Abstract
We screened 313 ceftazidime-resistant Enterobacteriaceae isolates obtained in the United States from 1999 to 2004 for all three known qnr genes. A qnr gene was present in 20% of Klebsiella pneumoniae isolates, 31% of Enterobacter sp. isolates, and 4% of Escherichia coli isolates. qnrA and qnrB occurred with equivalent frequencies and, except for qnrB in enterobacters, were stable over time. qnrS was absent.
Plasmids carrying qnr genes have been found to transmit quinolone resistance (5). These genes encode pentapeptide repeat proteins that block the action of ciprofloxacin on bacterial DNA gyrase and topoisomerase IV (7-9), resulting in low-level quinolone resistance with an increase in the MIC of ciprofloxacin for wild-type Escherichia coli J53 from 0.016 to 0.25 μg/ml. This reduced susceptibility is likely most important in that it facilitates the selection of mutants with higher-level resistance (5).
The geographical distribution of qnrA genes is known to be wide (6), but those of the newer qnr types qnrB (4) and qnrS (3) have not been studied; prior studies have also not evaluated temporal changes in prevalence. We thus surveyed 313 unique clinical isolates of Enterobacteriaceae collected in 1999, 2000, 2001, and 2004 throughout the United States for qnrA, qnrB, and qnrS by multiplex PCR screening.
Test isolates were drawn from Focus BioInova's TRUST study collection, which consists of equal numbers of nonrepeat clinical isolates of specified enterobacterial genera collected by specified laboratories in each of the nine continental U.S. census regions annually (Table 1). All Klebsiella pneumoniae, Enterobacter sp., and E. coli isolates with ceftazidime MICs of ≥16 μg/ml and ciprofloxacin MICs of ≥0.25 μg/ml were selected for this study. CLSI susceptibility criteria were used (1). Ceftazidime resistance was an inclusion criterion because of the strong association between qnr genes and plasmids carrying cephalosporinase genes; the ciprofloxacin MIC was the minimum expected for Enterobacteriaceae containing a qnr gene. Of 323 isolates with this phenotype, 313 (97%) were available. Another 32 isolates of K. pneumoniae and E. coli from 2002 were tested as part of a pilot study but were not included for statistical analysis because screening for 2002 was incomplete. A qnrB variant from one such isolate was sequenced, as discussed below. An isolate of Salmonella enterica serotype Bovismorbificans was used as the qnrS-positive control (2).
TABLE 1.
Characteristics of tested isolates
| Parameter | Value
|
||
|---|---|---|---|
| K. pneumoniae | Enterobacter spp. | E. coli | |
| Total no. of isolates | 106 | 160 | 47 |
| No. (%) of ciprofloxacin-susceptible isolates | 31 (29) | 54 (34) | 0 (0) |
| Patient characteristics | |||
| Age (yr) (mean [range]) | 54 (0-88) | 52 (0-89) | 56 (0-89) |
| No. (%) of inpatients | 65 (61) | 91 (57) | 20 (43) |
| No. of isolates from region | |||
| East North Central | 12 | 22 | 3 |
| East South Central | 6 | 27 | 4 |
| Middle Atlantic | 56 | 30 | 16 |
| Mountain | 1 | 9 | 6 |
| New England | 11 | 14 | 2 |
| Pacific | 4 | 21 | 7 |
| South Atlantic | 6 | 21 | 7 |
| West North Central | 0 | 6 | 0 |
| West South Central | 10 | 10 | 2 |
Screening was carried out by multiplex PCR amplification of qnrA, qnrB, and qnrS. Colonies were transferred to water in an Eppendorf tube and boiled to prepare DNA templates for PCR. Primers used were as follows: for qnrA, 5′-ATTTCTCACGCCAGGATTTG and 5′-GATCGGCAAAGGTTAGGTCA, to give a 516-bp product; for qnrB, 5′-GATCGTGAAAGCCAGAAAGG and 5′-ACGATGCCTGGTAGTTGTCC, to give a 469-bp product; and for qnrS, 5′-ACGACATTCGTCAACTGCAA and 5′-TAAATTGGCACCCTGTAGGC, to give a 417-bp product. All six primers were added to the template DNA and PCR Supermix High Fidelity (Invitrogen, Carlsbad, CA). The PCR conditions were 94°C for 45 s, 53°C for 45 s, and 72°C for 60 s, with a cycle number of 32. Positive (containing strains with known qnr genes) and negative (without DNA template) controls were included in each run. Amplification products were provisionally identified from their sizes in ethidium bromide-stained agarose gels. Positive results were confirmed by direct sequencing of PCR products or by amplification with primers 5′-TCAGCAAGAGGATTTCTCA and 5′-GGCAGCACTATTACTCCCA for qnrA and 5′-ATGACGCCATTACTGTATAA and 5′-GATCGCAATGTGTGAAGTTT for qnrB. qnrB3 was amplified using primers based on the 5′- and 3′-terminal sequences of qnrB1 for sequencing. qnrB4 was cloned into pBC SK (Stratagene, La Jolla, CA) as a 14-kb PstI fragment and sequenced using primers derived from the original qnrB4 amplification product.
