Abstract
The commercial production of microbiologically safe and stable sauces containing acetic acid is guided by the Comité des Industries des Mayonnaises et Sauces Condimentaires de la Communauté Économique Européenne's (CIMSCEE) code. The CIMSCEE safety value is calculated using a linear regression equation combining weighted contributions of pH and aqueous-phase concentrations of undissociated acetic acid, NaCl, and sugars. By implication, the CIMSCEE safety equation predicts that increasing concentrations of hurdles will always increase inactivation of the target pathogen. In this study, the time to achieve a 3-log10 reduction of an acid-resistant, acid-adapted, Shiga toxin-producing Escherichia coli (STEC) O157 isolate was determined experimentally for 81 formulations at various pHs and acetic acid, NaCl, and sucrose concentrations in a broth model. The combinations were intended to simulate the aqueous phase of acidic sauces and dressings. Experimental data were fitted to the log logistic model to estimate the time to 3-log10 reduction (t3D). Comparison of fitted t3D estimates with CIMSCEE values showed agreement in predicting safety (as defined by CIMSCEE) for the majority of formulations. However, CIMSCEE safety predictions were “fail dangerous” for 13 of 81 formulations. Among these formulations and others, the observed E. coli t3D initially increased and then decreased with increasing osmolalities (NaCl and sucrose). Relative protection increased with exposure time where the protective effect of NaCl predominated. While commercial acidic sauces are not considered high-risk vehicles for STEC, interactions among hurdles that decrease their combined effectiveness are deserving of further investigation because they may reveal mechanisms of broader relevance in the inactivation of pathogens in foods.
The microbiological safety and stability of cold-filled acidic sauces and dressings are conferred by the combination of low pH (usually achieved by the addition of acetic acid), NaCl, and sugars. The commercial production of microbiologically safe and stable emulsified and nonemulsified sauces and dressings containing acetic acid is currently guided by the Comité des Industries des Mayonnaises et Sauces Condimentaires de la Communauté Économique Européenne (CIMSCEE) code. CIMSCEE defines an intrinsically microbiologically safe product as one in which a 3-log10 reduction (t3D) in the number of viable Escherichia coli cells (as a model for Salmonella enterica) occurs in less than 72 h, and this is reflected in a CIMSCEE safety value, Σs, of >63 (2).
Σs is calculated by using the following linear regression equation:
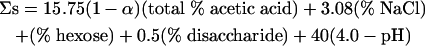 |
(1) |
where (1 − α) is the proportion of the total acetic acid that is in the undissociated form; CIMSCEE assumes that the pKa for acetic acid is 4.76 and does not take into account the effect of ionic strength on pKa. The CIMSCEE formula for prediction of the microbiological stability of acidic sauces was originally derived from a linear regression of molal salt plus sugars versus molal undissociated acetic acid on water. To predict product safety, a pH term was then added to this stability formula. The form of equation 1 embodies the concept that increasing concentrations of the various hurdles can only increase the inactivation of microorganisms.
Recently, protective effects of NaCl and sugars on the survival and growth of Shiga toxin-producing E. coli (STEC) O157 in acidic environments have been reported (1, 5). Also, McKellar et al. (6) constructed a probability model to describe the interface between survival and death of STEC in a mayonnaise model system (6). Upon comparing their observed results with predictions by CIMSCEE, they noted that CIMSCEE gave “fail-dangerous” predictions (i.e., false-negative results, predicting death when survival occurred) when the concentration of NaCl and/or sucrose in their mayonnaise models exceeded 2.5 and/or 4%, respectively (6).
Epidemiological evidence and likelihood-of-contamination assessments by other workers suggest that commercially produced acidic sauces and dressings are not a high-risk vehicle for STEC (3). The now almost universal use of pasteurized eggs in the commercial production of acidic sauces and dressings has perhaps also led to a decline in the importance of other pathogenic Enterobacteriaceae, including Salmonella, in this class of foods. Despite this observation, understanding the responses of STEC and other acid-resistant pathogens to various hurdle combinations remains important, given their importance to the broader food industry. The combination of low pH (acid) and low water activity (aw) (sucrose and NaCl) is a classic example of the hurdle approach and is used to preserve a large and diverse range of foods, including fermented meats, cheeses, and preserved vegetables. Given the widespread use of this approach in food manufacture, any protective effects afforded to a cell by one hurdle against another hurdle deserve further investigation, since such studies may reveal conditions previously regarded as safe that permit pathogens to survive.
The purpose of this study was firstly to determine the ability of an acid-resistant, acid-adapted strain of E. coli (STEC O157) to survive in a broth model formulated to simulate the aqueous phase of acetic acid-based sauces and dressings at different levels of pH, total acetic acid, NaCl, and sucrose. The second aim of the study was to compare experimental t3D values with those predicted by CIMSCEE and, particularly, to characterize and quantify any observed antagonistic effects of NaCl and sucrose on acid/pH-induced inactivation.
MATERIALS AND METHODS
Experimental matrix design and calculation of Σs.
The effects of four factors at each of three levels were assessed. Acetic acid (0.7, 1.4, and 2.1% [wt/wt]), NaCl (1, 3, and 8% [wt/wt]), sucrose (10, 20, and 30% [wt/wt]), and pH (3.2, 3.5, and 4.0) combinations were tested in a full factorial design, giving a total of 81 experimental combinations. Σs was calculated for each formulation according to equation 1, and the values are shown in Table 1. Of the 81 formulations tested, 36 were predicted to be microbiologically safe (Σs > 63) by CIMSCEE. Those combinations are denoted in bold in Table 1.
TABLE 1.
