Abstract
We developed dinoflagellate-specific 18S rRNA gene primers. PCR amplification using these oligonucleotides for a picoplanktonic DNA sample from Long Island Sound yielded 24 clones, and all but one of these clones were dinoflagellates primarily belonging to undescribed and Amoebophrya-like lineages. These results highlight the need for a systematic investigation of picodinoflagellate diversity in both coastal and oceanic ecosystems.
Dinoflagellates have received considerable attention due to their ecological and economical significance and their remarkable cytological and genetic features (6, 7, 20). However, our knowledge of the species diversity of these organisms remains limited even though novel living (2, 3, 10, 14) and fossil lineages (4, 19) have been discovered. Knowledge of the diversity of “small” dinoflagellates is particularly deficient. The recent discovery of ultraplanktonic (<5-μm) and picoplanktonic (<3-μm) dinoflagellates in Antarctica and the Pacific Ocean (13, 15), respectively, is the first demonstration of a rich biodiversity of small dinoflagellates that have escaped routine microscopic detection. A better understanding of dinoflagellate biodiversity requires targeted approaches, particularly for picoplanktonic species.
Development of dinoflagellate-oriented primers.
Based on a large database of nuclear small-subunit (18S) rRNA genes, we designed PCR primers that target dinoflagellates. A total of 140 18S rRNA gene sequences, including sequences from dinoflagellates, diatoms, chlorophytes, haptophytes, cryptophytes, and other algae, were obtained from GenBank and were aligned using ClustalW1.8; 11 of the dinoflagellate species were sequenced in this study (GenBank accession no. DQ388456 to DQ388466). Regions unique to dinoflagellates were used to design three forward and two reverse PCR primers (Table 1), which were paired with previously described eukaryotic 18S rRNA gene universal primers (22) for DNA amplification.
TABLE 1.
18S rRNA gene PCR primers designed in the present study
| Primera | Sequence (5′-3′) | Source or reference |
|---|---|---|
| Dino18SF1 | AAGGGTTGTGTTYATTAGNTACARAAC | This study |
| Dino18SF2 | ATTAATAGGGATAGTTGGGGGC | This study |
| Dino18SF3 | GTCAGAGGTGAAATTCTTGGATTTGTT | This study |
| Dino18SR1 | GAGCCAGATRCDCACCCA | This study |
| Dino18SR2 | TGCTTTCGCAGTAGTYYGTCTTTAAC | This study |
| 18ScomF1 | GCTTGTCTCAAAGATTAAGCCATGC | 22 |
| 18ScomR1 | CACCTACGGAAACCTTGTTACGAC | 22 |
In primer designations, F indicates a forward primer and R indicates a reverse primer.
The primers were tested with 33 genera of cultured dinoflagellates (35 species, including Oxyrrhis marina), as well as nine other taxa (Table 2). Algal cultures were grown in f/2 medium (28‰ or 15‰ salinity), cells were harvested, and DNA was purified as previously described (23). Briefly, after cell lysis in 1 ml DNA buffer (100 mM EDTA [pH 8.0], 0.5% sodium dodecyl sulfate, 200 μg ml−1 proteinase K), DNA was purified using DNA Clean and Concentrator columns (Zymo Research, Orange, CA). With these DNA samples as templates, PCR was performed using five combinations of the primers, as follows: primers 18ScomF1 and Dino18SR1 (expected product size, 0.65 kb), primers 18ScomF1 and Dino18SR2 (0.92 kb), primers Dino18F1 and 18Scom R1 (1.60 kb), primers Dino18F2 and 18Scom R1 (0.92 kb), and primers Dino18F3 and 18S com R1 (0.90 kb). All primer sets except the Dino18SF2-18ScomR1 set exhibited specificity for dinoflagellate 18S rRNA genes, which allowed amplification from most taxa examined (Table 2). The only exceptions were O. marina (often referred to as an ancestral dinoflagellate [17] or a predinoflagellate [18]) and Exuviaella cassubica, for which all primers failed, likely because their 18S rRNA gene sequences are significantly divergent. Of the four pairs of dinoflagellate-specific primers, 18ScomF1-Dino18SR1 and Dino18F1-18ScomR1 showed superior sensitivity and were able to detect 1 to 10 cells/reaction mixture for most of the dinoflagellates tested. Primers Dino18F1 and 18Scom R1 was chosen for further study because they spanned a larger 18S rRNA gene region (1.6 kb).