Variables were compared using Fisher's exact test. Significant associations between demographic variables and qnr status found by univariate analysis were analyzed further by multiple logistic regression methods. Temporal trends were examined with the Mantel-Haenszel chi-square test. SAS software (SAS Institute, Cary, NC) was used for analyses.
qnrA and qnrB were detected in 15 (14%) and 6 (6%) of 106 K. pneumoniae isolates, respectively, in 18 (11%) and 32 (20%) of 160 Enterobacter sp. isolates, respectively, and in 1 (2%) and 1 (2%) of 47 E. coli isolates, respectively, during the study period. No isolates carried qnrS, and none had both qnrA and qnrB (Table 2). There was no significant change in the prevalence of either qnrA or qnrB in all isolates over time, but qnrB was detected in Enterobacter sp. isolates more often in 2004 (32%) than in 1999 (11%) (P = 0.02). qnr-positive K. pneumoniae isolates were found in all regions except the East North Central, West North Central, New England, and Pacific regions, and qnr-positive Enterobacter isolates were found in all nine census regions, with no geographic clustering of either gene.
TABLE 2.
Prevalence of qnr genes in Enterobacteriaceae isolates from the United States (1999-2004)
| Year | qnr locus | No. of isolates with locus/total no. of isolates (%)
|
||
|---|---|---|---|---|
| K. pneumoniae | Enterobacter spp. | E. coli | ||
| 1999 | A | 4/21 (19) | 6/45 (13) | 0/9 |
| B | 2/21 (10) | 5/45 (11) | 0/9 | |
| S | 0/21 | 0/45 | 0/9 | |
| Any | 6/21 (29) | 11/45 (24) | 0/9 | |
| 2000 | A | 4/33 (12) | 1/38 (3) | 0/8 |
| B | 2/33 (6) | 7/38 (18) | 1/8 (13) | |
| S | 0/33 | 0/38 | 0/8 | |
| Any | 6/33 (18) | 8/38 (21) | 1/8 (13) | |
| 2001 | A | 2/20 (10) | 7/39 (18) | 0/5 |
| B | 1/20 (5) | 8/39 (21) | 0/5 | |
| S | 0/20 | 0/39 | 0/5 | |
| Any | 3/20 (15) | 15/39 (38) | 0/5 | |
| 2004 | A | 5/32 (16) | 4/38 (11) | 1/25 |
| B | 1/32 (3) | 12/38 (32) | 0/25 | |
| S | 0/32 | 0/38 | 0/25 | |
| Any | 6/32 (19) | 16/38 (42) | 1/25 (4) | |
| Total | 21 (20) | 50 (31) | 2 (4) | |
In a univariate analysis, only inpatient status and gentamicin or trimethoprim-sulfamethoxazole resistance in enterobacters were associated with the presence of a qnr gene (Table 3). In a multivariable model, resistance to trimethoprim-sulfamethoxazole and resistance to gentamicin were independently associated with the presence of a qnr gene, but patient location was not.
TABLE 3.
Characteristics of qnr-positive and qnr-negative isolates
| Characteristic | No. with characteristic/total no. with qnr result (%)
|
Odds ratio (95% confidence interval) | P valuea | |
|---|---|---|---|---|
| Positive | Negative | |||
| Patient characteristics | ||||
| Age of ≥65 yr | 22/70 (31) | 77/196 (39) | 0.7 (0.4-1.3) | 0.25 |
| Male | 38/71 (53) | 102/194 (53) | 1.0 (0.6-1.8) | 1 |
| Inpatient | 49/68 (72) | 111/191 (58) | 1.86 (1.0-3.4) | 0.04 |
| Strain characteristicsb | ||||
| K. pneumoniae | ||||
| CIP resistant | 13/21 (62) | 62/85 (73) | 0.6 (0.2-1.6) | 0.42 |
| GEN resistant | 12/21 (57) | 50/85 (59) | 0.9 (0.4-2.5) | 1 |
| SXT resistant | 17/21 (81) | 67/85 (79) | 1.1 (0.3-3.8) | 1 |
| Enterobacter spp. | ||||
| CIP resistant | 35/50 (70) | 71/110 (65) | 1.28 (0.6-2.6) | 0.59 |
| GEN resistant | 43/50 (86) | 38/110 (35) | 11.6 (4.8-28.4) | <0.001 |
| SXT resistant | 47/50 (94) | 46/110 (42) | 21.8 (6.4-74.0) | <0.001 |
P values were determined by Fisher's two-tailed exact test.
CIP, ciprofloxacin; GEN, gentamicin; SXT, trimethoprim-sulfamethoxazole.