Calculated CIMSCEE safety values for 81 broth formulations (E8 to M16) simulating the aqueous phase of acidic saucesa
| pH | Total % (wt/wt) acetic acid | Σs at indicated NaCl concn (% [wt/wt])
|
Formulation no. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10% (wt/wt) sucrose
|
20% (wt/wt) sucrose
|
30% (wt/wt) sucrose
|
|||||||||
| 1 (E) | 3 (F) | 8 (G) | 1 (H) | 3 (I) | 8 (J) | 1 (K) | 3 (L) | 8 (M) | |||
| 3.2 | 0.7 | 51 | 57 | 72 | 56 | 62 | 77 | 61 | 67 | 82 | 8 |
| 3.5 | 0.7 | 39 | 45 | 60 | 44 | 50 | 65 | 49 | 55 | 70 | 9 |
| 4 | 0.7 | 17 | 24 | 39 | 22 | 29 | 44 | 27 | 34 | 49 | 10 |
| 3.2 | 1.4 | 62 | 68 | 83 | 67 | 73 | 88 | 72 | 78 | 93 | 11 |
| 3.5 | 1.4 | 49 | 55 | 71 | 54 | 60 | 76 | 59 | 65 | 81 | 12 |
| 4 | 1.4 | 27 | 33 | 48 | 32 | 38 | 53 | 37 | 43 | 58 | 13 |
| 3.2 | 2.1 | 72 | 78 | 94 | 77 | 83 | 99 | 82 | 88 | 104 | 14 |
| 3.5 | 2.1 | 59 | 66 | 81 | 64 | 71 | 86 | 69 | 76 | 91 | 15 |
| 4 | 2.1 | 36 | 42 | 58 | 41 | 47 | 63 | 46 | 52 | 68 | 16 |
Numbers in bold indicate product formulations predicted to be intrinsically microbiologically safe (i.e., Σs > 63) by CIMSCEE.
Calculation of molal undissociated acetic acid and NaCl plus sucrose.
Molal undissociated acetic acid and the molal NaCl-plus-sucrose concentration on water were calculated as follows. The pKa′ for acetic acid in the presence of NaCl ions was calculated using the specific ion interaction theory model, as suggested by Sortwell (8):
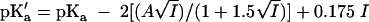 |
(2) |
where A is the Debye-Hückel coefficient (0.5091 at 25°C [7] and assumed to be equivalent at 23°C) and I is the molal ionic strength of the solution, which for NaCl is assumed to equal the molal concentration in each formulation.
As previously employed in studies on the microbiological stability of acidic sauces (4), the pKa was taken to be 4.75, which is intermediate between the values used by Sortwell (8) and CIMSCEE (2).
The fraction of total acetic acid in the undissociated form is given by the Henderson-Hasselbach equation, as follows:
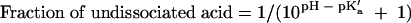 |
(3) |
and was used to calculate the percentage (wt/wt) of undissociated acetic acid in each formulation.
The molal concentration on water of each solute in each formulation (see Table 5) was calculated by the following equation:
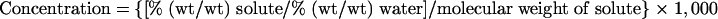 |
(4) |
where the molecular weight of the solute is 58.44 g liter−1 for NaCl, 60.05 g liter−1 for undissociated acetic acid, and 342.3 g liter−1 for sucrose.
TABLE 5.
k1 values (semilogarithmic inactivation rate) in log logistic curve fits describing survival of E. coli SERL 2 in 81 broth formulations (E8 to M16) simulating the aqueous phase of acidic saucesa
| pH | Total % (wt/wt) acetic acid |
k1 value at indicated NaCl concn (% [wt/wt])
|
Formulation no. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10% (wt/wt) sucrose
|
20% (wt/wt) sucrose
|
30% (wt/wt) sucrose
|
|||||||||
| 1 (E) | 3 (F) | 8 (G) | 1 (H) | 3 (I) | 8 (J) | 1 (K) | 3 (L) | 8 (M) | |||
| 3.2 | 0.7 | 0.048 | 0.030 | 0.057 | 0.040 | 0.013 | 0.050 | 0.029 | 0.050 | NDb | 8 |
| 3.5 | 0.7 | 0.040 | 0.017 | 0.029 | 0.030 | 0.010 | 0.047 | 0.019 | 0.037 | ND | 9 |
| 4 | 0.7 | 0.020 | 0.008 | 0.007 | 0.008 | 0.007 | 0.013 | 0.011 | 0.013 | 0.018 | 10 |
| 3.2 | 1.4 | 0.091 | 0.054 | 0.072 | 0.103 | 0.059 | 0.783 | 0.086 | 0.090 | ND | 11 |
| 3.5 | 1.4 | 0.056 | 0.031 | 0.056 | 0.070 | 0.056 | 0.092 | 0.071 | 0.082 | ND | 12 |
| 4 | 1.4 | 0.031 | 0.014 | 0.011 | 0.015 | 0.011 | 0.022 | 0.027 | 0.039 | 0.034 | 13 |
| 3.2 | 2.1 | 0.111 | 0.075 | 0.972 | 0.137 | 0.118 | 1.089 | 0.185 | 1.139 | ND | 14 |
| 3.5 | 2.1 | 0.097 | 0.073 | 0.197 | 0.072 | 0.074 | 0.669 | 0.074 | 0.654 | ND | 15 |
| 4 | 2.1 | 0.055 | 0.012 | 0.026 | 0.029 | 0.022 | 0.033 | 0.052 | 0.050 | ND | 16 |
Values in bold indicate product formulations predicted as microbiologically safe by CIMSCEE. Underlining indicates that the fitted time to 3-log10 reduction was >72 h, and hence the formulation does not meet CIMSCEE criteria for an intrinsically microbiologically safe formulation.
ND, not determined due to insufficient data for curve fitting following very rapid inactivation.
The combined molal NaCl-plus-sucrose concentration on water was determined by addition of the respective calculated values.
Tables 2 and 3 show the calculated molal undissociated acetic acid and NaCl-plus-sucrose molalities.
TABLE 2.
