34 μg of kanamycin per ml.
Enzyme activity assays.
In assays of inhibition of 24-SMT, soluble protein extracts from E. coli BL21(DE3)/pET28TbSMT cells were used. Trypanosoma brucei supsp. brucei recombinant 24-SMT is produced as a His-tagged fusion protein and is overexpressed when induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 4 h. Cells were disrupted by sonication in a buffer containing 50 mM Tris-HCl (pH 7.4), 2 mM MgCl2, 4 mM CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 0.5% (vol/vol) Tween 80, and protease inhibitors. The sonicate was centrifuged at 12,000 rpm for 30 min at 4°C to obtain the soluble fraction, which contained the active form of the enzyme. When parasite extracts were analyzed, bloodstream forms were collected at 2 × 106 cells per ml, while procyclic forms were collected at 7.5 × 106 cells per ml. Cultures were centrifuged at 2,600 rpm for 10 min at 4°C; parasites were washed twice with PBS (pH 7.2) and lysed by sonication in the same buffer as that used for bacterial cells. Total extracts obtained after sonication were used in the enzymatic assay. A standard 24-SMT activity assay contained 1.7 mg/ml of bacterial soluble extracts or 3.3 mg/ml of parasitic total extracts in buffer containing 50 mM Tris-HCl (pH 7.4), 2 mM MgCl2, 4 mM CHAPS, 0.5% (vol/vol) Tween 80, 100 μM desmosterol, and 200 μM [14C]S-adenosyl-l-methionine (6 × 105 dpm or 1 × 106 dpm per reaction). Desmosterol was dissolved in chloroform, which was evaporated before the rest of the components were added. The inhibitor was resuspended first in a minimal volume of its corresponding solvent and later added to the reaction mixture as an aqueous solution. The reaction was started with the enzyme. Incubations were performed at 30°C for 45 min and terminated with 0.5 ml of 10% KOH dissolved in 80% (vol/vol) methanol. To quantify the efficiency of the extraction, [3H]cholesterol (3 mg, 30,000 dpm per reaction) was added as an internal standard. The methylated sterol product was extracted three times with 1 ml of hexane and the resulting organic layer washed once with Tris-HCl buffer to remove the [14C]S-adenosyl-l-methionine that was not incorporated. One milliliter of the organic layer was added to 10 ml of hydrofluor (National Diagnostics), and the radioactivity was measured in a scintillation counter. Values for 50% inhibitory concentration (IC50) were obtained from hyperbolic plots of percentage of inhibition versus concentration of inhibitor.
Northern blot analysis.
Total RNA from both procyclic and bloodstream forms was obtained using the TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Total RNA (15 μg/lane) was fractionated by electrophoresis on a 1.5% formaldehyde agarose gel under denaturing conditions and transferred overnight to a nylon membrane (Hybond-N; Amersham Life Sciences, Inc.). The membrane was subjected to overnight hybridization of a 32P-radiolabeled, single-stranded DNA 24-SMT probe. The probe was obtained by PCR amplification under the same conditions and using the same primers as those described above. The obtained PCR product was added to the hybridization buffer at a final concentration of 106 cpm/ml. After hybridization, the membrane was washed and analyzed using X-OMATAR film (Kodak).
Western blot analysis.
For the preparation of T. brucei lysates, the parasites were washed twice in PBS (pH 7.2) and lysed by sonication in the same buffer supplemented with protease inhibitors. Twenty-one micrograms of protein from total extract was subjected to 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto an Immobilon-P membrane (Millipore) at 10 V for 30 min, using a semidry transfer sell (Bio-Rad). The membrane was blocked overnight at 4°C with 5% (wt/vol) nonfat powdered milk in PBS and incubated with a 1:2,500 dilution of a polyclonal antibody generated against purified recombinant Leishmania major SMT from E. coli inclusion bodies and purified by chromatography through a protein A Sepharose CL-4B column (Amersham biosciences) according to the manufacturer's instructions. As a secondary antibody, horseradish peroxidase-conjugated anti-rabbit immunoglobulin G diluted 1:5,000 was used. Bound antibody was visualized using enhanced-chemiluminescence immunodetection reagents (Amersham).
Studies of lipid composition.
