Abstract
Daptomycin has in vitro activity against gram-positive anaerobic bacteria, although limited numbers of species have been tested. We studied the in vitro activities of daptomycin, vancomycin, and penicillin against more than 100 strains each of Clostridium difficile, C. perfringens, Finegoldia magna, and Propionibacterium acnes. Daptomycin Etest MICs and results from time-kill studies were determined for selected strains. For 392 of 421 strains (93%), daptomycin was inhibitory at ≤1 μg/ml, including 15 of 16 strains of C. difficile with elevated linezolid MICs of 8 and 16 μg/ml, all 32 strains with moxifloxacin MICs of ≥4 μg/ml, and all 16 strains resistant to clindamycin. Daptomycin MICs were also ≤1 μg/ml for all 16 F. magna strains resistant to clindamycin and all 32 strains resistant to tetracycline. Only one strain, a C. perfringens strain, had a MIC of >2 μg/ml to daptomycin. Eighty-five and 92.5% of the Etest MICs were within 1 dilution of the agar dilution method for all drugs at 24 and 48 h, respectively. In time-kill studies, a C. difficile strain was inhibited by both daptomycin and vancomycin at 1, 2, 4, 8, and 24 h; colony counts were decreased by 2.3 to 2.9 log at 24 h. Vancomycin was not bactericidal for C. perfringens; however, daptomycin showed bactericidal activity as early as 1 h at four and eight times the MIC and at 2 and 4 h at two and four times the MIC.
Daptomycin is a cyclic lipopeptide antibiotic produced by Streptomyces roseosporus with bactericidal activity against resistant aerobic gram-positive organisms, such as methicillin-resistant Staphylococcus aureus, community-acquired methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and penicillin-resistant pneumococci (3, 8), including multidrug-resistant strains (18).
It has been found to be safe and effective in complicated skin and skin structure infections (2, 21) and does not disrupt the anaerobic microflora or promote colonization by vancomycin-resistant enterococci or extended-spectrum-β-lactamase-producing Klebsiella pneumoniae in mice (17). While antibiotic resistance is an increasing worldwide problem, daptomycin has shown low spontaneous resistance rates in vitro (19). Resistant strains have rarely been reported thus far (13, 15, 16, 20); however, in one instance (13), daptomycin at a high concentration retained bactericidal activity against resistant isolates of S. aureus.
Daptomycin has been shown to have in vitro activity against a variety of anaerobic gram-positive bacterial pathogens, such as Clostridium perfringens, Finegoldia magna, and Propionibacterium acnes, that may be present in polymicrobial infections (4, 9-11), although limited numbers of each species have been tested previously. To explore its in vitro efficacy further, we tested more than 100 strains each of Clostridium difficile, C. perfringens, Finegoldia magna, and Propionibacterium acnes and compared the daptomycin MICs with those of vancomycin and penicillin. Additionally, daptomycin Etest MICs were determined for selected strains in each group and time-kill studies with daptomycin and vancomycin were performed on one strain of C. difficile and two strains of C. perfringens.
MATERIALS AND METHODS
The in vitro activities of daptomycin, vancomycin, and penicillin against more than 100 strains each of Clostridium difficile, C. perfringens, Finegoldia magna, and Propionibacterium acnes were determined. The strains selected included F. magna isolates known to be resistant to clindamycin and tetracycline and C. difficile isolates resistant to linezolid, clindamycin, and fluoroquinolones (5). Control strains included Staphylococcus aureus ATCC 29213, Eubacterium lentum ATCC 40335, and Bacteroides fragilis ATCC 25285.
Susceptibility testing was performed according to the reference agar dilution method previously described in the CLSI M11-A6 document (7). Antimicrobial agents obtained were daptomycin (Cubist Pharmaceuticals, Inc., Lexington, MA), penicillin (Sigma Chemical Co., St. Louis, MO), and vancomycin (Eli Lilly & Co., Indianapolis, IN). The antimicrobial agents were reconstituted according to the manufacturers' instructions, serially diluted, and added to molten-supplemented brucella agar. For daptomycin plates, the calcium (Ca2+) concentration in brucella agar was determined prior to testing using a Ca2+ electrode and prepared agar was supplemented to a final concentration of 50 mg/liter of Ca2+. Plates were inoculated on the day of preparation. Drug-free plates were included as growth controls.