Molal undissociated acetic acid on water for 81 broth formulations (E8 to M16) simulating the aqueous phase of acidic sauces
| pH | Total % (wt/wt) acetic acid | Molal acetic acid at indicated NaCl concn (% [wt/wt])
|
Formulation no. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10% (wt/wt) sucrose
|
20% (wt/wt) sucrose
|
30% (wt/wt) sucrose
|
|||||||||
| 1 (E) | 3 (F) | 8 (G) | 1 (H) | 3 (I) | 8 (J) | 1 (K) | 3 (L) | 8 (M) | |||
| 3.2 | 0.7 | 0.13 | 0.13 | 0.14 | 0.14 | 0.15 | 0.16 | 0.16 | 0.17 | 0.19 | 8 |
| 3.5 | 0.7 | 0.12 | 0.12 | 0.13 | 0.14 | 0.14 | 0.15 | 0.16 | 0.16 | 0.18 | 9 |
| 4 | 0.7 | 0.10 | 0.10 | 0.12 | 0.11 | 0.12 | 0.13 | 0.13 | 0.14 | 0.16 | 10 |
| 3.2 | 1.4 | 0.26 | 0.26 | 0.28 | 0.29 | 0.30 | 0.32 | 0.33 | 0.34 | 0.38 | 11 |
| 3.5 | 1.4 | 0.24 | 0.25 | 0.27 | 0.28 | 0.28 | 0.31 | 0.32 | 0.33 | 0.37 | 12 |
| 4 | 1.4 | 0.21 | 0.21 | 0.23 | 0.23 | 0.24 | 0.27 | 0.27 | 0.27 | 0.32 | 13 |
| 3.2 | 2.1 | 0.39 | 0.40 | 0.43 | 0.44 | 0.45 | 0.49 | 0.50 | 0.52 | 0.57 | 14 |
| 3.5 | 2.1 | 0.37 | 0.38 | 0.41 | 0.42 | 0.43 | 0.47 | 0.48 | 0.50 | 0.56 | 15 |
| 4 | 2.1 | 0.31 | 0.31 | 0.35 | 0.35 | 0.36 | 0.41 | 0.40 | 0.41 | 0.49 | 16 |
TABLE 3.
Molal NaCl plus sucrose on water for 81 broth formulations (E8 to M16) simulating the aqueous phase of acidic sauces
| pH | Total % (wt/wt) acetic acid | Molal NaCl plus sucrose at indicated NaCl concn (% [wt/wt])
|
Formulation no. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10% (wt/wt) sucrose
|
20% (wt/wt) sucrose
|
30% (wt/wt) sucrose
|
|||||||||
| 1 (E) | 3 (F) | 8 (G) | 1 (H) | 3 (I) | 8 (J) | 1 (K) | 3 (L) | 8 (M) | |||
| 3.2 | 0.7 | 0.53 | 0.94 | 2.06 | 0.97 | 1.45 | 2.77 | 1.55 | 2.12 | 3.71 | 8 |
| 3.5 | 0.7 | 0.53 | 0.94 | 2.06 | 0.97 | 1.45 | 2.77 | 1.55 | 2.12 | 3.71 | 9 |
| 4 | 0.7 | 0.53 | 0.94 | 2.06 | 0.97 | 1.45 | 2.77 | 1.55 | 2.12 | 3.71 | 10 |
| 3.2 | 1.4 | 0.53 | 0.95 | 2.08 | 0.98 | 1.47 | 2.80 | 1.57 | 2.14 | 3.75 | 11 |
| 3.5 | 1.4 | 0.53 | 0.95 | 2.08 | 0.98 | 1.47 | 2.80 | 1.57 | 2.14 | 3.75 | 12 |
| 4 | 1.4 | 0.53 | 0.95 | 2.08 | 0.98 | 1.47 | 2.80 | 1.57 | 2.14 | 3.75 | 13 |
| 3.2 | 2.1 | 0.54 | 0.96 | 2.10 | 0.99 | 1.48 | 2.83 | 1.58 | 2.17 | 3.80 | 14 |
| 3.5 | 2.1 | 0.54 | 0.96 | 2.10 | 0.99 | 1.48 | 2.83 | 1.58 | 2.17 | 3.80 | 15 |
| 4 | 2.1 | 0.54 | 0.96 | 2.10 | 0.99 | 1.48 | 2.83 | 1.58 | 2.17 | 3.80 | 16 |
| Avg osmolality | 0.54 | 0.95 | 2.08 | 0.98 | 1.47 | 2.80 | 1.57 | 2.14 | 3.75 | ||
Culture conditions.
SERL 2, an outbreak strain of Shiga toxin-producing E. coli O157 previously shown to have good resistance to acetic acid, was kindly provided by T. Humphrey of the University of Bristol. The culture was maintained as a glycerol stock culture at −80°C and activated by transferring a loopful from the stock culture to 10 ml of nutrient broth (NB; Oxoid, United Kingdom) prepared according to the manufacturer's instructions. NB cultures were then incubated at 37°C (±1°C) for 22 h. For experiments, 10 μl of a 22-h NB culture was transferred to 10 ml tryptone soy broth (TSB; Oxoid) with 1% total glucose (TSB1%G) and incubated with shaking at 200 rpm for 22 h at 37°C (±1°C). At the conclusion of incubation, the pHs of TSB1%G cultures were checked using pH indicator papers (type CS, pH 3.8 to 5.5; Whatman International Ltd., United Kingdom) to ensure that they were approximately 4.2, indicating that acid conditioning of the cells was promoted during growth.
Broth model preparation.
Broth models simulating the aqueous phase of acidic sauces and dressings were prepared using NB as a base and supplemented with acetic acid, NaCl, and sucrose (Sigma Chemical Co.). NaCl (0.5% [wt/vol]) is normally present in NB when it is prepared according to the manufacturer's directions, and this was included in the final calculated nominal NaCl concentrations of the broth models. When required, the pH was adjusted with concentrated HCl or 10 M KOH and measured using a pH meter (Beckman model 390 with probe 511080; Beckman Coulter, Inc.). Broth models were prepared and filter sterilized (0.22 μm; Millipore) the day before use and were equilibrated at 23°C (±1°C) overnight. On the day of use, the pH of a subsample of each broth was rechecked, and the aw of each broth model was checked using an AquaLab water activity meter (CX-2; Decagon Devices).