TABLE 2.
Specificity of primers (in five pairs) with various protists
| Organism | Amplification with the indicated primer set
|
||||
|---|---|---|---|---|---|
| 18ScomF1-Dino18SR1 | 18ScomF1-Dino18SR2 | Dino18SF1-18ScomR1 | Dino18SF2-18ScomR1 | Dino18SF3-18ScomR1 | |
| Nondinoflagellates | |||||
| Ditylum brightwellii | − | − | − | − | − |
| Dunaliella tertiolecta | − | − | − | − | − |
| Emiliania huxleyii | − | − | − | + | − |
| Isochrysis galbana | − | − | − | + | − |
| Neoparamoeba aestuarina | − | − | − | + | − |
| Nitzschia alba | − | − | − | − | − |
| Perkinsus marinus | − | + | + | + | + |
| Rhodomonas sp. | − | − | − | + | − |
| Skeletonema costatum | − | − | − | − | − |
| Dinoflagellates | |||||
| Adenoides eludens | + | + | + | + | + |
| Akashiwo sanguinea | + | + | + | + | + |
| Alexandrium affine | + | + | + | + | + |
| Alexandrium tamarense | + | + | + | + | + |
| Amoebophrya sp. | − | − | + | + | − |
| Amphidinium carterae | + | + | − | + | − |
| Ceratium longipes | + | + | + | + | + |
| Ceratocorys horrida | + | + | + | − | + |
| Coolia monotis | + | − | + | − | + |
| Crypthecodinium cohnii | + | + | − | + | + |
| Cryptoperidiniopsis sp. strain CCMP1828 | + | + | + | + | + |
| Dinophysis acuminata | + | + | + | + | + |
| Exuviaella cassubica (synonym, Prorocentrum cassubicum) | + | + | + | + | + |
| Exuviaella pusilla (synonym, Prorocentrum nanum) | − | − | − | − | − |
| Gambierdiscus toxicus | + | − | − | + | − |
| Gonyaulax cochlea | + | + | − | + | + |
| Gymnodinium catenatum | + | + | + | + | + |
| Gyrodinium dorsum | + | + | + | + | + |
| Heterocapsa triquetra | + | + | + | + | + |
| Karenia brevis | + | + | + | + | + |
| Karlodinium micrum (synonym, Gyrodinium galatheanum) | + | + | + | + | + |
| Katodinium rotundatum | + | + | + | + | + |
| Lingulodinium polyedrum | + | + | + | − | + |
| Noctiluca scintillans | − | + | + | + | + |
| Oxyrrhis marina | − | − | − | − | − |
| Peridinium foliaceum (= Kryptoperidinium foliaceum) | − | + | + | + | + |
| Pfiesteria piscicida | + | + | + | + | + |
| Pseudopfiesteria shumwayae | + | + | + | + | + |
| Prorocentrum lima | + | + | + | − | − |
| Prorocentrum minimum | + | + | + | + | + |
| Pyrocystis lunula | + | + | − | + | + |
| Pyrodinium bahamense | + | + | + | + | + |
| Scrippsiella sweeneyae | + | + | + | + | + |
| Symbiodinium microadriaticum | + | + | + | + | + |
| Thecadinium inclinatum | + | + | + | + | + |
Detection of picodinoflagellates in Long Island Sound.