The isolates were obtained from human clinical specimens, including blood, skin and skin structure, intraabdominal, and feces, identified by standard methods (14) and maintained in skim milk at −70°C. The strains were taken from the freezer and transferred at least twice on brucella agar (Anaerobe Systems, Morgan Hill, CA) supplemented with hemin, vitamin K1, and 5% sheep blood (brucella blood agar [BBA]) to ensure purity and good growth. Colonies were suspended in brucella broth to a density equal to a 0.5 McFarland standard. The suspensions were applied to the antibiotic plates with a Steers replicator that delivered a final inoculum of approximately 105 CFU/spot. The plates were incubated in an anaerobic chamber at 36°C for 44 to 48 h. The MIC was defined as the concentration of the drug that completely inhibited growth or caused a marked reduction in the appearance of growth compared to a drug-free growth control.
The Etest was performed by suspending strains in brucella broth to a turbidity of a 0.5 to 1 McFarland standard, inoculating the suspension onto BBA according to the manufacturer's directions, and then applying the Etest strips containing a gradient of daptomycin plus calcium. The plates were incubated in the anaerobic chamber as described above and examined after 24 and 44 h. The MIC was recorded as the point where the ellipse intersected the interpretive scale on the strip.
Time-kill studies were performed in reduced brucella broth supplemented with vitamin K1 and hemin. Drug concentrations used were two, four, and eight times the MIC as previously determined by agar dilution; strains with higher MICs for daptomycin were selected. Drugs and test strains were added to tubes, which were then capped with Hungate-type caps, in the anaerobic chamber and incubated in an ambient air incubator. Aliquots were removed with a tuberculin syringe at 0, 1, 2, 4, 8, and 24 h and plated using an automated spiral plater (Spiral Biotech, Inc., Norwood, MA) onto prereduced, anaerobically sterilized 150-mm-diameter BBA plates (Anaerobe Systems). Plates were incubated for 24 h for Clostridia and 48 h for the other strains; colonies were quantitated according to the instrument's usage guidelines. Bactericidal activity was defined as a minimum 3-log reduction in bacterial counts at 24 h.
RESULTS AND DISCUSSION
The agar dilution MICs are shown in Table 1. Although interpretive daptomycin breakpoints for anaerobes are not yet available, most strains were inhibited by ≤1 μg/ml and MICs were generally the same or 1 dilution higher than vancomycin MICs. A few strains (14 of 102 C. difficile, 11 of 101 C. perfringens, 2 of 117 P. acnes, and 3 of 101 F. magna strains) required 2 μg/ml for inhibition. Only one strain, a C. perfringens strain, had a daptomycin MIC of >2 μg/ml, 8 μg/ml by agar dilution (tested twice) and 6 μg/ml by Etest; however, this strain also had a linezolid MIC of 4 μg/ml and a quinupristin-dalfopristin MIC of 4 μg/ml (determined previously). The daptomycin MIC was ≤1 μg/ml for 15 of 16 strains of C. difficile that were previously determined to have elevated linezolid MICs of 8 and 16 μg/ml, all 32 strains that had moxifloxacin MICs of ≥4 μg/ml, and all 16 strains that were resistant to clindamycin. The daptomycin MIC was also ≤1 μg/ml for all 16 F. magna strains resistant to clindamycin and all 32 strains resistant to tetracycline.
TABLE 1.