The specific gravity of each formulation in the broth model was calculated from the density measured using a density bottle. The specific gravity was used to correct microbiological counts from aliquot size, measured by volume, to standardize formulation effects on a per-gram basis.
Survival experiments.
Broth models were dispensed aseptically by weight. Just prior to broth inoculation, TSB1%G cultures were diluted 1 in 10 in 0.1% (wt/vol) bacteriological peptone diluent (RM 263; Amyl Media, Australia), and 20 μl of the diluted culture was used to inoculate 20 g of each broth model in sterile 28-ml screw-cap polypropylene containers. The initial concentration of cells in each broth model was targeted to be approximately 105 CFU/g and was confirmed by plating of the inoculum. Within 30 min of inoculation, a 1-ml sample of the inoculated broth model was withdrawn and decimally diluted twice in buffered peptone water (Oxoid). Dilutions were surface plated (0.1 ml) onto duplicate tryptone soy agar (Oxoid) plates. Plates were incubated aerobically at 37°C (±1°) for 48 h prior to counting. Inoculated broth models were incubated at 23°C (±1°C). Broth models were sampled at predetermined time intervals for up to 120 h, with a maximum of 16 time points (0.5, 4, 20, 24, 28, 44, 48, 52, 68, 72, 76, 92, 96, 100, 116, and 120 h [±0.5 h]), and survivors were enumerated as described above.
Curve fitting.
Each raw data point, N(t), expressed in CFU/g, was converted to the log10 of the survival fraction, log [N(t)/N0], where N(t) and N0 are the momentary and initial numbers of cells, respectively. The detection threshold of the experiment was approximately 50 CFU/g. Using the Solver function in Microsoft Excel (Microsoft Corporation), data were fitted to the log logistic model, as follows:
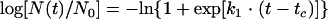 |
(5) |
where tc (h) is a measure of the shoulder period, or “delay time,” and k1 is a constant representing the semilogarithmic inactivation rate when t ≫ tc. By rearranging for time, the following equation was created:
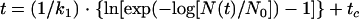 |
(6) |
and the t3D was determined for each of the 81 formulations.
Fitted t3D estimates were compared with corresponding Σs values. In addition, the derived values for t3D, k1 and tc were analyzed against each of the four factors examined (pH and acetic acid, NaCl, and sucrose concentrations) to establish the relationship of survival with these variables. The range of mean square errors for the data fitted to equation 6 was 0.001 to 0.099.
Statistical analysis.
Statistical analysis of the observed t3Ds of E. coli was undertaken on normalized (average relative kill) data sets, as follows. For each combination of pH and acetic acid, it was assumed that the greatest log10 reduction would be observed for formulations with the highest molal NaCl-plus-sucrose concentration (i.e., formulations from series J [Table 1]). Data from formulation series M could not be included in the analysis, as E. coli t3Ds were not accurately determined due to very rapid inactivation. Additionally, data from combinations with pH 4.0 and an acetic acid concentration of 0.7% (wt/wt) (i.e., E10, F10, G10, H10, I10, J10, K10, and L10) were not included in the analysis, as these combinations appeared marginal for inactivation of E. coli (upon plotting of normalized average relative kill data versus osmolalities for the entire data set; data not shown). Thus, the results include average relative kill values for eight series of eight formulations at a given (averaged) molal NaCl-plus-sucrose concentration. The average molal NaCl-plus-sucrose concentration was calculated from all formulations with given concentrations of NaCl and sucrose (Table 3).
The average relative kill was calculated for all formulations with a given pH, acetic acid concentration, and (average) osmolality, and treatments were compared using single-factor analysis of variance in Microsoft Excel. The statistical significance (P < 0.05) of differences between treatments was tested. In this way, comparisons between series representing different osmolalities (with different concentrations [wt/wt] of NaCl and sucrose) were made at sample times of 24, 48, and 72 h.
RESULTS
Comparison of Σs values and log logistic fitted times to 3-log10 reduction.
For 13 of 36 formulations predicted to be “safe” by CIMSCEE (i.e., having a ≥3-log10 reduction in <72 h), the estimate of t3D for E. coli SERL 2 obtained by fitting equation 3 exceeded 72 h (indicated by underlining in Table 4). Hence, CIMSCEE predictions for these formulations could be regarded as fail dangerous. All other formulations were predicted to be “unsafe” by CIMSCEE, which agreed with the log logistic fitted estimates of t3D for E. coli SERL 2, all of which exceeded 72 h (Table 4).
TABLE 4.
Log logistic fitted times to 3-log10 reduction (h) of E. coli SERL 2 in 81 broth formulations (E8 to M16) simulating the aqueous phase of acidic sauces and comparison with CIMSCEE safety scoresa
| pH | Total % (wt/wt) acetic acid | Time to 3-log10 reduction (h) of E. coli SERL 2 at indicated NaCl concn (% [wt/wt])
|
Formulation no. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10% (wt/wt) sucrose
|
20% (wt/wt) sucrose
|
30% (wt/wt) sucrose
|
|||||||||
| 1 (E) | 3 (F) | 8 (G) | 1 (H) | 3 (I) | 8 (J) | 1 (K) | 3 (L) | 8 (M) | |||
| 3.2 | 0.7 | 90 | 188 | 95 | 135 | 343 | 84 | 171 | 123 | NDb | 8 |
| 3.5 | 0.7 | 110 | 312 | 166 | 183 | 454 | 123 | 247 | 135 | ND | 9 |
| 4 | 0.7 | 268 | 667 | 635 | 679 | 685 | 428 | 436 | 387 | 249 | 10 |
| 3.2 | 1.4 | 77 | 125 | 54 | 98 | 111 | 6 | 81 | 37 | ND | 11 |
| 3.5 | 1.4 | 104 | 180 | 94 | 120 | 133 | 39 | 101 | 67 | ND | 12 |
| 4 | 1.4 | 179 | 395 | 419 | 337 | 497 | 220 | 209 | 152 | 97 | 13 |
| 3.2 | 2.1 | 61 | 77 | 5 | 64 | 46 | 3 | 26 | 4 | ND | 14 |
| 3.5 | 2.1 | 84 | 100 | 19 | 89 | 76 | 6 | 65 | 7 | ND | 15 |
| 4 | 2.1 | 122 | 436 | 185 | 186 | 265 | 123 | 130 | 109 | ND | 16 |
Values in bold indicate product formulations predicted as microbiologically safe by CIMSCEE. Underlining indicates that the fitted time to 3-log10 reduction was >72 h, and hence the formulation does not meet CIMSCEE criteria for an intrinsically microbiologically safe formulation.