For the analysis of the lipid composition in T. brucei subsp. brucei, total lipids of bloodstream forms and procyclic forms were extracted and fractionated into neutral and polar lipid fractions by silicic acid column chromatography and gas-liquid chromatography (8, 9). The neutral lipid fractions were first analyzed by thin-layer chromatography (on Merck 5721 silica gel plates with heptane-isopropyl ether-glacial acetic acid [60:40:4] as the developing solvent) and conventional gas-liquid chromatography (isothermic separation in a 4-m glass column packed with 3% OV-1 on Chromosorb 100/200 mesh, with nitrogen as the carrier gas at 24 ml/min−1, and flame ionization detection in a Varian 3700 gas chromatograph). For quantitative analysis and structural assignments, the neutral lipids were separated in a capillary high-resolution Ultra-2 column (25 m by 0.20 mm [inner diameter]; 5% phenyl-methyl-siloxane; film thickness, 0.33 μm) in a Hewlett-Packard 6890 series II gas chromatograph equipped with an HP5973A mass-sensitive detector. The lipids were injected in chloroform, and the column was kept at 50°C for 1 min, and then the temperature was increased to 270°C at a rate of 25°C · min−1 and finally to 300°C at a rate of 1°C · min−1.The carrier gas (He) flow was kept constant at 0.5 ml · min−1. The injector temperature was 250°C, and the detector was kept at 280°C.
RESULTS
Inhibitor synthesis and evaluation of action on parasite growth.
The inhibitors, 1a to 1g, were synthesized by standard methods using a convergent strategy (Table 1). The full details of this strategy will be published separately.
TABLE 1.
Activities of azasterol compounds against recombinant L. major 24-SMT, T. brucei subsp. brucei 24-SMT, 24-SMT from T. brucei subsp. brucei parasite extracts, and bloodstream forms of T. brucei subsp. rhodesiensea
CI, confidence interval; SI, selectivity index.
Values in parentheses are for 24-SMT from T. brucei subsp. brucei extracts. ND, not determined.
In vitro growth assays were carried out using the clinically relevant BSF of T. brucei subsp. rhodesiense and against mammalian KB cells to give an idea of toxicity in mammalian cells (Table 1). All compounds analyzed showed potent growth inhibition of T. brucei subsp. rhodesiense, with the most active compound having an ED50 of 12 nM. There appeared to be an optimum chain length; growth inhibition increased as chain length increased, with an optimum chain length of three carbon atoms. When chain length was further increased, activity slowly decreased. The compounds showed greater activity against T. brucei subsp. rhodesiense than the lead compound, AZA, which had an ED50 of 3.3 μM. The growth inhibition of KB cells was significantly lower than that of T. brucei subsp. rhodesiense, implying that there is a good selectivity for parasite cells over mammalian cells. There appeared to be a slight increase in toxicity as chain length was increased; notwithstanding, compounds appeared to have lower toxicity than the lead compound, AZA. The compound with a chain length of three carbon atoms shows the greatest selectivity index, about 1,600-fold more active against the parasite, and all of these new analogues showed greater selectivity than AZA.
An analysis of growth inhibition was also performed in the presence of ergosterol in the culture medium in order to evaluate whether the availability of alkylated sterols would counteract the effect of potential 24-SMT inhibition. The resulting inhibition data are shown in Table 2. T. brucei subsp. brucei BSFs were cultured for 72 h in the presence of different concentrations of compounds 1e and 1f, and ED50 values were calculated as described above. The selected compounds were not active against procyclic forms at concentrations as high as 1 μM. In the case of BSFs, ergosterol in the medium had no effect on the resulting ED50, which was somewhat higher than the value obtained for T. brucei subsp. rhodesiense BSFs.
TABLE 2.
Effects of ergosterol on the growth inhibition of procyclic forms and BSFs from T. brucei subsp. brucei by azasterolsa
| Inhibitor | ED50 value (μM) for:
|
|||
|---|---|---|---|---|
| Procyclic forms with no addition | Procyclic forms + 1 μg/ml ergosterol | BSFs with no addition | BSFs + 1 μg/ml ergosterol | |
| 1e | >1 | >1 | 0.127 ± 0.044 | 0.101 ± 0.022 |
| 1f | >1 | >1 | 0.080 ± 0.032 | 0.118 ± 0.057 |
Values are the averages ± standard deviations obtained from five different drug concentrations assayed in triplicate.
Cloning and overexpression of T. brucei subsp. brucei 24-SMT.
