Abstract
Bacillus anthracis lethal toxin (LT) produces symptoms of anthrax in mice and induces rapid lysis of macrophages derived from certain inbred strains. LT is comprised of a receptor binding component, protective antigen (PA), which delivers the enzymatic component, lethal factor (LF), into cells. We found that mouse macrophages were protected from toxin by the antitumor drug cis-diammineplatinum (II) dichloride (cisplatin). Cisplatin was shown to inhibit LT-mediated cleavage of cellular mitogen-activated protein kinases (MEKs) without inhibiting LF's in vitro proteolytic activity. Cisplatin-treated PA lost 100% of its ability to function in toxicity assays when paired with untreated LF, despite maintaining the ability to bind to cells. Cisplatin-treated PA was unable to form heptameric oligomers required for LF binding and translocation. The drug was shown to modify PA in a reversible noncovalent manner. Not surprisingly, cisplatin also blocked the actions of anthrax edema toxin and of LF-Pseudomonas aeruginosa exotoxin A fusion peptide (FP59), both of which require PA for translocation. Treatment of BALB/cJ mice or Fischer F344 rats with cisplatin at biologically relevant concentrations completely protected the animals from a coadministered lethal dose of LT. However, treatment with cisplatin 2 hours before or after animals received a lethal bolus of toxin did not protect them.
Anthrax toxin consists of three polypeptides that combine to express two toxic actions. Protective antigen (PA) and lethal factor (LF) constitute the lethal toxin (LT), whereas PA and edema factor (EF) constitute edema toxin (ET). PA binds to cellular receptors present on all tested cell types and is cleaved to its 63-kDa form (PA63), which is required for oligomerization and binding of LF and EF. Oligomerized PA63 translocates LF and EF to the cell cytosol (8). LF is a metalloproteinase which cleaves and inactivates members of the mitogen-activated protein kinase family (MEKs) (9, 40, 41). EF is a calmodulin-dependent adenylate cyclase (22). Anthrax LT is considered the major virulence factor of Bacillus anthracis, in part because LT injection into animals induces symptoms of anthrax and lethality (5, 11, 26). Macrophage cell lines such as RAW264.7 and J774.A1, as well as primary macrophages from certain inbred strains of mice, such as BALB/cJ mice, are uniquely sensitive to rapid lysis by LT (12). Thus far, a link between MEK cleavage and macrophage lysis has not been established (29). However, it is clear that the proteolytic function of LF and its successful translocation to the cytosol are required for macrophage lysis (21).
Although there is a potential contribution of macrophage sensitivity to higher mouse susceptibilities in select backgrounds, possibly through a cytokine response (26, 27), other genetic elements play a role in animal susceptibility to LT (25, 27). Despite questions surrounding the actual role of LT-mediated macrophage lysis in pathogenesis, the value of the macrophage cytotoxicity test for rapid analysis of toxin function and deciphering of the steps involved in toxin binding and uptake to cells cannot be overstated. The concern that anthrax might be used as a bioterrorism weapon has encouraged much research to identify new therapies directed against the bacterium and its toxins. The macrophage cytotoxicity test also provides a convenient system with which to screen for materials that block LT action. It would be particularly valuable to find drugs approved for human use that have protective actions against anthrax. In the course of such a screen, we obtained evidence that the widely used antitumor agent cisplatin (CIS) blocks LT action. Cisplatin is one of the most effective chemotherapeutic anticancer agents, acting primarily by the formation of DNA adducts and the induction of apoptosis (6). Cisplatin has a wide range of other effects, some of which include inhibition of protein synthesis (30), thiol depletion (20), inhibition of mitochondrial function (42), and interference with HSP90 chaperone function (32).
In the present study, we show that cisplatin protects LT-sensitive macrophages from lysis by inhibiting LF translocation to the cytosol. Cisplatin modifies PA in a reversible noncovalent manner that does not interfere with its cell binding and processing but prevents the oligomer formation that is required for LF binding. The administration of cisplatin-pretreated lethal doses of LT affords total protection in BALB/cJ mice and Fischer 344 rats. However, the administration of cisplatin to mice before or after toxin administration does not provide protection against lethality. The potential of cisplatin as a therapeutic agent for anthrax is also discussed.
MATERIALS AND METHODS
Materials.
PA, LF, EF, and FP59 (the LF-Pseudomonas exotoxin A fusion protein) were purified as previously described (4, 22, 39). For cytotoxicity assays, toxin was prepared in serum-free Dulbecco's modified Eagle medium (DMEM) prior to addition to cells. Toxin for animal injections was prepared in sterile phosphate-buffered saline (PBS). Concentrations and doses of LT refer to the amounts of each component (i.e., 1,000 ng LT/ml is 1,000 ng PA plus 1,000 ng LF/ml and 100 μg LT is 100 μg PA plus 100 μg LF). Rabbit polyclonal antibodies to PA and LF were developed in our laboratory. A MEK1 N-terminal antibody and glutathione S-transferase (GST)-tagged purified MEK1 were purchased from Upstate Biotechnologies (Waltham, MA). The polyclonal antibody SC-71 against c-Rel was purchased from Santa Cruz Biotech (Santa Cruz, CA). Cisplatin [cis-diammineplatinum (II) dichloride] was purchased from Sigma (St. Louis, MO).
Cytotoxicity assays.
RAW264.7 cells (ATCC, Manassas, VA) were grown in DMEM with 10% fetal calf serum, 2 mM Glutamax, 2 mM HEPES, and 50 μg/ml gentamicin (all from Invitrogen, Carlsbad, CA) at 37°C in 5% CO2. Cells were seeded in 96-well plates 24 to 48 h prior to assay. For macrophage protection assays where the drug was introduced first, cells were treated in duplicate with cisplatin at various concentrations for 10 min prior to the addition of a set concentration of LT. Cell viability was assessed after 150 min by the addition of MTT [3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma, St. Louis, MO) at a final concentration of 0.5 mg/ml. Cells were then further incubated with MTT for 40 min, and the blue pigment produced by viable cells was dissolved by removing all medium, adding 50 μl/well of 0.5% (wt/vol) sodium dodecyl sulfate (SDS)-25 mM HCl in 90% (vol/vol) isopropanol, and shaking the plates for 5 min prior to reading the A570 by using a microplate reader. For experiments where PA was pretreated with cisplatin in PBS (100 μg/ml treated with 2.5 to 50 μg/ml cisplatin [see below]), twofold dilutions of cisplatin-treated PA were added to the wells first, prior to adding untreated LF at 10 μg/ml. After 150 min, MTT was added for assessment of cell viability as described above. For FP59 toxicity assays, cells were treated with twofold dilutions of cisplatin for 10 min prior to the addition of a single concentration of PA plus FP59. After 45 min of toxin incubation, the medium was removed and cells were washed once with serum-free medium, followed by the addition of 100 μl/well of complete medium. Cells were then incubated for 16 h prior to the addition of MTT and assessment of cell viability as described above.