ND, not determined due to insufficient data for curve fitting following very rapid inactivation.
Σs is a relative measure. Where ΣsFormulation1 ≫ ΣsFormulation2, it would generally be predicted that a 3-log10 reduction of E. coli would be most rapidly achieved in formulation 1. To more fully characterize the predictive limits of the CIMSCEE code, (i.e., equation 1), the Σs predicted for each formulation was compared with the fitted t3D (Tables 1 and 4). While all formulations with Σs values of <63 produced t3ds of >72 h and all formulations with Σs values of >78 produced t3ds of <72 h, it was found that not all formulations with Σs values between 64 and 78 resulted in E. coli t3Ds of <72 h. Among this subgroup of 22 formulations predicted to be safe by equation 1, the t3D varied between 7 h (formulation L15; Σs = 78) or less (formulations M9 and M16, for which insufficient data were available to allow curve fitting) and 125 h (formulation F11; Σs = 68). The unreliability of equation 1 for this subgroup of formulations is illustrated by comparing the t3Ds of E. coli for formulations H14, J8, J12, and L15, which were 64, 84, 39, and 7 h (Table 4), respectively, with Σs values of 77, 77, 76, and 76, respectively (Table 1).
Effect of pH and acetic acid concentration (% [wt/wt]).
As expected from equation 1, when all other factors were held constant, the t3D generally increased with increasing pHs and with decreasing acetic acid concentrations (% [wt/wt]) (Table 4). These trends were also reflected in fitted values for the log logistic curve parameter k1 (Table 5), which decreased with increasing pHs and decreasing % (wt/wt) acetic acid, and for tc (Table 6), which increased with increasing pHs and decreasing % (wt/wt) acetic acid.
TABLE 6.
tc values (delay time) in log logistic curve fits describing survival of E. coli SERL 2 in 81 broth formulations (E8 to M16) simulating the aqueous phase of acidic saucesa
| pH | Total % (wt/wt) acetic acid |
tc value (delay time [h]) at indicated NaCl concn (% [wt/wt])
|
Formulation no. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10% (wt/wt) sucrose
|
20% (wt/wt) sucrose
|
30% (wt/wt) sucrose
|
|||||||||
| 1 (E) | 3 (F) | 8 (G) | 1 (H) | 3 (I) | 8 (J) | 1 (K) | 3 (L) | 8 (M) | |||
| 3.2 | 0.7 | 29 | 90 | 43 | 60 | 116 | 26 | 68 | 64 | NDb | 8 |
| 3.5 | 0.7 | 37 | 135 | 66 | 85 | 159 | 60 | 92 | 55 | ND | 9 |
| 4 | 0.7 | 118 | 298 | 228 | 304 | 267 | 198 | 165 | 161 | 84 | 10 |
| 3.2 | 1.4 | 44 | 70 | 14 | 69 | 61 | 2 | 46 | 4 | ND | 11 |
| 3.5 | 1.4 | 51 | 85 | 41 | 78 | 80 | 7 | 60 | 31 | ND | 12 |
| 4 | 1.4 | 83 | 178 | 152 | 136 | 237 | 84 | 102 | 77 | 10 | 13 |
| 3.2 | 2.1 | 35 | 38 | 2 | 43 | 21 | 1 | 10 | 1 | ND | 14 |
| 3.5 | 2.1 | 54 | 60 | 4 | 48 | 36 | 1 | 25 | 2 | ND | 15 |
| 4 | 2.1 | 69 | 192 | 70 | 85 | 131 | 33 | 73 | 50 | ND | 16 |
Values in bold indicate product formulations predicted as microbiologically safe by CIMSCEE. Underlining indicates that the fitted time to 3-log10 reduction was >72 h, and hence the formulation does not meet CIMSCEE criteria for an intrinsically microbiologically safe formulation.
ND, not determined due to insufficient data for curve fitting following very rapid inactivation.
Effects of NaCl and sucrose concentrations.
Of the 27 series of formulations where all factors were constant except for the NaCl concentration (i.e., 1, 3, or 8% [wt/wt] NaCl), 16 formulation series did not show the expected reduction in t3D with increasing NaCl concentrations. Three alternative responses to increasing NaCl concentrations were observed. For 11 formulation series, t3D was slowest at 3% (wt/wt) NaCl, followed by 1% NaCl and then 8% NaCl, i.e., 3 > 1 > 8%. Four formulation series showed a t3D response profile of 3 > 8 > 1% (wt/wt) NaCl, and one formulation series showed a t3D response profile of 8 > 3 > 1% (wt/wt) NaCl. The results are detailed in Table 4.
Similarly, for the 27 series of three formulations in which only the % (wt/wt) sucrose varied, 14 formulation series followed the expected response of having shorter t3Ds with increasing sucrose concentrations (i.e., time to 3-log10 reduction at 10 > that at 20 > that at 30% [wt/wt] sucrose). Of the 13 other data sets, 7 formulation series showed a t3D response profile of 20 > 10 > 30% (wt/wt) sucrose, 4 formulation series showed a t3D response profile of 20 > 30 > 10% (wt/wt) sucrose, and 2 formulation series showed a t3D response profile of 30 > 20 > 10% (wt/wt) sucrose (Table 4).