Agar dilution MICs of daptomycin, vancomycin, and penicillin against C. difficile, C. perfringens, P. acnes, and F. magna
| Organism (no. of isolates) | Antimicrobial agent | MIC (μg/ml)a
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Clostridium difficile (102) | Daptomycin | 0.125-2 | 0.5 | 1 |
| Vancomycin | 0.25-2 | 0.5 | 1 | |
| Penicillin G | 1-8 | 1 | 4 | |
| Clostridium perfringens (101) | Daptomycin | 0.06-8b | 0.5 | 2 |
| Vancomycin | 0.25-0.5 | 0.5 | 0.5 | |
| Penicillin G | ≤0.015-0.25 | 0.06 | 0.125 | |
| Propionibacterium acnes (117) | Daptomycin | 0.25-1 | 0.5 | 1 |
| Vancomycin | 0.25-0.5 | 0.5 | 0.5 | |
| Penicillin G | ≤0.015-0.25 | 0.03 | 0.06 | |
| Finegoldia magna (101) | Daptomycin | ≤0.015-2 | 0.5 | 1 |
| Vancomycin | 0.125-0.5 | 0.25 | 0.5 | |
| Penicillin G | ≤0.015-1 | 0.125 | 0.25 | |
50% and 90%, MICs at which 50 and 90% of isolates are inhibited, respectively.
Single isolate at >2 μg/ml.
The Etest and agar dilution comparisons for daptomycin are shown in Table 2. Eighty-five and 92.5% of the MICs were within 1 dilution of the agar dilution reference method for all drugs at 24 and 48 h, respectively. C. perfringens was the only organism that did not fall within 1 dilution of the agar dilution method, with three (30%) of the strains reading 2 dilutions higher by Etest than the reference method; this was ascribed to the spreading nature of these strains.
TABLE 2.
Comparison of Etest with agar dilution daptomycin MICs after 24 and 48 h of incubation
| Organism | No. of isolates at indicated no. of dilutions away from agar dilution reference method value at:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h
|
48 h
|
|||||||||
| −2 | −1 | 0 | +1 | +2 | −2 | −1 | 0 | +1 | +2 | |
| C. difficile | 4 | 6 | 4 | 5 | 1 | |||||
| C. perfringens | 1 | 2 | 5 | 2 | 1 | 2 | 4 | 3 | ||
| P. acnes | 3 | 7 | 2 | 6 | 2 | |||||
| F. magna | 4 | 3 | 3 | 1 | 4 | 5 | ||||
| Total | 4 | 14 | 12 | 8 | 2 | 8 | 17 | 12 | 3 | |
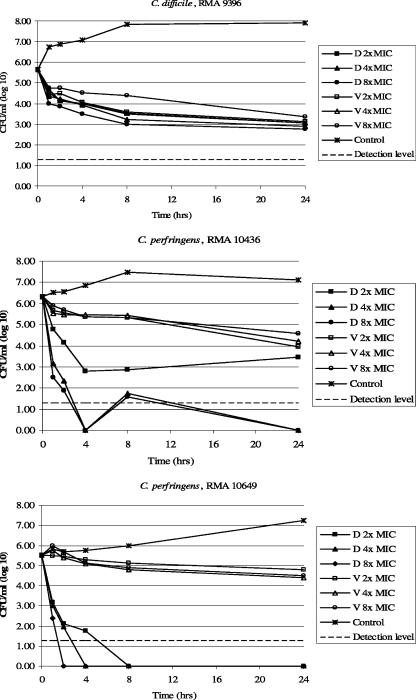
The time-kill assays for one strain of C. difficile and two strains of C. perfringens are shown in Fig. 1. MICs for daptomycin were 2 μg/ml for C. difficile RMA 9396 (Fig. 1, top) as well as C. perfringens RMA 10436 (Fig. 1, middle) and 8 μg/ml for C. perfringens RMA 10649 (Fig. 1, bottom); MICs for vancomycin were 0.5 μg/ml for all three strains. Test concentrations represent two, four, and eight times the MIC. The detection level was ≥20 CFU/ml. Although the C. difficile strain was inhibited similarly by daptomycin and vancomycin at each time tested, neither drug was fully bactericidal at 24 h (colony counts were decreased by 2.3 to 2.9 log). C. perfringens RMA 10436 was only slightly inhibited by vancomycin (a <1-log reduction from the control at 1, 2, 4, and 8 h) and was not bactericidal; however, daptomycin showed bactericidal activity as early as 1 h at four and eight times the MIC and at 4 h for all concentrations. Vancomycin was also not bactericidal for C. perfringens RMA 10649 (a <1-log reduction from the control at 1, 2, 4, 8, and 24 h), while daptomycin was bactericidal at 1 h at eight times the MIC and at 2 h for all concentrations.