Cisplatin pretreatment of toxin.
PA or LF at 100 μg/ml was treated with 1.0, 2.5, 10, or 25 μg/ml (3.3 μM, 8.25 μM, 33 μM, and 82.5 μM, respectively) cisplatin in PBS (pH 7.0) or 10 mM K2HPO4 (pH 9.1). Cisplatin stocks were prepared in dimethyl sulfoxide prior to being diluted in PBS. Samples were then either tested for activity in toxicity assays, as described above, or first dialyzed for 24 h against previously described buffers, using Pierce (Rockford, IL) Slide-A-Lyzer dialysis cassettes (30,000-molecular-weight cutoff), prior to testing for activity. Alternatively, cisplatin-treated PA (100 μg/ml) was first mixed with 100% fetal calf serum (1:1) or with PBS (1:1) and then rapidly separated from unbound cisplatin over PD-10 desalting columns (Amersham Pharmacia Biotech, Piscataway, NJ), using 50 mM HEPES, pH 7.0 (with or without 500 mM NaCl) as column buffer, prior to dilution. All collected column fractions were then tested for PA activity in a macrophage lysis assay, using excess (10 μg/ml) LF for activity. All samples described above were run in native gels, using the Pharmacia PhastGel system (Piscataway, NJ), or in 4 to 20% Tris-glycine gels from Invitrogen (Carlsbad, CA).
ET function assays.
RAW264.7 cells in 96-well plates were treated with cisplatin at various concentrations for 10 min prior to the addition of ET (250 ng/ml). Cells were incubated with ET for 60 min, and intracellular cyclic AMP (cAMP) production was assessed using the 96-well BioTRAK cAMP enzyme immunoassay from Amersham Pharmacia Biotech (Piscataway, NJ).
PA binding and MEK cleavage assays.
For MEK cleavage assays, RAW264.7 cells were grown in 10-cm plates to 90% confluence. Cells were treated with 1 mM cisplatin or medium (control) for 10 min prior to the addition of LT at 1,000 ng/ml. The medium was removed after 60 min, and cells were washed five times with ice-cold PBS, followed by lysis in RIPA buffer (1% Nonidet, 0.5% sodium deoxycholate, 0.1% SDS in PBS) plus EDTA-free Complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). Protein concentrations in lysates were quantified using a bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL) to ensure equal loading into SDS-polyacrylamide gels for Western blot analysis using anti-MEK1 NT (1:1,000) and c-Rel (1:1,000) antibodies. For PA binding and oligomer formation studies, CHO WTP4 cells, which allow better visualization of the PA63 oligomer (23), were used. Cells were grown to 90% confluence in alpha-modified essential medium supplemented exactly as described above for DMEM prior to treatment with LT (1,000 ng/ml) or with PA pretreated with cisplatin (25 μg/ml, or 82.5 μM) and untreated LF, with each toxin component added to a final concentration of 1,000 ng/ml. The reverse experiment was also performed by adding LF pretreated with cisplatin (25 μg/ml, or 82.5 μM) along with untreated PA, both at 1,000 ng/ml. In all experiments, cells were washed five times with PBS after 45 min to remove unbound toxin, lysates were made as described above, and Western blotting was performed with anti-PA (1:5,000).
Measurement of LF proteolytic activity in vitro.
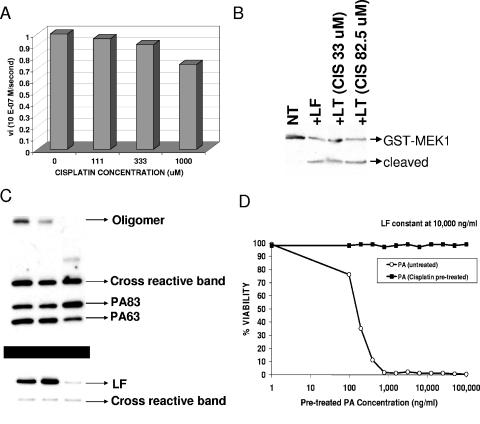
A colorimetric substrate assay (EMD Biosciences, San Diego, CA) was used to assess LF proteolytic function, as previously described (36). Briefly, 98-μl reaction mixtures containing 2 μg/ml LF in reaction buffer (25 mM Na2HPO4, 15 mM NaCl, pH 7.4, 2 μM ZnCl2) were set up, and 2 μl of cisplatin was added to a final concentration of 1,000, 333, or 111 μM. Controls received 2 μl of buffer or trypsin (2 μg/ml) instead of LF. Negative controls received 2 μl of EDTA (final concentration, 1 mM) or ZnCl2 (final concentration, 200 μM). The colorimetric substrate was added to a final concentration of 50 μM, and the A405 was read at intervals. Initial reaction rates were derived from the relationship ΔA/ΔT (where T is time) and converted to M/s using Beer's law, A = ɛcl, where ɛ is 9,920 M−1 cm−1 and the path length, l, is 1 cm. Alternatively, purified GST-tagged Mek1 fusion protein (37 ng) was cleaved by the addition of 1 ng of LF or LF pretreated with cisplatin (33 μM or 82.5 μM) in cleavage reaction buffer (10 mM HEPES, pH 7.4, 1 mM NaCl, 2 μM ZnCl2). Reactions were stopped by adding SDS loading buffer after 40 min. One-tenth (3.7 ng) of each reaction mix was subjected to SDS-polyacrylamide gel electrophoresis followed by Western blotting using the MEK1 NT antibody (1:1,000). The cleavage of the N terminus of MEK1 fused to GST results in a significant size change detected by Western blotting.