Effect of molal undissociated acetic acid and NaCl-plus-sucrose concentration on water.
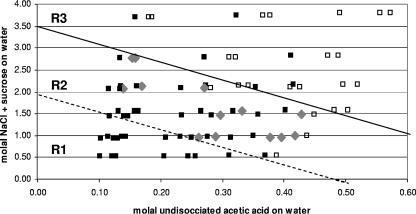
The molal undissociated acetic acid versus molal NaCl-plus-sucrose concentration on water was plotted for each formulation (Fig. 1). Formulations could be placed into three categories (R1, R2, and R3) (Fig. 1). For R1, CIMSCEE predictions agreed with experimental results, and all formulations were considered “unsafe” (i.e., fitted times to 3-log10 reduction were >72 h). For R3, CIMSCEE predictions were also supported by the experimental data but included formulations that were considered “safe” (i.e., >3-log reduction in 72 h) and those that were considered “unsafe.” In the latter case, all such formulations were found to have a high pH (i.e., pH 4.0) (Fig. 1, filled squares in R3). Category R2, however, was characterized by frequent disagreements between predictions of equation 1 and the experimental results. Within R2, the following three responses were observed: (i) CIMSCEE was found to correctly predict safe formulations (Fig. 1, unfilled squares in R2) in some cases where the pH was relatively low (pH 3.2); (ii) equation 1 correctly predicted unsafe formulations (filled squares in R2) where the pH was relatively high (pH 3.5 and pH 4.0); and (iii) predictions of equation 1 predicted “safe” formulations (gray diamonds in R2) where the pH was relatively low (pH 3.2 and pH 3.5) that were not supported by the experimental results. The last group represents the fail-dangerous predictions by CIMSCEE.
FIG. 1.
Molal undissociated acetic acid versus molal NaCl plus sucrose on water for 81 broth formulations (E8 to M16) simulating the aqueous phase of acidic sauces. Formulations predicted to be unsafe by CIMSCEE were experimentally determined to be unsafe (▪). Formulations predicted to be safe by CIMSCEE were experimentally determined to be safe (□) or unsafe (gray diamonds). Safe formulations were defined as those for which the log logistic fitted time to 3-log10 reduction of E. coli (SERL 2) was <72 h.
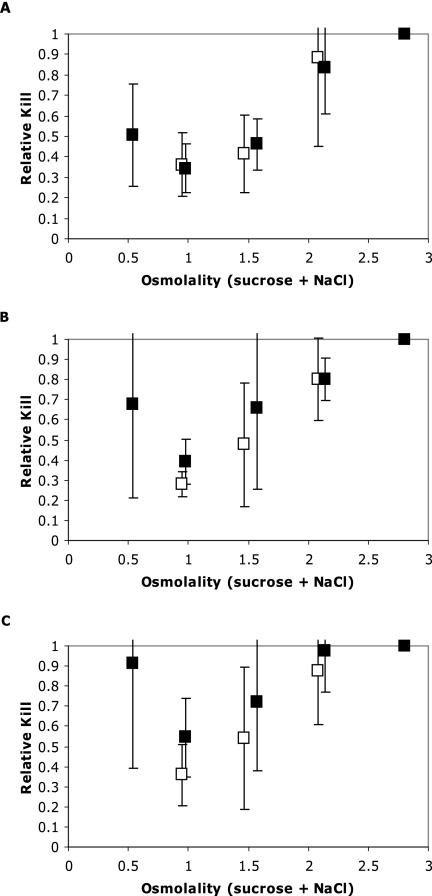
Statistical analysis of the effects of NaCl and sucrose was performed on the observed log10 reductions in all formulations after 24, 48, and 72 h by calculation of the average relative kill values. Average relative kill values, derived as described in Materials and Methods, are shown in Fig. 2, and the results of the statistical analysis are presented in Table 7.
FIG. 2.
Average (n = 8) relative kill values (versus series J formulations with an average molality [sucrose plus NaCl] of 2.80) for E. coli (SERL 2) in broth formulations simulating the aqueous phase of acidic sauces after 24 (A), 48 (B), and 72 (C) h. Error bars indicate the variance in average relative kill values at each sampling time; statistical significance is detailed in Table 7. The NaCl (□) predominance in formulations with similar average molalities is indicated. Average relative kill values at all osmolalities increased with increasing exposure times. However, the proportional increases in average relative kill values with increasing exposure times were smaller for formulations with similar average molalities in which NaCl predominated (i.e., for formulations with similar osmolalities, NaCl appears to be more protective than sucrose).
TABLE 7.
Analysis of variance of average relative kill values for series J formulations versus eight formulations in seven series (E to L) with different (averaged) molal NaCl-plus-sucrose concentrations on water at 24, 48, and 72 h
| Molal NaCl plus sucrose | Significance of variance in average relative kill values for series J vs indicated formulation (% [wt/wt] sucrose, % [wt/wt] NaCl, and molal NaCl plus sucrose)a
|
||||||
|---|---|---|---|---|---|---|---|
| E (10, 1, 0.54) | F (10, 3, 0.95) | H (20, 1, 0.98) | I (20, 3, 1.47) | K (30, 1, 1.57) | G (10, 8, 2.08) | L (30, 3, 2.14) | |
| 24 h | |||||||
| 0.54 | N | N | — | N | N | — | |
| 0.95 | — | N | — | S | S | ||
| 0.98 | N | N | — | — | |||
| 1.47 | — | — | S | ||||
| 1.57 | — | S | |||||
| 2.08 | — | ||||||
| 2.14 | |||||||
| 48 h | |||||||
| 0.54 | N | N | — | N | N | — | |
| 0.95 | — | N | — | S | S | ||
| 0.98 | N | N | — | — | |||
| 1.47 | — | — | S | ||||
| 1.57 | — | N | |||||
| 2.08 | — | ||||||
| 2.14 | |||||||
| 72 h | |||||||
| 0.54 | S, L | N | — | N | N | — | |
| 0.95 | — | N | — | S | S | ||
| 0.98 | N | N | — | — | |||
| 1.47 | — | — | S | ||||
| 1.57 | — | N | |||||
| 2.08 | — | ||||||
| 2.14 | |||||||
N, not significant at α level of 0.05; S, significantly different at α level of 0.05; L, the average relative kill with more NaCl was significantly less than that with less NaCl; —, invalid comparison; empty cells, replicate comparisons.