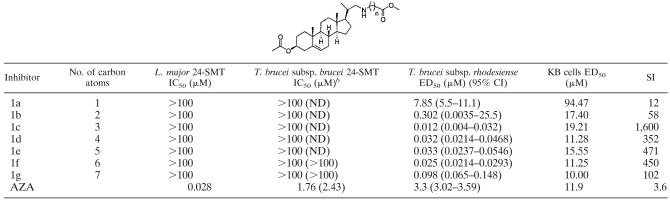
The 24-SMT sequence, exhibiting high similarity with the coding sequence from Leishmania major, was identified in chromosome 10 of the Trypanosoma brucei subsp. brucei genome. The sequence gives a deduced amino acid sequence of 359 residues and a pI of 5.2. The T. brucei subsp. brucei amino acid sequence exhibits a 62% identity with the L. major enzyme, and all of the highly conserved motifs characteristic of 24-SMTs are conserved, including the S-adenosyl-l-methionine and sterol binding sites (motifs II and I, respectively). Efforts to produce recombinant protein were successful when the entire coding region was placed in pET28a and the enzyme was produced with a His tag at the amino terminus. 24-SMT accounted for 1% of the total soluble protein, while high quantities were generated as inclusion bodies, accounting for 25% of the total protein in E. coli extracts (Fig. 2). Soluble active protein proved to be extremely unstable under different experimental conditions when immobilized-metal-affinity chromatography was attempted. We therefore decided to use E. coli BL21(DE3)/pET28TbSMT extracts for enzyme determinations.
FIG. 2.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of 24-SMT expression in Escherichia coli BL21(DE3)/pET28TbSMT cells. Lane 1, 25 μg of soluble extract of cells prior to induction with IPTG; lane 2, 25 μg of soluble extract after 4 h of induction with 1 mM IPTG at 25°C for 4 h; lane 3, 25 μg of the insoluble fraction of cells after 4 h of induction with 1 mM IPTG at 25°C for 4 h.
Inhibition of T. brucei subsp. brucei 24-SMT by azasterols.
Compounds were then assayed against T. brucei subsp. brucei 24-SMT. The assays were conducted using the freshly prepared whole-cell extracts of E. coli overexpressing recombinant 24-SMT, due to the difficulty in producing large quantities of soluble active enzyme, in a method similar to what we have reported for assays of L. major 24-SMT (9). A range of six concentrations of inhibitor was tested in duplicate. AZA was assayed against both the T. brucei subsp. brucei and the L. major enzymes, as a reference compound; the IC50 found against recombinant T. brucei subsp. brucei 24-SMT was 1.76 μM. This value was higher than what we have reported for L. major 24-SMT (0.028 μM), indicating that despite high similarities in sequence between the L. major and T. brucei subsp. brucei sequences, subtle differences in the active site may exist, leading to different IC50 values.
The newly synthesized azasterols were then evaluated using cell extracts of the recombinant T. brucei subsp. brucei 24-SMT, and IC50 values obtained are shown in Table 1. None of the compounds tested showed any significant inhibition, and IC50 values were estimated to all be higher than 100 μM.
Considering the possibility that the His-tagged recombinant enzyme might exhibit properties different from those of the native enzyme present in parasite extracts, procyclic cells were used for extract preparation and analyzed for inhibition on behalf of two of the compounds previously tested against the recombinant enzyme. In addition, the reference compound AZA was evaluated for inhibition against 24-SMT from parasite extracts. Compounds 1f and 1g gave inhibition percentages of 35 and 29%, respectively, when tested against the 24-SMT present in extracts at a concentration of 100 μM (IC50 values higher than 100 μM), while the IC50 value obtained for AZA was 2.43 μM under the same conditions.
Northern and Western blot analyses of 24-SMT expression in procyclic forms and BSFs.