FIG. 1.
Time-kill assays. MICs for daptomycin (D) were 2 μg/ml for C. difficile RMA 9396 (top) and C. perfringens RMA 10436 (middle) and 8 μg/ml for C. perfringens RMA 10649 (bottom). MICs for vancomycin (V) were 0.5 μg/ml for all three strains. Test concentrations for daptomycin and vancomycin were two, four, and eight times the MIC. The detection level is ≥20 CFU/ml.
Conclusion.
Daptomycin showed excellent in vitro activity, with most MICs at or below 1 μg/ml, against a large sampling of a variety of anaerobic gram-positive bacteria that are frequently isolated from clinical infections. Although there are no clinical data to support a breakpoint for anaerobes, ≤1 μg/ml is at or below the CLSI breakpoints for Staphylococcus aureus and β-streptococci (≤1 μg/ml) as well as vancomycin-sensitive Enterococcus faecalis (≤4 μg/ml) (6). Daptomycin is reversibly bound to human plasma proteins, primarily serum albumin protein, in a concentration-independent manner. The mean serum protein binding of daptomycin is approximately 92% at a dosage of 4 or 6 mg/kg of body weight (manufacturer's data), but studies have shown that serum increases the MIC by approximately twofold (12, 22). The Etest showed good correlation with the reference agar dilution method when incubated for 48 versus 24 h (92.5 versus 85%). The time-kill studies demonstrate daptomycin activity that is both concentration related and functional in an anaerobic environment. Although daptomycin was not fully bactericidal (colony counts showed a 2.6- to 2.9-log reduction) against the one strain of C. difficile tested, it showed good bactericidal activity (a >3-log reduction) against the two strains of C. perfringens tested. While a MIC of 8 μg/ml for the one strain of C. perfringens indicates in vitro resistance, it is difficult to predict clinical efficacy; however, the package insert provides maximum concentration of drug in serum values of 57.8, 98.6, and 133 μg/ml at dosages of 4, 6, and 8 mg/kg, which are above four and eight times the MIC for this strain. One study with vancomycin-sensitive enterococci shows that MICs of 2, 4, and 8 μg/ml are treatable in a mouse model (1); however, clinical data must be used to assess in vivo efficacies for organisms with MICs greater than the established breakpoint.
Acknowledgments
This study was supported by a grant from Cubist Pharmaceuticals, Inc., Lexington, MA.
REFERENCES
- 1.Alder, J., T. Li, D. Yu, L. Morton, J. Silverman, X. X. Zhang, I. Critchley, and G. Thorne. 2003. Analysis of daptomycin efficacy and breakpoint standards in a murine model of Enterococcus faecalis and Enterococcus faecium renal infection. Antimicrob. Agents Chemother. 47:3561-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbeit, R. D., D. Maki, F. P. Tally, E. Campanaro, and B. I. Eisenstein. 2004. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 38:1673-1681. [DOI] [PubMed] [Google Scholar]
- 3.Barry, A. L., P. C. Fuchs, and S. D. Brown. 2001. In vitro activities of daptomycin against 2,789 clinical isolates from 11 North American medical centers. Antimicrob. Agents Chemother. 45:1919-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow, A. W., and N. Cheng. 1988. In vitro activities of daptomycin (LY146032) and paldimycin (U-70,138F) against anaerobic gram-positive bacteria. Antimicrob. Agents Chemother. 32:788-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Citron, D. M., C. V. Merriam, K. L. Tyrrell, Y. A. Warren, H. Fernandez, and E. J. C. Goldstein. 2003. In vitro activities of ramoplanin, teicoplanin, vancomycin, linezolid, bacitracin, and four other antimicrobials against intestinal anaerobic bacteria. Antimicrob. Agents Chemother. 47:2334-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute/NCCLS. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 7.Clinical and Laboratory Standards Institute/NCCLS. 2004. Methods for antimicrobial susceptibility testing of anaerobic bacteria. Approved standard M11-A6. NCCLS, Wayne, Pa.