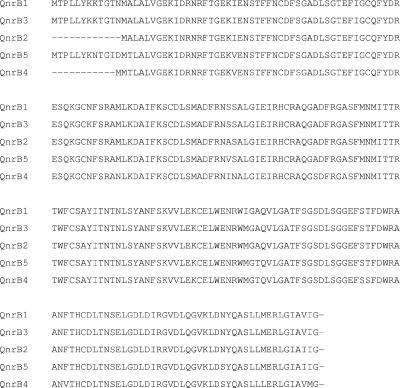
qnr genes were sequenced for 50 of 73 positive isolates from the study years and for two E. coli isolates from 2002 that were not included in the statistical analysis. All 29 sequenced qnrA genes were of the qnrA1 allele. Most qnrB genes (18/22) were the qnrB2 variant. Two other alleles, designated qnrB3 and qnrB4, each appeared twice. qnrB3 and qnrB4 have 98% and 86% nucleic acid identity, respectively, and 99% and 94% amino acid identity, respectively, with qnrB1 (Fig. 1).
FIG. 1.
Amino acid sequence alignment of qnrB variants. qnrB5 was recently identified in Salmonella enterica serotype Berta isolates from the United States.
Overall, in this survey of ceftazidime-resistant Enterobacteriaceae isolates from the United States, 23% of isolates were positive for either qnrA or qnrB, while qnrS was notably absent. qnr genes were significantly more prevalent in Enterobacter sp. and K. pneumoniae isolates than in E. coli isolates. The most striking finding was the wide geographical distribution of the qnr genes. qnrA was present in most regions, and qnrB was present in all census regions. The proportion of Enterobacter isolates harboring qnrB nearly tripled between 1999 and 2004, largely due to increased qnrB prevalence among ciprofloxacin-resistant isolates (3% to 31%). In contrast, no significant change in overall qnr gene prevalence occurred over the study years.
This study also suggests that there is considerable genetic diversity within the qnr genes themselves. At least three variants of qnrB—qnrB2, qnrB3, and qnrB4—circulate among U.S. Enterobacteriaceae. In addition, another variant, qnrB5, was recently detected in clinical isolates of Salmonella enterica serotype Berta from the United States (2).
Given the location of qnr genes in a variety of genetic environments, the most important limitation of this study was the inclusion of only ceftazidime-resistant organisms, a criterion that reflected the strong known association of qnr genes with cephalosporinases. Recent work (2), however, suggested that ceftazidime-susceptible isolates also harbor qnr genes. Another potential limitation comes from the use of specific primers for known qnr genes for PCR, since such primers might not amplify other, as yet unidentified qnr variants. Thus, our findings on the prevalence of qnr genes represent minimum estimates.
The association between qnr and gentamicin or trimethoprim-sulfamethoxazole resistance in Enterobacter spp. was expected because qnrA and qnrB are located in integrons; the lack of this association in K. pneumoniae was thus surprising. Notably, however, in both cases, qnr was similarly common in ciprofloxacin-susceptible and -resistant organisms. This finding may have clinical implications, since qnr genes found in organisms classified as susceptible may promote further selection from low- to high-level resistance when quinolones are used (5).
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study have been deposited in the EMBL/GenBank/DDBJ databases and assigned the following accession numbers: qnrB3, DQ303920; and qnrB4, DQ303921.
Acknowledgments
This work was supported in part by grants AI57576 (to D.C.H.) and AI43312 (to G.A.J.) from the National Institutes of Health, U.S. Public Health Service.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing, 15th informational supplement. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 2.Gay, K., A. Robicsek, J. Strahilevitz, C. H. Park, G. A. Jacoby, T. J. Barrett, F. Medalla, T. M. Chiller, and D. C. Hooper. Plasmid mediated quinolone resistance in non-Typhi serotypes of Salmonella enterica. Clin. Infect. Dis., in press. [DOI] [PubMed]
- 3.Hata, M., M. Suzuki, M. Matsumoto, M. Takahashi, K. Sato, S. Ibe, and K. Sakae. 2005. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob. Agents Chemother. 49:801-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacoby, G. A., K. E. Walsh, D. M. Mills, V. J. Walker, H. Oh, A. Robicsek, and D. C. Hooper. 2006. qnrB, another plasmid-mediated gene for quinolone resistance. Antimicrob. Agents Chemother. 50:1178-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Martinez, L., A. Pascual, and G. A. Jacoby. 1998. Quinolone resistance from a transferable plasmid. Lancet 351:797-799. [DOI] [PubMed] [Google Scholar]
- 6.Nordmann, P., and L. Poirel. 2005. Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae. J. Antimicrob. Chemother. 56:463-469. [DOI] [PubMed] [Google Scholar]
- 7.Tran, J. H., and G. A. Jacoby. 2002. Mechanism of plasmid-mediated quinolone resistance. Proc. Natl. Acad. Sci. USA 99:5638-5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran, J. H., G. A. Jacoby, and D. C. Hooper. 2005. Interaction of the plasmid-encoded quinolone resistance protein Qnr with Escherichia coli DNA gyrase. Antimicrob. Agents Chemother. 49:118-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran, J. H., G. A. Jacoby, and D. C. Hooper. 2005. Interaction of the plasmid-encoded quinolone resistance protein QnrA with Escherichia coli topoisomerase IV. Antimicrob. Agents Chemother. 49:3050-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]