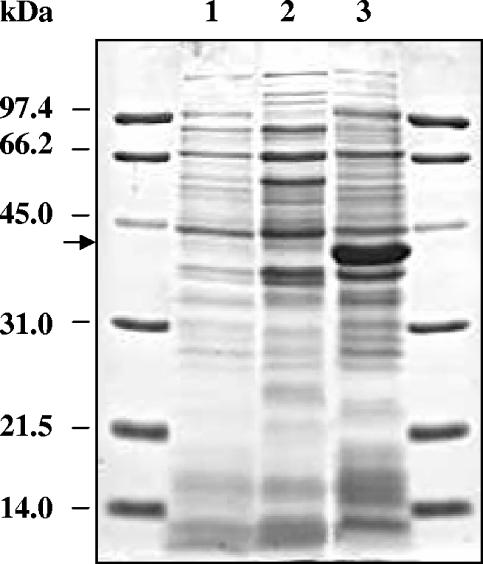
While growth inhibition assays in vitro clearly showed that the novel azasterols were particularly potent against T. brucei subsp. brucei, surprisingly, none of them showed significant activity against T. brucei subsp. brucei 24-SMT. It has been reported that bloodstream forms of T. brucei subsp. brucei do not synthesize sterols (2, 3) and that their cellular requirements are satisfied by the uptake of cholesterol for the culture media via a mechanism that involves an LDL receptor. We decided to investigate further the existence of 24-SMT activity in BSFs by Northern and Western blot analyses. Logarithmic-phase procyclic forms and BSFs were used for total RNA extraction, and the coding sequence of the 24-SMT gene was used as a probe. As shown in Fig. 3A, a single transcript of 2.6 kb was observed in both forms. Densitometric analysis illustrated that approximately threefold-higher levels are present in procyclic forms.
FIG. 3.
(A) Northern analysis of Trypanosoma brucei subsp. brucei 24-SMT gene expression. Total RNA was prepared from both procyclic and BSFs. The Northern blot was hybridized with a probe encompassing the entire coding sequence of T. brucei subsp. brucei 24-SMT. (B) Western blot analysis of SMT protein levels in T. brucei subsp. brucei. Polyclonal serum raised against Leishmania major 24-SMT was used. Lane 1, bloodstream forms; lane 2, procyclic forms.
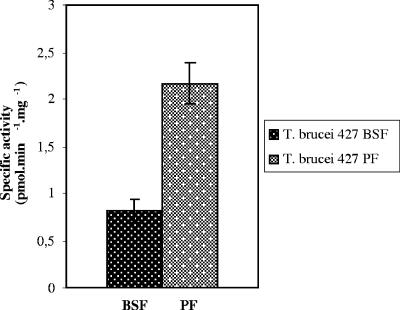
Western blot analysis was performed using an antibody raised against L. major 24-SMT and is shown in Fig. 3B. Again, a band of the expected molecular mass was evidenced in both procyclic and BSFs, although levels were approximately threefold higher in procyclic forms as determined densitometrically. Furthermore, protein detected by Western blot analysis corresponds to active protein. As shown in Fig. 4, the conversion of desmosterol to methylsterols by extracts of both life forms of T. brucei was detectable and measurable. Thus, the specific activities of 24-SMT were 2.17 and 0.82 pmol · min−1 · mg−1 in procyclic forms and BSFs, respectively.
FIG. 4.
Determination of 24-SMT activity in parasite extracts. 24-SMT activity measurements were performed at 200 μM [14C]S-adenosyl-l-methionine (8.33 dpm/pmol) and 100 μM desmosterol as indicated in Materials and Methods. Results are the averages of the values for specific activity obtained for three different protein extract concentrations, and assays were performed in triplicate. BSF, bloodstream forms; PF, procyclic forms.
Sterol composition.
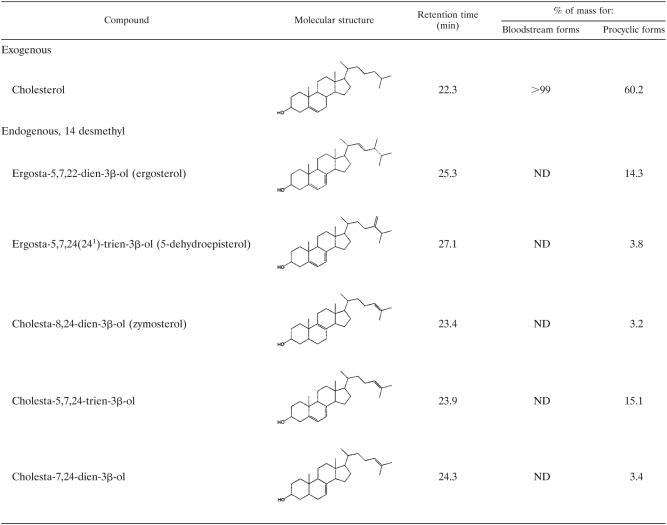
The sterol compositions of T. brucei subsp. brucei BSFs and procyclic forms were investigated. Table 3 shows that in procyclic forms, 60.2% of the mass of the sterol composition is represented by cholesterol, the rest being sterols alkylated in position 24, with ergosterol accounting for 14.3% of the total sterol mass. On the other hand, in BSFs, cholesterol accounts for more than 99% of the total sterol mass and no 24-alkylated sterols were detected.