Animals.
BALB/cJ mice (8 to 12 weeks old; 20 to 22 g) were purchased from Jackson Laboratory (Bar Harbor, Maine). Fischer F344 rats (220 g) were purchased from Taconic Farms (Germantown, NY). Mice were injected intraperitoneally (i.p.) with 1.0 ml of 100 μg LT alone (100 μg PA plus 100 μg LF/ml in PBS) or 100 μg LT plus cisplatin at various doses. For experiments involving pre- or posttreatment of mice with cisplatin, the drug was injected i.p. in a 1.0-ml volume or intravenously (i.v.) in a 100-μl volume according to the schedule described, and 100 μg LT/mouse was injected i.p. in a 0.5-ml volume at various times pre- or post-drug treatment. In some experiments, rats were injected i.v. with 100 μl of drug premixed with LT or with the drug first, followed 10 min later by LT (7.5 μg, lethal at 120 to 140 min). All statistical analyses on animal survival curves were performed using GraphPad Prism 4.0 software (San Diego, CA).
RESULTS
Cisplatin protects against LT-mediated macrophage lysis.
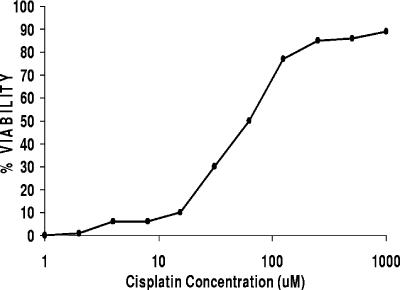
RAW264.7 macrophages and BALB/cJ peritoneal macrophages are rapidly lysed by LT. We treated these macrophages with various concentrations of cisplatin for 10 min prior to the addition of LT. Macrophages were protected from lysis by cisplatin in a dose-dependent manner (Fig. 1). Cisplatin alone produced 10 to 15% toxicity at the higher concentrations of 500 to 1,000 μM but was fully protective against LT at these concentrations.
FIG. 1.
Cisplatin protects macrophages from LT-mediated lysis. RAW264.7 cells were treated in duplicate wells with various concentrations of cisplatin for 10 min, treated with LT (1,000 ng/ml), and incubated for 150 min, and their viability was assessed by MTT assay. Viability was calculated based on the average of two treatment wells relative to the average of medium-treated control wells and is expressed as a percentage. The data shown are representative of >20 similar toxicity protection curves created with cisplatin.
Cisplatin inhibits LT-mediated MEK cleavage in cells.
LT-mediated cleavage of the N-terminal region of MEK1 in RAW264.7 cells can be assessed by monitoring the loss of an N-terminal epitope destroyed by the cleavage. Cells were treated with cisplatin or left untreated, followed by LT treatment for 40, 60, or 80 min. Cell lysates were monitored for MEK1 cleavage by Western blotting using a MEK1 N-terminal antibody specific to the cleaved epitope region. While LT cleavage of MEK1 was complete by 60 min in the absence of cisplatin, treatment with the drug completely prevented MEK1 cleavage (Fig. 2).
FIG. 2.
Cisplatin inhibits LT-mediated MEK1 cleavage. RAW264.7 cells were treated with cisplatin (1 mM) or medium prior to the addition of LT (1,000 ng/ml) for 60 min. MEK1 cleavage was assessed by Western blotting by following the loss of an N-terminal epitope using MEK1 NT antibody. An antibody against c-Rel was used simultaneously to demonstrate equal loading. The “X” represents a protein band cross-reactive with the c-Rel antibody. The gel shown is representative of five independent experiments.
Cisplatin does not inhibit LF proteolytic function.
To test whether cisplatin interfered with LF proteolytic function, we utilized a colorimetric substrate to measure toxin proteolytic activity in vitro. As shown in Fig. 3A, 1 mM cisplatin did produce a 25% inhibitory effect on LF proteolytic function. This weak effect seems insufficient to account for the complete inhibition of MEK cleavage in macrophages. We also tested cisplatin for inhibition of LF cleavage of a purified GST-tagged MEK1 protein. Pretreatment of a 100-μg/ml LT (PA plus LF) solution with 10 or 25 μg/ml cisplatin (33 or 82.5 μM) in PBS for 30 min resulted in a 100% loss of toxic ability in the macrophage toxicity assay (data not shown), but treating LF with the same concentrations of cisplatin had no effect on LF proteolytic function (Fig. 3B).
FIG. 3.
Cisplatin effects on LF proteolytic function and translocation. (A) A colorimetric LF substrate was utilized to assess the inhibition of toxin proteolytic function after incubation of LF with three concentrations of cisplatin (111, 333, and 1,000 μM) or reaction buffer alone. Initial proteolysis rates for each reaction were calculated based on changes in absorbance over 15-s increments for the first few minutes of each cleavage reaction and were converted to M/s by using Beer's law (see Materials and Methods). (B) Purified GST-tagged Mek1 fusion protein was cleaved by the addition of LF or LF pretreated with cisplatin (33 μM or 82.5 μM) and analyzed by Western blotting using MEK1 NT antibody. (C) CHO WTP4 cells were treated for 45 min with LT (lane 1), LF pretreated with cisplatin (25 μg/ml [82.5 μM]) and untreated PA (lane 2), or PA pretreated with cisplatin (25 μg/ml [82.5 μM]) along with untreated LF (lane 3). All toxin components were added to cells at 1,000 ng/ml. Cell lysates were analyzed by Western blotting performed with anti-PA (top panel) or anti-LF (bottom panel) antibody. (D) Unmodified PA or PA pretreated (in PBS) with cisplatin was tested at a range of concentrations in conjunction with a set concentration of unmodified LF (10 μg/ml) for toxicity against RAW264.7 macrophages. Viability was calculated based on the average of two treatment wells relative to the average of medium-treated control wells and is expressed as a percentage. The results shown are for an experiment which is representative of three independent studies.