At 24, 48, and 72 h, significant differences in average relative kill values were observed for formulations from series F (3% [wt/wt] NaCl and 10% [wt/wt] sucrose) and G (8% [wt/wt] NaCl and 10% sucrose), with average relative kill values increasing with increasing NaCl concentrations (Table 7). However, at 24 and 48 h, the average relative kill values for the E (1% [wt/wt] NaCl and 10% [wt/wt] sucrose) and F formulation series were not significantly different. Furthermore, the average relative kill values at 72 h were significantly different between the E and F formulation series, but with the average relative kill value decreasing with the increased NaCl concentration. Taken together, the results of the statistical analysis indicate that for formulations with 10% (wt/wt) sucrose, the survival of E. coli over time in storage is positively influenced by NaCl at a 3% concentration.
For formulations with 20% sucrose, the average relative kills with 1% and 3% (wt/wt) NaCl (formulation series H and I, respectively) were not significantly different. However, the average relative kill value observed in the presence of 8% (wt/wt) NaCl (formulation series J) was clearly greater than that observed with 1% (wt/wt) NaCl for 24-, 48-, and 72-h samples. In formulations with 30% sucrose, increasing the NaCl concentration from 1% (formulation series K) to 3% (formulation series L) significantly affected the average relative kill value only at the 24-h sample time; relative kill values for 30% (wt/wt) sucrose solutions with 1% and 3% (wt/wt) NaCl were not significantly different at 48 and 72 h.
Despite the observation of trends in the log logistic fitted values for times to 3-log10 reduction, the average relative kill values for formulations containing 1% (wt/wt) NaCl were not found to be significantly different when the concentration of sucrose was increased from 10% (formulation series E) to 20% (formulation series H) or 30% (formulation series K) at 24, 48, or 72 h. However, for formulations with 3% (wt/wt) NaCl, the relative kill of E. coli in the presence of 30% (wt/wt) sucrose (formulation series L) was found to be significantly greater than that with 10% sucrose (formulation series F) but not that with 20% (wt/wt) sucrose (formulation series I). This indicates that increasing the sucrose concentration in such formulations has little effect on the inactivation of E. coli unless very large amounts are added. In the presence of 8% NaCl, however, increasing the sucrose concentration from 10% (formulation series G) to 20% (formulation series J) or 30% (formulation series M) increased the rate of kill.
The overall effect of increasing osmolality on average relative kill values for E. coli in broth models simulating the aqueous phase of acidic sauces is illustrated in Fig. 2. A distinct U-shaped response in average relative kill values is observed with increasing NaCl-plus-sucrose concentrations, where E. coli is initially protected by increasing osmolalities. Average relative kill values at all osmolalities increased with increasing exposure times. However, the proportional increase in average relative kill values with increasing exposure times was smaller for formulations with similar average molalities in which NaCl predominated. That is, for formulations with similar osmolalities, NaCl appeared to be more protective than sucrose.
DISCUSSION
On the basis of the results presented here, it appears that the CIMSCEE safety equation provides a good prediction of the safety (defined in terms of a 3-log10 reduction in the number of E. coli within 72 h at ambient temperature) of acetic acid-containing sauces and dressings where the pH is high (formulations predicted to be unsafe), where the molal concentrations of both undissociated acetic acid and combined NaCl plus sucrose are low (formulations predicted to be unsafe), or where the molal concentrations of both undissociated acetic acid and combined NaCl plus sucrose are high (formulations predicted to be safe unless the pH is high). However, under some conditions of intermediate molal concentrations of undissociated acetic acid and combined NaCl plus sucrose, the CIMSCEE safety equation is unable to adequately predict product safety. In approximately one-third of cases where CIMSCEE predicted times to 3-log10 reduction of <72 h, the log logistic fitted times to 3-log10 reduction exceeded 72 h. For these fail-dangerous predictions, it was observed that increasing concentrations of NaCl and sucrose did not always increase the inactivation of E. coli.
Statistical analysis of the observed relative kill values after 72 h in the presence of increasing NaCl concentrations showed that for formulations containing 10% sucrose, E. coli is relatively protected by 3% (wt/wt) NaCl compared with the case for 1% (wt/wt) NaCl. In formulations with 20% (wt/wt) sucrose, increasing the NaCl concentration from 1% to 3% (wt/wt) had no significant effect in either direction on E. coli survival. Only in formulations with 30% (wt/wt) sucrose did increasing the concentration of NaCl from 1 to 3% (wt/wt) appear to more rapidly inactivate E. coli; however, this response was observed only over the first 24 h and no longer appeared significant (absence of an effect in either direction) after 48 and 72 h.
The average relative kill values for formulations containing 1% (wt/wt) NaCl were not found to be significantly different when the concentration of sucrose was increased from 10% to 20% or 30% (wt/wt). Only in formulations with 8% (wt/wt) NaCl did increasing the sucrose concentration from 10 to 20 to 30% (wt/wt) appear to have a significant effect on increasing the inactivation of E. coli. In formulations with 3% (wt/wt) NaCl, only an increase in the sucrose concentration to 30% (wt/wt) appeared to have a significant effect on increasing the inactivation of E. coli.
Previously, others have reported on the survival of STEC in broth models that simulate the aqueous phase of acidic sauces and dressings and have noted apparent protective effects of NaCl and sucrose under such conditions. McKellar et al. commented that in designing their inactivation experiments, it became necessary to test additional combinations of conditions, as it became more difficult to achieve the required reductions in viable cell numbers necessary to suit their probability model design when the salt and/or sucrose concentration was high (6). They further noted that their probability model provided some fail-dangerous predictions, generally under conditions where the NaCl concentration was >0.5% and the pH was ≤4 (at ambient temperature), but they also noted that some fail-safe predictions were made over the same ranges (6).