TABLE 3.
Free sterol composition of bloodstream and procyclic forms of T. b. bruceia
Sterols were extracted from cells in cultivated in vitro to late exponential growth phase; they were separated from polar lipids by silicic acid column chromatography and analyzed by quantitative capillary gas-liquid chromatography and mass spectrometry. Composition is expressed as mass percentages. ND, not detected.
DISCUSSION
All of the compounds described in the present report were active in vitro against T. brucei subsp. rhodesiense. In particular, compounds 1b to 1g showed antiproliferative effects in the nanomolar range against BSFs of T. brucei subsp. rhodesiense. Compound 1c appeared to be the most active, with an ED50 of 12 nM. Consequently, these compounds represent exciting leads for the development of new chemotherapeutic agents for the treatment of human African trypanosomiasis, yet their mode of action now appears to be independent of 24-SMT activity.
The potent activity observed for certain compounds with bloodstream forms of the parasite was somewhat surprising since it has been well documented that Trypanosoma brucei BSFs lack the ability to synthesize sterols and depend on the presence in the culture medium of plasma low-density lipoprotein particles, which are necessary for membrane assembly (2, 3). Conversely, procyclic forms may obtain sterols from both exogenous and endogenous sources, although in this case, the two pathways can compensate for each other as incorporation of exogenous lipid downregulates the isoprenoid biosynthetic machinery. Indeed, no growth inhibition was observed for this form of the parasite for a subset of the compounds tested. We were therefore interested in investigating the mode of action of these compounds and the role of 24-SMT in parasite viability.
Northern and Western blot analyses clearly indicated that the 24-SMT gene was transcribed and translated in both bloodstream and procyclic forms of T. brucei subsp. brucei, yet levels of enzyme were approximately threefold higher in procyclic forms. Having established that the 24-SMT was present, it was important to show that it was functional; this was achieved by carrying out enzyme assays with radiolabeled desmosterol and showing that it was converted to methylsterols. Again, enzyme activity in procyclic forms was approximately threefold higher than that measured in BSFs. Previous results obtained by Coppens and Courtoy (2, 3) together with our own analysis of the sterol composition of procyclic and bloodstream form T. brucei subsp. brucei showed that 24-alkylated sterols are present in the former but absent in the latter. An explanation for a functional 24-SMT in bloodstream forms of T. brucei subsp. brucei may be that a low level of biosynthesis of 24-alkylated sterols is required for a sparking role, as suggested for the yeast Saccharomyces cerevisiae (12), or alternatively that although this enzyme is still expressed at low levels, carbon flow leading to sterol production is undetectable.
Due to previous observations on the efficacy of azasterols as antitrypanosomal agents, compounds were developed as inhibitors of 24-SMT and we accordingly decided to analyze the interaction of inhibitors with the T. brucei enzyme. As found with the L. major enzyme, the purified protein has poor aqueous solubility and was difficult to purify on a large scale. However, it was possible to clone and overexpress the enzyme in E. coli cells and screen compounds against the enzyme using whole-cell extracts. None of the novel azasterol derivatives were active against the enzyme, suggesting that their primary mode of trypanocidal activity was not inhibition of 24-SMT or depletion of 24 alkyl sterols. Additionally, two compounds were selected to analyze inhibition of the native 24-SMT using procyclic parasite extracts as an enzyme source. Again, no significant inhibition was observed, ruling out possible differences in inhibitor susceptibility that may arise as a result of heterologous expression in E. coli.
The mode of action of this set of analogues remains to be established and is currently under investigation, although certain hypotheses may be set forth. One possibility is the existence of alternative extracellular or intracellular protein targets to which the sterol analogue specifically binds, and in this sense, certain structural requirements would exist for efficient binding. Other possibilities include the existence of specific membrane microdomains (25) where the analogues may be inserted and alter protein/membrane function. General toxic effects do not appear to exist at the concentrations tested, at least when ED50 values for KB cells are analyzed, yet the possibility of a wider-range toxic mode of action cannot be fully discarded. Proteomic studies as well as membrane function are currently under investigation for disclosure of possible molecular targets and mechanisms of action for this class of compounds. Likewise, further structure-activity relationship determinations could contribute to the understanding of requirements for inhibition and mode of action.