Cisplatin effects on PA binding, processing to PA63, and oligomer formation.
We next tested cisplatin's effects on PA binding and processing in CHO WTP4 cells. Either PA or LF was pretreated with cisplatin (100 μg/ml toxin treated with 25 μg/ml cisplatin [82.5 μM] in PBS). Cisplatin-treated PA was added to cells with untreated LF (both at 1 μg/ml) or untreated PA was added with cisplatin-treated LF (both at 1,000 μg/ml) for 45 min. As shown in Fig. 3C, cells treated with cisplatin-treated LF and untreated PA showed little change in PA binding, cleavage, oligomerization (top gel), or binding of LF (bottom gel) compared to the control LT-treated cells. Cisplatin treatment of PA, however, while only slightly affecting PA binding and cleavage, completely prevented oligomerization of PA and subsequent LF binding. An incomplete oligomeric species did form in the cisplatin-treated PA samples in some experiments, but not always (Fig. 3C, top panel, lane 3). In fact, cisplatin pretreatment of PA in PBS under these conditions was far more effective at inhibiting PA function than providing cisplatin in the cell medium prior to toxin addition (as described for the protection shown in Fig. 1). PA (100 μg/ml = 1.1 μM) treated with cisplatin at 1.0, 2.5, 10, or 25 μg/ml (3.3 to 83 μM) in PBS tested over a wide range of dilutions (100 μg/ml to 40 ng/ml) with a set concentration of untreated LF (10 μg/ml) had absolutely no activity at any concentration of PA. The data shown in Fig. 3D are for pretreatment with 10 μg/ml cisplatin.
The effects of cisplatin on PA function were independent of pH. The same loss of activity was seen when PA was treated at pH 7.0 or pH 9.1 (Fig. 4A). Cisplatin-treated PA showed a mobility shift in native gels indicating cisplatin binding and modification of the toxin (Fig. 4A, gel, lanes 2 and 4 show cisplatin-treated PA shifts). Interestingly, a 24-h dialysis of cisplatin-bound toxin resulted in the removal of cisplatin and restoration of full toxic activity against macrophages as well as a return to normal mobility in native gels (Fig. 4A). This reversion was pH independent (Fig. 4A). Rapid removal of free cisplatin from treated PA samples by gel filtration using PD10 columns, however, did not result in regained function (Fig. 4B). Additionally, incubation of the cisplatin-modified PA with a 50% final concentration of fetal calf serum prior to column separation in order to remove larger amounts of the free cisplatin through reaction with serum proteins did not result in any gain of PA function over 6 h, indicating that cisplatin dissociation is a slow process.
FIG. 4.
Noncovalent, reversible, pH-independent cisplatin modification of PA. (A) (Top panel) PA at 100 μg/ml was treated with 1.0, 2.5, 10, or 25 μg/ml (3.3 μM, 8.25 μM, 33 μM, and 82.5 μM, respectively) cisplatin in PBS (pH 7.0) or 10 mM K2HPO4 (pH 9.1) and tested with or without dialysis (24 h) for function in macrophage toxicity assays. (Bottom panel) PA samples corresponding to the toxicity curves in the top panel were run in a native gel and stained with Coomassie blue. The experimental results shown represent those obtained from two independent experiments and four independent native gels. (B) Activity of cisplatin-treated PA (100 μg/ml) separated from unbound cisplatin by column chromatography and tested with excess (10 μg/ml) LF at various times after column application, using the assay measuring toxicity against RAW264.7 macrophages. Viability was calculated based on the average of two treatment wells relative to the average of medium-treated control wells and is expressed as a percentage. The results shown are for an experiment which is representative of three similar experiments. Data shown are for pretreatment with 25 μg/ml cisplatin.
Cisplatin inhibits PA-mediated translocation of toxins to cytosol.
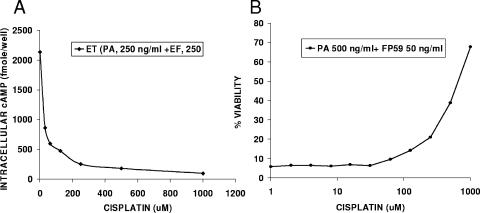
To confirm that cisplatin inhibits PA-mediated LF translocation to the cytosol, the drug was tested against two other toxins translocated by PA, the anthrax edema toxin and the cytotoxin FP59, a fusion of LF and exotoxin A from Pseudomonas aeruginosa (2, 3). As shown in Fig. 5A and B, cisplatin inhibited both toxins, indicating that its general effect is on PA-mediated translocation.
FIG. 5.
Cisplatin inhibits ET and FP59 actions. Treatment of macrophages with cisplatin inhibited cAMP production by ET (A) and cell death induced by FP59 (B). Cells were treated with various concentrations of cisplatin for 10 min prior to the addition of a set concentration of ET (250 ng/ml) or FP59 (500 ng/ml PA plus 50 ng/ml FP59). For ET inhibition studies, cAMP production was assessed 60 min after ET addition, while FP59 viability assays required washing and removal of extracellular drug and toxin after 45 min, followed by a 16-h incubation and assessment by MTT assay. The results shown are based on the average of two treatment wells for each dose used in a single experiment relative to the average of medium-treated control wells and are expressed as percentages of the medium controls. The results shown are for an experiment which is representative of two independent studies.
Cisplatin protects mice and rats from lethal doses of LT.
The maximum tolerated doses of cisplatin in rodents have been reported to range from 6 to 12 mg/kg of body weight, depending on the mouse strain (7, 14, 30, 38). Ten percent lethal dose values of 8 mg/kg (13) to 15.5 mg/kg (37) have been reported for rodents. Assuming distribution of the drug throughout the body, these doses would produce concentrations of approximately 30 μM in animals. Doses used successfully in the treatment of tumors in mice are in the 5- to 10-mg/kg range (13, 38), at which antitumor activity relative to toxicity is acceptable. We tested the effects of cisplatin on LT lethality at a range of drug doses (0.125 mg/kg to 10 mg/kg), using a single coadministration of each cisplatin dose with LT (always at 100 μg in 1 ml, or 1.1 μM). This dose of LT is lethal to BALB/cJ mice (26). As expected, cisplatin doses of 0.125 and 0.5 mg/kg (2.5 μg/ml and 10 μg/ml, respectively [8.3 and 33 μM]), which were shown to inhibit toxin activity, were also completely protective against LT mortality in the mouse model, with no signs of malaise in animals (Fig. 6A shows results for the 0.5-mg/kg dose). Cisplatin doses of >5.0 mg/kg showed toxicity to BALB/cJ mice, with 40% mortality at 5.0 mg/kg and 80% mortality at 10 mg/kg (data not shown).