Three water samples collected on 8 September 2005 along the boat dock of the University of Connecticut Avery Point campus (Long Island Sound) were combined and mixed. Microscopic examination of a subsample revealed that phytoplankton lineages such as Nitzschia, Navicula, Chaetoceros, and Eucampia were dominant. A 2-liter subsample was prescreened (100-μm mesh), and this was followed by passage through a 3-μm polycarbonate membrane (Nuclepore, Pleasanton, CA) under a low vacuum pressure (<10 lb/in2). One liter of the filtrate was collected and filtered onto a 0.2-μm-pore-size, 47-mm-diameter polycarbonate membrane (Nuclepore). The filter membrane was cut into small pieces using sterile scissors, placed in a 1.5-ml microcentrifuge tube, and stored at −80°C until DNA extraction. To examine whether any large plankton were present in the sample, 100 ml of the 3-μm filtrate was filtered onto a 0.2-μm-pore size, 25-mm-diameter black Poretics polycarbonate membrane (Osmonics Inc., Minnetonka, MN), fixed with 1% paraformaldehyde, and stained with 0.005% acridine orange. Observation with an epifluorescence microscope (Olympus BX51) revealed only small organisms except for one large cell (length, ∼12 μm) that appeared to be a Heterocapsa cell.
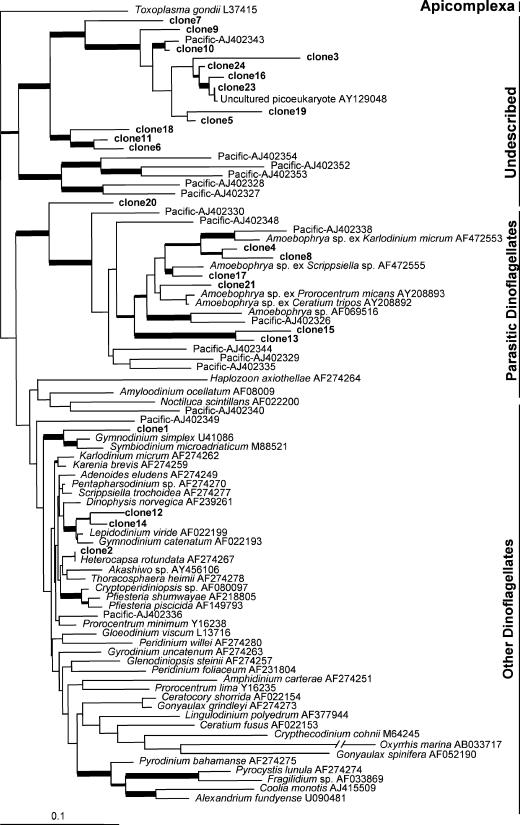
DNA extracted from the <3-μm plankton sample as described above was used for PCR amplification with primers Dino18F1 and 18Scom R1. The PCR was performed using Takara ExTaq DNA polymerase (Takara Mirus Bio, Madison, WI) following the manufacturer's instructions, with an annealing temperature of 58°C. The amplicon was cloned into a TA vector, and 24 of the clones were randomly selected and sequenced (GenBank accession no. DQ386737 to DQ386760). A BLAST search showed that all 24 clones were related to dinoflagellates. These sequences were aligned using CLUSTAL W (1.8) with a Kimura two-parameter model (11). A maximum likelihood tree was inferred using PHYML (5) based on the GTR + I + Γ model of nucleotide substitution, which was identified as the best-fit model by Modeltest3.7 (16). Species of chlorophytes (as an outgroup), cryptophytes, diatoms, ciliates, apicomplexa, and dinoflagellates (a total of 101 taxa) were initially included in the analysis to obtain a global view of the distribution of the dinoflagellate-like clones. One (clone 12) clustered with the recently recognized parasitic ciliate Cryptocaryon irritans (21) with robust bootstrap support, whereas the other 23 clones clustered with dinoflagellates. In further analyses we focused on dinoflagellates with Toxoplasma gondii (apicomplexa) as the outgroup. The results indicated that 12 of the 23 dinoflagellate-like clones (clones 3, 5 to 7, 9 to 11, 16, 18, 19, 23, and 24) were members of a clade of undescribed eukaryotes comprising previously isolated picoeukaryotes from the Pacific Ocean (Fig. 1). This clade received strong bootstrap support and diverged before all known dinoflagellates (Fig. 1), suggesting that it could be an ancestral clade of dinoflagellates or a lineage that is intermediate between dinoflagellates and apicomplexa. Of the remaining 11 clones, 7 formed a clade with the parasitic dinoflagellate Amoebophrya. Four of these seven clones (clones 4, 8, 17, and 21) clustered tightly with Amoebophrya sp., which infects various dinoflagellate species, and the other three (clones 13, 15, and 20) appeared to be more ancestral. These assignments had moderate to strong support. One of the four other clones (clone 1) was related to a Gymnodiniales-dominated clade and clustered tightly with Gymnodinium simplex and Symbiodinium microadriaticum, and two clones (clones 12 and 14) clustered with Gymnodinium catenatum/Lepidodinium viride. The last clone (clone 2) appeared to be a Heterocapsa species, likely related to the large cell observed under the microscope (see above). To our knowledge, this is the first documentation of the presence of picodinoflagellates in coastal waters.