In conclusion, we have prepared some novel azasterols which inhibit the growth of bloodstream forms of T. brucei subsp. rhodesiense with ED50 values in the nanomolar range, which represent good drug leads worthy of further study. The mode of action of the compounds does not seem to be associated with 24-SMT inhibition. Further work is required to analyze the mode of action of azasterols and the cellular basis for toxicity as well as the function of 24-SMT in T. brucei.
Acknowledgments
We acknowledge the European Union INCO-DEV program (ICA4-CT-2001-10074), the Plan Nacional de Investigación (SAF2004-03828), and the Welsh School of Pharmacy for funding. Ludovic Gros was a fellow of the Marie Curie Training Site (QLK2-CT-2001-60091). This investigation received financial support from the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR).
The EPSRC National Mass Spectrometry Service Centre is acknowledged for accurate mass spectroscopy.
REFERENCES
- 1.Chance, M. L., and L. J. Goad. 1997. Sterol metabolism of Leishmania and trypanosomes: potential for chemotherapeutic exploitation, p. 163-167. In G. Hide, G. H. Coombs, and P. H. Holmes (ed.), Trypanosomiasis and leishmaniasis: biology and control. CAB International, Wallingford, United Kingdom.
- 2.Coppens, I., and P. J. Courtoy. 2000. The adaptative mechanisms of Trypanosoma brucei for sterol homeostasis in its different life-cycle environments. Annu. Rev. Microbiol. 54:129-156. [DOI] [PubMed] [Google Scholar]
- 3.Coppens, I., and P. J. Courtoy. 1995. Exogenous and endogenous sources of sterols in the culture-adapted procyclic trypomastigotes of Trypanosoma brucei. Mol. Biochem. Parasitol. 73:179-188. [DOI] [PubMed] [Google Scholar]
- 4.Croft, S. L. 1997. The current status of antiparasite chemotherapy. Parasitology 114(Suppl.):S3-S15. [PubMed] [Google Scholar]
- 4a.Gros, L., S. Orenes Lorente, C. Jimenez Jimenez, V. Yardley, L. Rattray, H. Wharton, S. Little, S. L. Croft, L. M. Ruiz-Perez, D. Gonzalez-Pacanowska, and I. H. Gilbert. Evaluation of azasterols as anti-parasitics. J. Med. Chem., in press. [DOI] [PubMed]
- 5.Haughan, P. A., M. L. Chance, and L. J. Goad. 1995. Effects of an azasterol inhibitor of sterol 24-transmethylation on sterol biosynthesis and growth of Leishmania donovani promastigotes. Biochem. J. 308:31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirumi, H., and K. Hirumi. 1989. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 75:985-989. [PubMed] [Google Scholar]
- 7.Lorente, S. O., C. J. Jimenez, L. Gros, V. Yardley, K. de Luca-Fradley, S. L. Croft, J. A. Urbina, L. M. Ruiz-Perez, D. G. Pacanowska, and I. H. Gilbert. 2005. Preparation of transition-state analogues of sterol 24-methyl transferase as potential anti-parasitics. Bioorg. Med. Chem. 13:5435-5453. [DOI] [PubMed] [Google Scholar]
- 8.Lorente, S. O., J. C. Rodrigues, C. Jimenez Jimenez, M. Joyce-Menekse, C. Rodrigues, S. L. Croft, V. Yardley, K. de Luca-Fradley, L. M. Ruiz-Perez, J. Urbina, W. de Souza, D. Gonzalez Pacanowska, and I. H. Gilbert. 2004. Novel azasterols as potential agents for treatment of leishmaniasis and trypanosomiasis. Antimicrob. Agents Chemother. 48:2937-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magaraci, F., C. J. Jimenez, C. Rodrigues, J. C. Rodrigues, M. V. Braga, V. Yardley, K. de Luca-Fradley, S. L. Croft, W. de Souza, L. M. Ruiz-Perez, J. Urbina, D. Gonzalez Pacanowska, and I. H. Gilbert. 2003. Azasterols as inhibitors of sterol 24-methyltransferase in Leishmania species and Trypanosoma cruzi. J. Med. Chem. 46:4714-4727. [DOI] [PubMed] [Google Scholar]
- 10.Nes, W. D. 2000. Sterol methyl transferase: enzymology and inhibition. Biochim. Biophys. Acta. 1529:63-88. [DOI] [PubMed] [Google Scholar]