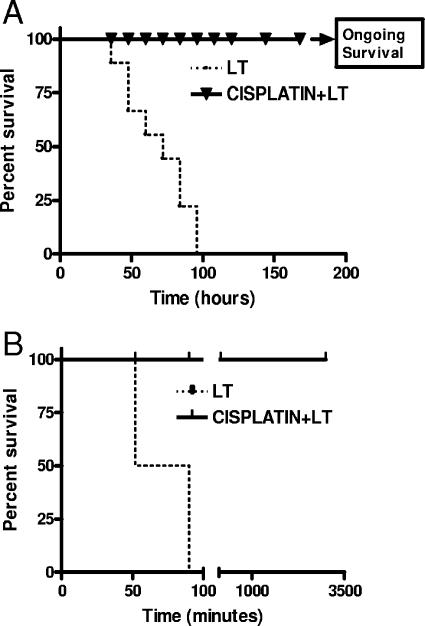
FIG. 6.
Cisplatin protects BALB/cJ mice and Fischer 344 rats from LT-mediated lethality when coadministered with toxin. (A) Survival of BALB/cJ mice injected with 100 μg LT mixed with cisplatin (0.5 mg/kg) compared to that of mice treated with LT alone. Survival was monitored every 12 h postinjection. Survival percentages are based on an n value of 10. The curves are significantly different by the log rank test (P < 0.0001). (B) Survival curves for Fischer 344 rats treated with 100 μg PA plus 40 μg LF coadministered with or without cisplatin (2.6-mg/kg dose). Survival percentages are based on an n value of 4. The curves are significantly different by the log rank test (P < 0.0001).
Tests of cisplatin were then performed with Fischer F344 rats, which are uniquely sensitive to LT and succumb in as early as 1 h (10). Cisplatin (2.6 mg/kg) was coinjected i.v. with 100 μg PA and 40 μg LF and completely protected against lethal toxin, with no signs of malaise (Fig. 6B).
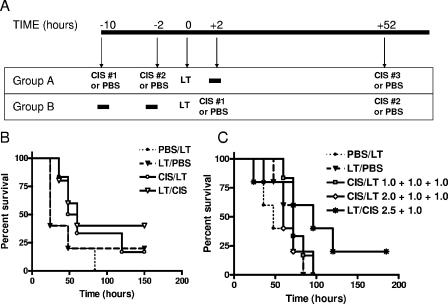
To investigate if cisplatin treatment prior to or after a high-dose-bolus toxin challenge was protective, we performed two types of experiment. For pretreatment, experiments were performed in which mice were pretreated with cisplatin (0.5, 1.0, or 2.0 mg/kg) 10 h and 2 h prior to a lethal toxin bolus (100 μg LT). Control animals received PBS instead of cisplatin. For postchallenge experiments, the toxin was administered to animals first, and 2 h later a single dose of cisplatin (1.0 or 2.5 mg/kg) or PBS (control) was administered. In both sets of experiments, a dose of cisplatin (1.0 mg/kg) was administered 52 h after toxin injection as well (Fig. 7A). The use of low-dose combinations (pretreatment with 0.5- plus 0.5-mg/kg and posttreatment with 1.0-mg/kg doses) resulted in a slight delay in time to death (TTD) but in no statistically significant protection (Fig. 7B). Maximal tolerated dose combinations (pretreatment 2.0- and 1.0-mg/kg doses, pretreatment with 1.0- and 1.0-mg/kg doses, and posttreatment with a 2.5-mg/kg dose) were also not protective against a lethal bolus of LT (Fig. 7C). Because cisplatin is cleared very rapidly from the circulation, we also performed experiments in which the drug (1.0 mg/kg) was provided 1 min, 15 min, and 25 min prior to or after LT injection. Cisplatin did not afford any statistically significant protection in any of these experiments, in which it was not premixed with toxin (data not shown). In parallel experiments with the supersensitive Fischer rat, which succumbs to a 7.5-μg dose of LT in 120 to 140 min, we injected six rats with 10 mg/kg cisplatin i.v., followed by a 7.5-μg LT bolus i.v. 10 min later and compared the TTDs with those for four rats injected with LT alone. The TTDs for the cisplatin group increased by an average of 38 min, but again, no protection was afforded (data not shown). This result was in direct contrast to the full inhibition of toxin function at cisplatin doses as low as 0.125 mg/kg if the drug and toxin were premixed prior to injection into rats (data not shown).
FIG. 7.