FIG. 1.
Maximum likelihood tree of dinoflagellates constructed using Phyml V2.4.4. T. gondii was used as the outgroup. The parameters of the GTR + I + Γ model of nucleotide substitution were estimated as follows: the frequencies of nucleotides were 0.23931 for A, 0.31386 for T, 0.18944 for C, and 0.25739 for G; the rate parameters were 1.1979 for A ↔ C, 3.3746 for A ↔ G, 1.3953 for A ↔ T, 0.9178 for C ↔ G, 5.1569 for C ↔ T, and 1.0 (fixed) for G ↔ T; the fraction of invariant sites was 0.2824; the shape parameter (α) was 0.6838; and the likelihood value (loglk) was −34037. The robustness of species groups was assessed using the bootstrap approach with 100 resamplings. The thickest lines indicate bootstrap values of >80%, the thick lines indicate bootstrap values of >50%, and the thin lines indicate bootstrap values of <50%. Scale bar = 0.1 substitution per base.
An increasing number of studies have indicated that field DNA samples derived from mixed microbial assemblages are prone to formation of PCR chimeras by cDNA strands from different species, thus creating artificial, novel genes (1, 9). To examine whether any of the sequences obtained in this study was a chimera of different species in the water sample, sequences of the field-derived clones, as well as previously reported dinoflagellate sequences used in this study, were analyzed using the program Bellerophon (8). The details of the analysis are shown in Table S1 and Fig. S1 in the supplemental material. The results indicated that most clones appeared to be nonchimeric sequences; the only exception was one clone (clone 20) that was ambiguous.
Wide distribution of diverse and predominantly unknown picodinoflagellates.
Strikingly, one-half of the 24 clones identified in this study were closely related to undescribed picodinoflagellate lineages from the Pacific Ocean. Furthermore, the seven clones that clustered with the parasitic dinoflagellate Amoebophrya were allied with lineages from the Pacific Ocean. Separate analyses that included the shorter sequences from Antarctic deep water (13) revealed that these sequences also were closely related to the Amoebophrya-like lineages detected in the Pacific Ocean and Long Island Sound (results not shown). In addition, lineages close to Gymnodinium/Symbiodinium have also been found in the Pacific Ocean. Therefore, the distribution of picodinoflagellates extends from the open ocean to coastal waters, suggesting that small dinoflagellates are cosmopolitan. Moreover, the similarity between the dinoflagellate species composition in Long Island Sound surface water and the dinoflagellate species composition in the aphotic zone of Antarctica and the Pacific Ocean (13, 15), especially the presence of Amoebophrya, suggests that heterotrophic and parasitic dinoflagellates may be more common than currently thought. The ease with which novel lineages were isolated also suggests that dinoflagellate diversity has been underestimated. However, this observation should not be surprising given that even in the larger cell size range new species have frequently been discovered (e.g., Stoeckeria, Takayama, Polarella, and Pfiesteria [2, 3, 10, 14]). The dearth of information regarding the lineages of small dinoflagellates is a result of a lack of targeted analyses.