The mechanism by which NaCl and sucrose might positively protect cells under acidic conditions cannot be elucidated from the results of this study. It was observed that the protective effect was generally manifested both in the shoulder period prior to inactivation (as reflected by larger tc values in the log logistic fit to the experimental data) and in the rate of inactivation of the cell population (as reflected in smaller k1 values). However, it was found that multiplication of tc and k1 for the different formulations did not yield a constant product, thus revealing differences in the relative effects of the formulation hurdles on these two parameters and suggesting differences in the mechanisms of inactivation.
It has been suggested that the protective effect of NaCl under acidic conditions might be mediated by the coupling of Na+ import to H+ export, thus permitting STEC to maintain the internal pH, allowing survival (1). It has also been suggested that at lower water activities, water is lost from the cytoplasm, resulting in a decrease in cell volume that might effectively concentrate the cytoplasmic constituents and thereby raise the internal pH of the cell (1). The latter might also be used to explain the protection of STEC in acidic environments by sugars and combinations of salts and sugars. However, it is evident from our results that the protective effect of NaCl is greater than that of sucrose at nearly identical osmolalities.
During pH adjustment of the broth models, it was noted that the addition of increasing concentrations of NaCl resulted in pH depression, as measured using a pH meter. We have previously noted this pH depression with NaCl additions, and the phenomenon has been confirmed using pH indicator dyes (data not shown). Consequently, broth models containing high concentrations of NaCl generally also contained larger amounts of KOH than broth models with smaller concentrations of NaCl at the same pH. The possibility that the K+ concentration is of importance in determining the survival of E. coli SERL 2 in the described system has been considered, but no clear correlation of K+ concentration with the observed NaCl protective effects is apparent from the experimental data (data not shown).
It would be of interest to know how widespread the phenomenon of NaCl protection under acidic conditions is, both among O157 strains and among other serotypes of STEC. A more comprehensive study of this phenomenon might also include other acid-tolerant organisms, such as Salmonella. It would also be worthwhile to assess whether prior acid adaptation is required for this protective effect to be in evidence. CIMSCEE does not specify a requirement for acid adaptation of E. coli prior to challenge testing of dressings and sauces for microbiological safety (2).
The results of this study show that the CIMSCEE equation for the microbiological safety of sauces is potentially inadequate for predicting the effect (decrease) of increasing salt concentrations on pathogen inactivation and the sometimes lack of effect of large increases in sucrose concentration on pathogen inactivation. This study highlights the need to develop a more mechanistic understanding of the manner by which solutes might protect acid-tolerant pathogens in acidic environments to allow the continued development of strategies to minimize the risks to human health posed by these organisms. Alternatively, at a time when there is considerable consumer pressure to reduce the concentrations of acid, salt, and sugars in a quest for better tasting and healthier foods, such studies may be of benefit in guiding manufacturers to achieve these ends without compromising product safety.
Acknowledgments
We thank S. Ferguson, formerly of Food Science Australia, for her technical assistance, M. Corradini and M. Peleg of the University of Massachusetts for their suggestions regarding log logistic curve fitting, and E. Szabo, formerly of Food Science Australia, for her suggestions regarding preparation of the manuscript.
We acknowledge the financial support of The Australian Food Safety Centre of Excellence, an initiative of the Australian Federal Government's National Food Industry Strategy and a consortium of the Tasmanian Institute of Agricultural Research and Food Science Australia.
REFERENCES
- 1.Casey, P. G., and S. Condon. 2002. Sodium chloride decreases the bacteriocidal effect of acid pH on Escherichia coli O157:H45. Int. J. Food Microbiol. 76:199-206. [DOI] [PubMed] [Google Scholar]
- 2.Comité des Industries des Mayonnaises et Sauces Condimentaires de la Communauté Économique Européenne. 1992. CIMSCEE code for the production of microbiologically safe and stable emulsified and non-emulsified sauces containing acetic acid. Comité des Industries des Mayonnaises et Sauces Condimentaires de la Communauté Économique Européenne, Brussels, Belgium.
- 3.Erickson, J. P., J. W. Stamer, M. Hayes, D. N. McKenna, and L. A. Vanalstine. 1995. An assessment of Escherichia coli O157:H7 contamination risks in commercial mayonnaise from pasteurized eggs and environmental sources, and behaviour in low-pH dressings. J. Food Prot. 58:1059-1064. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins, P., P. G. Poulos, M. B. Cole, M. H. Vandeven, and J. D. Legan. 2000. The boundary for growth of Zygosaccharomyces bailii in acidified products described by models for time to growth and probability of growth. J. Food Prot. 63:222-230. [DOI] [PubMed] [Google Scholar]
- 5.Jordan, K. N., and K. W. Davies. 2001. Sodium chloride enhances recovery and growth of acid-stressed E. coli O157:H7. Lett. Appl. Microbiol. 32:312-315. [DOI] [PubMed] [Google Scholar]
- 6.McKellar, R. C., X. Lu, and P. J. Delaquis. 2002. A probability model describing the interface between survival and death of Escherichia coli O157:H7 in a mayonnaise model system. Food Microbiol. 19:235-247. [Google Scholar]
- 7.Mizera, J., A. H. Bond, G. R. Choppin, and R. C. Moore. 1999. Dissociation constants of carboxylic acids at high ionic strengths, p. 113-124. In D. T. Reed, S. B. Clark, and L. Rao (ed.), Actinide speciation in high ionic strength media. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 8.Sortwell, D. R. 2001. The boundary for growth of Zygosaccharomyces bailii in acidified products described by models for time to growth and probability of growth, a comment on. J. Food Prot. 64:439-440. (Letter to the editor.) [DOI] [PubMed] [Google Scholar]