Cisplatin does not protect mice against LT when administered pre- or post-toxin treatment. (A) Injection schedule for mice. For cisplatin pretreatment experiments (group A), groups of five animals each were injected with cisplatin at various doses (0.5, 1.0, or 2.0 mg/kg) 10 to 12 h prior to receiving a second identical dose of cisplatin. Mice received LT (100 μg/ml) or PBS 2 h after the second dose of drug. A third dose of cisplatin was injected 52 h after toxin or PBS injection. For cisplatin posttreatment experiments (group B), mice were injected with LT (100 μg/ml) or PBS 2 h prior to receiving the first dose of cisplatin (at 1.0 or 2.0 mg/kg). Animals with a 2.0-mg/kg posttreatment also received a second dose of cisplatin (1.0 mg/kg) 52 h after toxin injection. Control animals received PBS instead of cisplatin. (B) Comparison of survival curves for low-dose (0.5 mg/kg) pre- and post-LT drug treatments. In these experiments, five animals received two 0.5-mg/kg pretreatment doses (10 and 2 h prior to toxin injection) of cisplatin, while five animals received a post-toxin-treatment dose of 1.0 mg/kg 2 h after toxin injection. Only a single post-LT drug injection was given in these experiments (no injection at 52 h). Control groups of five mice received PBS instead of drug in the same pre- and post-toxin treatment schedule. None of the curves are statistically different from each other by the log rank test (for PBS/LT group versus LT/PBS group, P = 0.4151; for PBS/LT group versus cisplatin CIS/LT group, P = 0.1241; for PBS/LT group versus LT/CIS group, P = 0.1149; for LT/PBS group versus CIS/LT group, P = 0.4546; and for LT/PBS group versus LT/CIS group, P = 0.4151). (C) Comparison of survival curves for high-dose pre- and post-LT drug treatment. In these experiments, five animals received 2.0- and 1.0-mg/kg doses of cisplatin prior to toxin injection (10 and 2 h before injection) as well as a 1.0-mg/kg dose 52 h after LT injection. Five animals received 1.0-mg/kg doses of cisplatin prior to (10 and 2 h before injection) as well as 52 h after LT injection. Five mice received 1.0-mg/kg drug doses only after toxin injection (2 h and 52 h after injection). Two control groups with five mice in each group received PBS at 10 h and 2 h pre-toxin treatment and 52 h after LT treatment or, alternatively, 2 and 52 h after LT treatment. None of the curves are statistically different from each other by the log rank test (for PBS/LT group versus LT/PBS group, P = 0.4151; for PBS/LT group versus CIS/LT group, P = 0.5488; for PBS/LT group versus CIS/LT [1.0 + 1.0 + 1.0] group, P = 0.1637; for PBS/LT group versus CIS/LT [2.0 + 1.0 + 1.0] group, P = 0.4961; for PBS/LT group versus LT/CIS [2.5 + 1.0] group, P = 0.0919; for CIS/LT group versus CIS/LT [1.0 + 1.0 + 1.0] group, P = 0.3088; for CIS/LT group versus CIS/LT [2.0 + 1.0 + 1.0] group, P = 0.9103; and for CIS/LT group versus LT/CIS [2.5 + 1.0] group, P = 0.1097).
DISCUSSION
Cisplatin is efficacious in the treatment of many human cancers, but it has well-recognized dose-limiting toxicities, mediated in part through oxidative stress induction (17). We discovered that cisplatin protects LT-sensitive mouse macrophages from lysis and does so by preventing LF delivery to the cytosol. Experiments showed that cisplatin treatment of macrophages prevented LF-mediated MEK cleavage by inhibiting proper oligomerization of PA. Cisplatin's parallel inhibitory activities on two other toxins (ET and FP59) which utilize PA for translocation into the cell cytosol support this inhibition of PA function. A search of the extensive cisplatin literature yielded evidence of potential inhibition of receptor-mediated endocytosis by this drug through inhibition of the vacuolar H+ ATPase, resulting in an increase in endosomal pH (35). Endosomal acidification is a crucial step required for formation of the PA oligomer (for a review, see reference 8). However, it is also clear from our studies that cisplatin can modify PA directly, potentially at residues involved in oligomerization.
While the concentrations of cisplatin required for protection against lysis of macrophages are relatively high if the drug is added to cells first (100 to 1,000 μM, a 104 - to 105-fold molar excess of cisplatin relative to toxin), we discovered that mixing cisplatin with toxin prior to its addition to cells or medium allowed for very low doses of cisplatin and a far lower molar ratio (an 8-fold molar excess of cisplatin relative to PA) to completely inhibit PA-mediated LF translocation into cells. Even a 1.5-fold molar excess of cisplatin mixed with PA in PBS was sufficient for 50% protection in subsequent macrophage assays. The drug bound to PA, as demonstrated by an altered mobility in native gels, but the modification was noncovalent and fully reversible in a time-dependent fashion, as demonstrated by our dialysis studies.
Cisplatin is an electrophilic agent for which thiols act as effective nucleophiles, displacing chlorides to produce sulfhydryl conjugates. This explains the reactivity of cisplatin with many thiol-containing proteins. Although cisplatin has been shown to form complexes with histidine, aspartic acid, glutamic acid, and glycine, it is unlikely that these complexes will form in plasma or within cells in preference to complexes with protein thiol groups (1, 16, 24, 31). The much higher reactivity of cisplatin with thiol groups is well established. It is likely that the potent inactivation of anthrax PA at low cisplatin concentrations in PBS prior to exposure to cells or medium results from the lack of the competing thiol groups present in serum and medium.
In fact, levels of tumor resistance to cisplatin correlate with the glutathione contents of cancer cells (15). Agents that sensitize tumors to cisplatin often do so by glutathione depletion or reduction of glutathione S-transferase activity (18, 19, 34). In a normal cell system or animal setting, cisplatin reacts primarily with the multitude of available thiols, leaving little drug to react with amino acid side chains of the cysteine-free anthrax toxin proteins. This clearly explains why pretreatment of LT in PBS with cisplatin at very low molar ratios prior to injection into animals was fully effective at inhibiting all toxic activity, while injection at 2 h pre- or post-toxin treatment was completely ineffective at protecting animals.
Animal studies indicate that cisplatin is cleared from the plasma very rapidly (26 ml/min/kg) and has a very short half-life in blood, with no drug present in plasma 60 min after injection (13, 33), and this may contribute to the ineffectiveness we observed for the drug administered before or after the toxin. However, the very low doses of pretreated cisplatin able to inhibit toxin activity make it unlikely that cisplatin inhibited toxin function by a method independent of PA modification (including inhibition of endosomal acidification) in our animal studies. We conclude that a direct cisplatin modification of LT is responsible for its inhibitory effect on the toxin and that this reaction is only efficient in the absence of competing thiol groups, making the drug an unsuitable candidate for therapy in its current form.