Interestingly, dinoflagellates have normally been categorized as larger phytoplankton. Recently, LaJeunesse et al. (12) suggested that Symbiodinium (4 to 13 μm) is the lineage containing the smallest dinoflagellates. It is clear now that smaller dinoflagellates are present in both oceanic and neritic waters and that the smallest organism is likely yet to be described. Once the widespread distribution of these picodinoflagellates is verified by culture and morphological analyses, their role in the microbial loop in the world's ocean can begin to be assessed. In addition, given the pressing need for a complete dinoflagellate genome sequence, free-living picodinoflagellates like those described here are likely to be the best candidates for such an attempt. The “normal-size” taxa have human-size (or much larger) genomes (12), which makes the use of current sequencing approaches with these organisms infeasible.
Concluding remarks.
This is the first report of dinoflagellate-oriented primers and the first documentation of the presence of picodinoflagellates in coastal waters. Although the number of clones sequenced was limited, our findings nevertheless revealed the high level of diversity and the dominance of novel lineages of picodinoflagellates. Therefore, larger-scale targeted analyses of different oceanic regions are essential for determining the true biodiversity of these taxa.
Nucleotide sequence accession numbers.
The nucleotide sequences of the 24 field-retrieved picoplankton clones have been deposited in the GenBank database under accession numbers DQ386737 to DQ386760, and the nucleotide sequences of the 11 dinoflagellate cultures have been deposited in the GenBank database under accession numbers DQ388456 to DQ388466.
.
Supplementary Material
Acknowledgments
We are grateful to Terry Gaasterland of Scripps Genome Center for access to her computing facility for the phylogenetic analysis and to Christopher Dungan of the Maryland DNR Cooperative Oxford Laboratory for kindly providing Perkinsus species.
This work was supported by National Science Foundation grants DEB-0344186 (to S.L. and H.Z.) and DEB-0107754 and EF-0431117 (to D.B.).
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Berhey, C., J. Fahrni, and J. Pawlowski. 2004. How many novel eukaryotic ‘kingdoms’? Pitfalls and limitations of environmental DNA surveys. BMC Biol. 2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burkholder, J. M., E. J. Noga, C. H. Hobbs, and H. B. Glasgow. 1992. New “phantom” dinoflagellate is the causative agent of major estuarine fish kills. Nature 358:407-410. [DOI] [PubMed] [Google Scholar]
- 3.de Salas, F. M., C. J. S. Bolch, L. Botes, G. Nash, S. W. Wright, and G. M. Hallegraeff. 2003. Takayama gen. nov. (Gymnodiniales, Dinophyceae), a new genus of unarmored dinoflagellates with sigmoid apical grooves, including the description of two new species. J. Phycol. 39:1233-1246. [Google Scholar]
- 4.de Schepper, S., M. J. Head, and S. Louwye. 2004. New dinoflagellate cyst and incertae sedis taxa from the Poliocene of northern Belgium, Southern North Sea basin. J. Paleontol. 78:625-644. [Google Scholar]
- 5.Guindon, S., and O. Gascuel. 2003. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst. Biol. 52:696-704. [DOI] [PubMed] [Google Scholar]
- 6.Hackett, J. D., D. M. Anderson, D. Erdner, and D. Bhattacharya. 2004. Dinoflagellates: a remarkable evolutionary experiment. Am. J. Bot. 91:1523-1534. [DOI] [PubMed] [Google Scholar]