- 11.Oehlschlager, A. C., R. H. Angus, A. M. Pierce, H. D. Pierce, Jr., and R. Srinivasan. 1984. Azasterol inhibition of delta 24-sterol methyltransferase in Saccharomyces cerevisiae. Biochemistry 23:3582-3589. [DOI] [PubMed] [Google Scholar]
- 12.Parks, L. W., S. J. Smith, and J. H. Crowley. 1995. Biochemical and physiological effects of sterol alterations in yeast—a review. Lipids 30:227-230. [DOI] [PubMed] [Google Scholar]
- 13.Rahier, A., M. Taton, and P. Benveniste. 1990. Inhibition of sterol biosynthesis enzymes in vitro by analogues of high-energy carbocationic intermediates. Biochem. Soc. Trans. 18:48-52. [DOI] [PubMed] [Google Scholar]
- 14.Raz, B., M. Iten, Y. Grether-Buhler, R. Kaminsky, and R. Brun. 1997. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop. 68:139-147. [DOI] [PubMed] [Google Scholar]
- 15.Roberts, C. W., R. McLeod, D. W. Rice, M. Ginger, M. L. Chance, and L. J. Goad. 2003. Fatty acid and sterol metabolism: potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa. Mol. Biochem. Parasitol. 126:129-142. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues, J. C., M. Attias, C. Rodriguez, J. A. Urbina, and W. Souza. 2002. Ultrastructural and biochemical alterations induced by 22,26-azasterol, a Δ24(25)-sterol methyltransferase inhibitor, on promastigote and amastigote forms of Leishmania amazonensis. Antimicrob. Agents Chemother. 46:487-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roux, C. 1964. Action teratogene du triparanol chez l'animal. Arch. Fr. Pediatr. 21:451-464. [PubMed] [Google Scholar]
- 18.Tong, Y., B. S. McCourt, D. A. Guo, A. T. Mangla, W. X. Zhou, M. D. Jenkins, W. Zhou, M. Lopez, and W. D. Nes. 1997. Stereochemical features of C-methylations on the path to Δ24(28)-methylene and Δ24(28)-ethylidene sterols: studies on the recombinant phytosterol methyl transferase from Arabidopsis thaliana. Tetrahedron Lett. 38:6115-6118. [Google Scholar]
- 19.Urbina, J. A. 1997. Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology 114(Suppl.):S91-S99. [PubMed] [Google Scholar]
- 20.Urbina, J. A., G. Visbal, L. M. Contreras, G. McLaughlin, and R. Docampo. 1997. Inhibitors of Δ24(25) sterol methyltransferase block sterol synthesis and cell proliferation in Pneumocystis carinii. Antimicrob. Agents Chemother. 41:1428-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urbina, J. A., J. Vivas, K. Lazardi, J. Molina, G. Payares, M. M. Piras, and R. Piras. 1996. Antiproliferative effects of Δ24(25) sterol methyl transferase inhibitors on Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Chemotherapy 42:294-307. [DOI] [PubMed] [Google Scholar]
- 22.Urbina, J. A., J. Vivas, G. Visbal, and L. M. Contreras. 1995. Modification of the sterol composition of Trypanosoma (Schizotrypanum) cruzi epimastigotes by Δ24(25)-sterol methyl transferase inhibitors and their combinations with ketoconazole. Mol. Biochem. Parasitol. 73:199-210. [DOI] [PubMed] [Google Scholar]
- 23.Vivas, J., J. A. Urbina, and W. de Souza. 1996. Ultrastructural alterations in Trypanosoma (Schizotrypanum) cruzi induced by Δ24(25) sterol methyl transferase inhibitors and their combinations with ketoconazole. Int. J. Antimicrob. Agents 7:235-240. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. 1. June 2006, last date modified. The UNICEF-UNDP-World Bank-WHO special programme for research and training in tropical diseases. [Online.] http://www.who.int/tdr.
- 25.Zhang, W., A. L. McIntosh, H. Xu, D. Wu, T. Gruninger, B. Atshaves, J. C. Liu, and F. Schroeder. 2005. Structural analysis of sterol distributions in the plasma membrane of living cells. Biochemistry 44:2864-2884. [DOI] [PubMed] [Google Scholar]