Identification of the residue or residues modified by cisplatin which lead to inhibition of oligomerization without affecting PA binding or processing may provide useful information in structure/function studies of LT. The low molar ratios of drug to toxin and the gel shift assays seem to indicate modification of a very small number of residues by cisplatin. PA has no cysteines, and the likely candidates for modification are the 10 methionines present in the protein. The reversibility of the modification by dialysis, however, makes it unlikely that derivatization of a methionine residue is involved. This may be due to inaccessibility of these residues to the drug within the structure of PA. The reversibility of the modification at alkaline pHs makes it unlikely that the modified residue is a histidine (1). Residues previously identified by mutagenesis as being important in oligomer formation include aspartic acid residues 512 and 520 (28), so these may be the targets of cisplatin modification. However, it is also possible that other, previously unidentified residues crucial to oligomerization are modified by this drug. The identification of cisplatin-targeted residues awaits further study.
Acknowledgments
This research was supported by the Intramural Research Program of the NIAID, NIH.
REFERENCES
- 1.Appleton, T. G. 1999. Diammine and diammineplatinum complexes with non-sulfur containing amino acids and peptides, p. 363-376. In B. Lippert (ed.), Cisplatin: chemistry and biochemistry of a leading anticancer drug. John Wiley and Sons, New York, N.Y.
- 2.Arora, N., K. R. Klimpel, Y. Singh, and S. H. Leppla. 1992. Fusions of anthrax toxin lethal factor to the ADP-ribosylation domain of Pseudomonas exotoxin A are potent cytotoxins which are translocated to the cytosol of mammalian cells. J. Biol. Chem. 267:15542-15548. [PubMed] [Google Scholar]
- 3.Arora, N., and S. H. Leppla. 1993. Residues 1-254 of anthrax toxin lethal factor are sufficient to cause cellular uptake of fused polypeptides. J. Biol. Chem. 268:3334-3341. [PubMed] [Google Scholar]
- 4.Arora, N., and S. H. Leppla. 1994. Fusions of anthrax toxin lethal factor with Shiga toxin and diphtheria toxin enzymatic domains are toxic to mammalian cells. Infect. Immun. 62:4955-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beall, F. A., and F. G. Dalldorf. 1966. The pathogenesis of the lethal effect of anthrax toxin in the rat. J. Infect. Dis. 116:377-389. [DOI] [PubMed] [Google Scholar]
- 6.Boulikas, T., and M. Vougiouka. 2003. Cisplatin and platinum drugs at the molecular level. Oncol. Rep. 10:1663-1682. [PubMed] [Google Scholar]
- 7.Christova, T. Y., G. A. Gorneva, S. I. Taxirov, D. B. Duridanova, and M. S. Setchenska. 2003. Effect of cisplatin and cobalt chloride on antioxidant enzymes in the livers of Lewis lung carcinoma-bearing mice: protective role of heme oxygenase. Toxicol. Lett. 138:235-242. [DOI] [PubMed] [Google Scholar]
- 8.Collier, R. J., and J. A. T. Young. 2003. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 19:45-70. [DOI] [PubMed] [Google Scholar]
- 9.Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, T. D. Copeland, N. G. Ahn, M. K. Oskarsson, K. Fukasawa, K. D. Paull, and G. F. Vande Woude. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280:734-737. [DOI] [PubMed] [Google Scholar]
- 10.Ezzell, J. W., B. E. Ivins, and S. H. Leppla. 1984. Immunoelectrophoretic analysis, toxicity, and kinetics of in vitro production of the protective antigen and lethal factor components of Bacillus anthracis toxin. Infect. Immun. 45:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fish, D. C., F. Klein, R. E. Lincoln, J. S. Walker, and J. P. Dobbs. 1968. Pathophysiological changes in the rat associated with anthrax toxin. J. Infect. Dis. 118:114-124. [DOI] [PubMed] [Google Scholar]
- 12.Friedlander, A. M., R. Bhatnagar, S. H. Leppla, L. Johnson, and Y. Singh. 1993. Characterization of macrophage sensitivity and resistance to anthrax lethal toxin. Infect. Immun. 61:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fulco, R. A., M. Vannozzi, P. Collecchi, F. Merlo, B. Parodi, D. Civalleri, and M. Esposito. 1990. Effect of normal saline on cisplatin pharmacokinetics and antitumor activity in mice bearing P388 leukemia. Anticancer Res. 10:1603-1610. [PubMed] [Google Scholar]
- 14.Giacomelli, S., D. Gallo, P. Apollonio, C. Ferlini, M. Distefano, P. Morazzoni, A. Riva, E. Bombardelli, S. Mancuso, and G. Scambia. 2002. Silybin and its bioavailable phospholipid complex (IdB 1016) potentiate in vitro and in vivo the activity of cisplatin. Life Sci. 70:1447-1459. [DOI] [PubMed] [Google Scholar]
- 15.Godwin, A. K., A. Meister, P. J. O'Dwyer, C. S. Huang, T. C. Hamilton, and M. E. Anderson. 1992. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc. Natl. Acad. Sci. USA 89:3070-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn, M., M. Kleine, and W. S. Sheldrick. 2001. Interaction of cisplatin with methionine- and histidine-containing peptides: competition between backbone binding, macrochelation and peptide cleavage. J. Biol. Inorg. Chem. 6:556-566. [DOI] [PubMed] [Google Scholar]
- 17.Hannemann, J., and K. Baumann. 1988. Cisplatin-induced lipid peroxidation and decrease of gluconeogenesis in rat kidney cortex: different effects of antioxidants and radical scavengers. Toxicology 51:119-132. [DOI] [PubMed] [Google Scholar]
- 18.Hofs, H. P., T. D. Wagener, V. Valk-Bakker, H. van Rennes, W. H. Doesburg, H. C. Ottenheijm, and W. J. de Grip. 1997. The effect of ethyldeshydroxy-sparsomycin and cisplatin on the intracellular glutathione level and glutathione S-transferase activity. Anticancer Drugs 8:349-357. [DOI] [PubMed] [Google Scholar]