- 8.Fuchs, P. C., A. L. Barry, and S. D. Brown. 2002. In vitro bactericidal activity of daptomycin against staphylococci. J. Antimicrob. Chemother. 49:467-470. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein, E. J. C., D. M. Citron, C. V. Merriam, Y. A. Warren, K. L. Tyrrell, and H. T. Fernandez. 2003. In vitro activities of daptomycin, vancomycin, quinupristin-dalfopristin, linezolid, and five other antimicrobials against 307 gram-positive anaerobic and 31 Corynebacterium clinical isolates. Antimicrob. Agents Chemother. 47:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein, E. J. C., D. M. Citron, C. V. Merriam, Y. A. Warren, K. L. Tyrrell, H. T. Fernandez, and A. Bryskier. 2005. Comparative in vitro activities of XRP 2868, pristinamycin, quinupristin-dalfopristin, vancomycin, daptomycin, linezolid, clarithromycin, telithromycin, clindamycin, and ampicillin against anaerobic gram-positive species, actinomycetes, and lactobacilli. Antimicrob. Agents Chemother. 49:408-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenwood, D., and J. Palfreyman. 1987. Comparative activity of LY146032 against anaerobic cocci. Eur. J. Clin. Microbiol. 6:682-684. [DOI] [PubMed] [Google Scholar]
- 12.Hanberger, H., L. E. Nilsson, R. Maller, and B. Isaksson. 1991. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob. Agents Chemother. 35:1710-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayden, M. K., K. Rezai, R. A. Hayes, K. Lolans, J. P. Quinn, and R. A. Weinstein. 2005. Development of daptomycin resistance in vivo in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43:5285-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jousimies-Somer, H. R., P. Summanen, D. M. Citron, E. J. Baron, H. M. Wexler, and S. M. Finegold. 2002. Wadsworth-KTL anaerobic bacteriology manual. Star Publishing, Belmont, Calif.
- 15.Mangili, A., I. Bica, D. R. Snydman, and D. H. Hamer. 2005. Daptomycin-resistant, methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 40:1058-1060. [DOI] [PubMed] [Google Scholar]
- 16.Marty, F. M., W. W. Yeh, C. B. Wennersten, L. Venkataraman, E. Albano, E. P. Alyea, H. S. Gold, L. R. Baden, and S. K. Pillai. 2006. Emergence of a clinical daptomycin-resistant Staphylococcus aureus isolate during treatment of methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. J. Clin. Microbiol. 44:595-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pultz, N. J., U. Stiefel, and C. J. Donskey. 2005. Effects of daptomycin, linezolid, and vancomycin on establishment of intestinal colonization with vancomycin-resistant enterococci and extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae in mice. Antimicrob. Agents Chemother. 49:3513-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sader, H. S., J. M. Streit, T. R. Fritsche, and R. N. Jones. 2004. Antimicrobial activity of daptomycin against multidrug-resistant gram-positive strains collected worldwide. Diagn. Microbiol. Infect. Dis. 50:201-204. [DOI] [PubMed] [Google Scholar]
- 19.Silverman, J. A., N. Oliver, T. Andrew, and T. Li. 2001. Resistance studies with daptomycin. Antimicrob. Agents Chemother. 45:1799-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skiest, D. J. 2006. Treatment failure resulting from resistance of Staphylococcus aureus to daptomycin. J. Clin. Microbiol. 44:655-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein, G. E. 2005. Safety of newer parenteral antibiotics. Clin. Infect. Dis. 41(Suppl. 5):S293-S302. [DOI] [PubMed] [Google Scholar]
- 22.Wise, R., J. M. Andrews, and J. P. Ashby. 2001. Activity of daptomycin against gram-positive pathogens: a comparison with other agents and the determination of a tentative breakpoint. J. Antimicrob. Chemother. 48:563-567. [DOI] [PubMed] [Google Scholar]