- 7.Hackett, J. D., T. E. Scheetz, H. S. Yoon, M. B. Soares, M. F. Bonaldo, T. L. Casavant, and D. Bhattacharya. 2005. Insights into a dinoflagellate genome through expressed sequence tag analysis. BMC Genomics 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber, T., G. Faulkner, and P. Hugenholtz. 2004. Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317-2319. [DOI] [PubMed] [Google Scholar]
- 9.Hugenholtz, H., and T. Huber. 2003. Chimeric 16S rDNA sequences of diverse origin are accumulating in the public databases. Int. J. Syst. Evol. Microbiol. 53:289-293. [DOI] [PubMed] [Google Scholar]
- 10.Jeong, H. J., J. S. Kim, J. Y. Park, J. H. Kim, S. Kim, I. Lee, S. H. Lee, J. H. Ha, and W. H. Yih. 2005. Stoeckeria algicida n. gen., n. sp. (Dinophyceae) from the coastal waters off southern Korea: morphology and small subunit ribosomal DNA gene sequence. J. Eukaryot. Microbiol. 52:382-390. [DOI] [PubMed] [Google Scholar]
- 11.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 12.LaJeunesse, T. C., G. Lambert, R. A. Anderson, M. A. Coffroth, and D. W. Galbraith. 2005. Symbiodinium (Pyrrhophyta) genome sizes (DNA content) are smallest among dinoflagellates. J. Phycol. 41:880-886. [Google Scholar]
- 13.Lopez-Garcia, P., F. Rodriguez-Valera, C. Pedros-Allo, and D. Moreira. 2001. Unexpected diversity of small eukaryotes in deep-sea Antarctic plankton. Nature 409:603-607. [DOI] [PubMed] [Google Scholar]
- 14.Montresor, M., G. Procaccini, and D. K. Stoecker. 1999. Polarella glacialis gen. nov., sp. nov. (Dinophyceae): Suessiaceae are still alive. J. Phycol. 35:186-197. [Google Scholar]
- 15.Moon-van der Staay, S. Y., R. De Wachter, and D. Vaulot. 2001. Oceanic 18S rDNA sequences from picoplankton reveal unsuspected eukaryotic diversity. Nature 409:607-610. [DOI] [PubMed] [Google Scholar]
- 16.Posada, D., and K. A. Crandall. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817-818. [DOI] [PubMed] [Google Scholar]
- 17.Saldarriaga, J. F., M. L. McEwan, N. M. Fast, F. J. R. Taylor, and P. J. Keeling. 2003. Multiple protein phylogenies show that Oxyrrhis marina and Perkinsus marinus are early branches of the dinoflagellate lineage. Int. J. Syst. Evol. Microbiol. 53:355-365. [DOI] [PubMed] [Google Scholar]
- 18.Saunders, G. W., D. R. A. Hill, J. P. Sexton, and R. A. Andersen. 1997. Small-subunit ribosomal RNA sequences from selected dinoflagellates: testing classical evolutionary hypotheses with molecular systematic methods. Plant Syst. Evol. 11(Suppl.):237-259. [Google Scholar]
- 19.Smith, G., and I. C. Harding. 2004. New dinoflagellate cyst species from Upper Jurassic to Lower Cretaceous sediments of the Volgian lectostratotype sections at Gorodische and Kashpir, Volga Basin, Russia. Rev. Palaeobot. Palynol. 128:355-379. [Google Scholar]
- 20.Taylor, F. J. R. (ed.). 1987. The biology of dinoflagellates, p.143-173. Blackwell Scientific Publications, Boston, Mass.
- 21.Wright, A.-D. G., and A. Colorni. 2002. Taxonomic re-assignment of. Cryptocaryon irritans, a marine fish parasite. Eur. J. Protistol. 37:375-378. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, H., and S. Lin. 2005. Phylogeny of dinoflagellates based on mitochondrial cytochrome b and nuclear small subunit rDNA sequence comparisons. J. Phycol. 41:411-420. [Google Scholar]
- 23.Zhang, H., and S. Lin. 2005. Development of a cob-18S dual-gene real-time PCR assay for Pfiesteria shumwayae and quantification of this species in the natural environment. Appl. Environ. Microbiol. 71:7053-7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.