- 19.Hromas, R. A., P. A. Andrews, M. P. Murphy, and C. P. Burns. 1987. Glutathione depletion reverses cisplatin resistance in murine L1210 leukemia cells. Cancer Lett. 34:9-13. [DOI] [PubMed] [Google Scholar]
- 20.Khynriam, D., and S. B. Prasad. 2002. Changes in glutathione-related enzymes in tumor-bearing mice after cisplatin treatment. Cell Biol. Toxicol. 18:349-358. [DOI] [PubMed] [Google Scholar]
- 21.Klimpel, K. R., N. Arora, and S. H. Leppla. 1994. Anthrax toxin lethal factor contains a zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol. Microbiol. 13:1093-1100. [DOI] [PubMed] [Google Scholar]
- 22.Leppla, S. H. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 79:3162-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, S., and S. H. Leppla. 2002. Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalization. J. Biol. Chem. 278:5227-5234. [DOI] [PubMed] [Google Scholar]
- 24.Marchan, V., E. Pedroso, and A. Grandas. 2004. Insights into the reaction of transplatin with DNA and proteins: methionine-mediated formation of histidine-guanine trans-Pt(NH3)2 cross-links. Chemistry 10:5369-5375. [DOI] [PubMed] [Google Scholar]
- 25.McAllister, R. D., Y. Singh, W. D. Du Bois, M. Potter, T. Boehm, N. D. Meeker, P. D. Fillmore, L. M. Anderson, M. E. Poynter, and C. Teuscher. 2003. Susceptibility to anthrax lethal toxin is controlled by three linked quantitative trait loci. Am. J. Pathol. 163:1735-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moayeri, M., D. Haines, H. A. Young, and S. H. Leppla. 2003. Bacillus anthracis lethal toxin induces TNF-α-independent hypoxia-mediated toxicity in mice. J. Clin. Investig. 112:670-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moayeri, M., N. W. Martinez, J. Wiggins, H. A. Young, and S. H. Leppla. 2004. Mouse susceptibility to anthrax lethal toxin is influenced by genetic factors in addition to those controlling macrophage sensitivity. Infect. Immun. 72:4439-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mogridge, J., M. Mourez, and R. J. Collier. 2001. Involvement of domain 3 in oligomerization by the protective antigen moiety of anthrax toxin. J. Bacteriol. 183:2111-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellizzari, R., C. Guidi-Rontani, G. Vitale, M. Mock, and C. Montecucco. 2000. Lethal factor of Bacillus anthracis cleaves the N-terminus of MAPKKs: analysis of the intracellular consequences in macrophages. Int. J. Med. Microbiol. 290:421-427. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Soler, R., A. R. Khokhar, P. Claringbold, L. P. Kasi, and G. Lopez-Berestein. 1986. Effects on the monocyte-macrophage system and antitumor activity against L1210 leukemia of cis-bis-cyclopentenecarboxylato-trans-R,R-1,2-diaminocyclohexane-platinum (II) encapsulated in multilamellar vesicles. JNCI 77:1137-1143. [PubMed] [Google Scholar]
- 31.Pizzo, S. V., P. A. Roche, S. R. Feldman, and S. L. Gonias. 1986. Further characterization of the platinum-reactive component of the alpha 2-macroglobulin-receptor recognition site. Biochem. J. 238:217-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenhagen, M. C., C. Soti, U. Schmidt, G. M. Wochnik, F. U. Hartl, F. Holsboer, J. C. Young, and T. Rein. 2003. The heat shock protein 90-targeting drug cisplatin selectively inhibits steroid receptor activation. Mol. Endocrinol. 17:1991-2001. [DOI] [PubMed] [Google Scholar]
- 33.Siddik, Z. H., D. R. Newell, F. E. Boxall, and K. R. Harrap. 1987. The comparative pharmacokinetics of carboplatin and cisplatin in mice and rats. Biochem. Pharmacol. 36:1925-1932. [DOI] [PubMed] [Google Scholar]
- 34.Spitz, D. R., J. W. Phillips, D. T. Adams, C. M. Sherman, D. F. Deen, and G. C. Li. 1993. Cellular resistance to oxidative stress is accompanied by resistance to cisplatin: the significance of increased catalase activity and total glutathione in hydrogen peroxide-resistant fibroblasts. J. Cell Physiol. 156:72-79. [DOI] [PubMed] [Google Scholar]
- 35.Takano, M., N. Nakanishi, Y. Kitahara, Y. Sasaki, T. Murakami, and J. Nagai. 2002. Cisplatin-induced inhibition of receptor-mediated endocytosis of protein in the kidney. Kidney Int. 62:1707-1717. [DOI] [PubMed] [Google Scholar]
- 36.Tonello, F., P. Ascenzi, and C. Montecucco. 2003. The metalloproteolytic activity of the anthrax lethal factor is substrate-inhibited. J. Biol. Chem. 278:40075-40078. [DOI] [PubMed] [Google Scholar]
- 37.van Hennik, M. B., W. J. van der Vijgh, I. Klein, F. Elferink, J. B. Vermorken, B. Winograd, and H. M. Pinedo. 1987. Comparative pharmacokinetics of cisplatin and three analogues in mice and humans. Cancer Res. 47:6297-6301. [PubMed] [Google Scholar]
- 38.van Moorsel, C. J., H. M. Pinedo, G. Veerman, J. B. Vermorken, P. E. Postmus, and G. J. Peters. 1999. Scheduling of gemcitabine and cisplatin in Lewis lung tumour bearing mice. Eur. J. Cancer 35:808-814. [DOI] [PubMed] [Google Scholar]
- 39.Varughese, M., A. Chi, A. V. Teixeira, P. J. Nicholls, J. M. Keith, and S. H. Leppla. 1998. Internalization of a Bacillus anthracis protective antigen-c-Myc fusion protein mediated by cell surface anti-c-Myc antibodies. Mol. Med. 4:87-95. [PMC free article] [PubMed] [Google Scholar]
- 40.Vitale, G., L. Bernardi, G. Napolitani, M. Mock, and C. Montecucco. 2000. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem. J. 352:739-745. [PMC free article] [PubMed] [Google Scholar]
- 41.Vitale, G., R. Pellizzari, C. Recchi, G. Napolitani, M. Mock, and C. Montecucco. 1998. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem. Biophys. Res. Commun. 248:706-711. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, J. G., and W. E. Lindup. 1994. Cisplatin nephrotoxicity: decreases in mitochondrial protein sulphydryl concentration and calcium uptake by mitochondria from rat renal cortical slices. Biochem. Pharmacol. 47:1127-1135. [DOI] [PubMed] [Google Scholar]