Abstract
A previously characterized O157-specific lytic bacteriophage KH1 and a newly isolated phage designated SH1 were tested, alone or in combination, for reducing intestinal Escherichia coli O157:H7 in animals. Oral treatment with phage KH1 did not reduce the intestinal E. coli O157:H7 in sheep. Phage SH1 formed clear and relatively larger plaques on lawns of all 12 E. coli O157:H7 isolates tested and had a broader host range than phage KH1, lysing O55:H6 and 18 of 120 non-O157 E. coli isolates tested. In vitro, mucin or bovine mucus did not inhibit bacterial lysis by phage SH1 or KH1. A phage treatment protocol was optimized using a mouse model of E. coli O157:H7 intestinal carriage. Oral treatment with SH1 or a mixture of SH1 and KH1 at phage/bacterium ratios ≥102 terminated the presence of fecal E. coli O157:H7 within 2 to 6 days after phage treatment. Untreated control mice remained culture positive for >10 days. To optimize bacterial carriage and phage delivery in cattle, E. coli O157:H7 was applied rectally to Holstein steers 7 days before the administration of 1010 PFU SH1 and KH1. Phages were applied directly to the rectoanal junction mucosa at phage/bacterium ratios calculated to be ≥102. In addition, phages were maintained at 106 PFU/ml in the drinking water of the phage treatment group. This phage therapy reduced the average number of E. coli O157:H7 CFU among phage-treated steers compared to control steers (P < 0.05); however, it did not eliminate the bacteria from the majority of steers.
Since its association with disease in 1982 (33), Escherichia coli O157:H7 has become a worldwide threat to public health and is one of today's most troubling food-borne pathogens. Human illness with E. coli O157:H7 ranges from self-limited watery diarrhea and hemorrhagic colitis to life-threatening manifestations such as the hemolytic uremic syndrome and thrombotic thrombocytopenic purpura. Cattle and sheep, important domestic ruminants, are the primary reservoirs for this human pathogen and are the most common sources for food-borne, waterborne, and direct-animal-contact infections (9-11, 18, 48, 51). In contrast to humans, the carrier ruminants remain healthy, and E. coli serotype O157:H7 is a transient member of the ruminant gastrointestinal flora. Persistence of E. coli O157:H7 carriage in naturally and experimentally infected animals varies from days to months (6, 8, 12, 13, 19, 35, 38).
Successful strategies to control or reduce the carriage and prevalence of E. coli O157:H7 in live ruminants would reduce the risk of human exposure to this pathogen. There is currently no reliable intervention or animal vaccine available to curb carriage of E. coli O157:H7. Probiotics aimed at creating an intestinal microenvironment inhibitory to E. coli O157:H7 have been tested, but without consistent success (14, 22, 28, 29, 50). A lytic bacteriophage that would specifically target the E. coli O157 serotype is another appealing approach because phage therapy has been successful in animal trials against a broad range of bacterial pathogens such as enteropathogenic E. coli (3, 39), Staphylococcus aureus (23, 41), Pseudomonas aeruginosa (41), and vancomycin-resistant Enterococcus faecium (7).
Isolating E. coli O157-specific phages and testing their use to control E. coli O157:H7 in vivo and in vitro have been done by our laboratory and by others (15, 21, 25, 27, 44). However, control of E. coli O157:H7 with phage treatment in natural farm settings is limited. To our knowledge, the only published data on the assessment of the ability of phage to control E. coli O157:H7 in live animals are by Bach et al. for sheep (2) and Tanji et al. (45) for mice. In the sheep study, lambs orally dosed with E. coli O157:H7 and then treated with a single dose of O157-specific phage DC22 did not clear the pathogen, although phage DC22 was effective at controlling E. coli O157:H7 in an artificial rumen system (2). In the mouse study, daily administration of a phage cocktail (SP15-21-22) reduced the number of fecal E. coli O157:H7 CFU among the treated mice compared to the untreated mice but did not clear the bacteria from the intestinal tract (45).
Two recent breakthroughs in understanding the relationship between ruminants and E. coli O157:H7 will likely impact the ability of phage treatment as an intervention strategy. The primary site of E. coli O157:H7 colonization in cattle is the rectoanal junction (RAJ) (16, 26). Also, application of E. coli O157:H7 directly to the bovine RAJ mucosa, as opposed to an oral dose, is a method that reliably colonizes cattle with these bacteria (38).
Using phage to control E. coli O157:H7 has been a goal of our laboratory for years (21). Here we investigated various phage treatments to reduce or eliminate E. coli O157:H7 in vivo. Experiments included (i) testing the previously characterized O157-specific lytic phage KH1 (21) for its ability to limit E. coli O157 carriage in sheep experimentally infected with an oral dose of E. coli O157:H7, (ii) the isolation and characterization of a new lytic phage designated SH1, (iii) the use of a mixture of the phages KH1 and SH1 to limit E. coli O157:H7 carriage in a mouse model, and (iv) the use of a novel phage treatment that placed a mixture of KH1 and SHI directly onto the bovine RAJ mucosa.
MATERIALS AND METHODS
All personnel followed strict biosafety procedures when handling E. coli O157:H7 and/or animals, and all procedures were approved by the Institutional Animal Care and Use and Biosafety committees.
Bacteria.
E. coli O157:H7 strains included clinical human isolate ATCC 43894 (American Type Culture Collection, Manassas, Va.) and bovine isolates WSU180, WSU400, and WSU588 (32). All four E. coli O157:H7 strains have distinct pulsed-field gel electrophoresis profiles and possess the Shiga toxin genes (stx1 and stx2). When mixtures of bacteria were administered to animals, the bacteria were prepared individually in 250-ml flasks and equal volumes were combined just before they were given to the animals. In addition, E. coli strains JM103, O55:H6, O111:H12, O111:H21, O111:NM, and O113:H21 and 120 bovine non-O157 E. coli strains were used to test phage host range. Bacterial cultures were grown in Luria-Bertani (LB) broth (BBL/Becton Dickinson, Detroit, Mich.) at 37°C for 18 h with aeration (150 rpm).
Phage isolation, preparation, and titration.
E. coli O157:H7-specific lytic phage KH1 was previously isolated from bovine feces (21). Phage SH1 was isolated by a standard enrichment procedure (37) from raw sewage taken from a municipal sewage treatment system (Pullman, WA). Briefly, 15-ml sewage samples were centrifuged at 3,500 × g for 25 min at 10°C. The supernatants were filtered through a 0.45-μm-pore-size filter, 8.6 ml of filtrate was added to 1 ml 10× LB broth, and 100 μl (∼108 CFU) of each E. coli O157:H7 strain (ATCC 43894, WSU180, WSU400, and WSU588) was added. The mixture was incubated at 37°C for 18 h with aeration (150 rpm). Debris and bacteria were removed by centrifugation at 10,000 × g for 10 min, and supernatants were filtered through a 0.22-μm-pore-size filter. Phage activity in the supernatant was tested by a spot assay that entailed placing 5 μl of the supernatant on LB agar seeded with E. coli O157:H7. The plates were checked for plaques after 18 h at 37°C. Lysis-positive supernatants were serially diluted, and plaques were isolated and purified using a soft agar overlay technique with E. coli O157:H7 ATCC 43894 (34). One phage that produced plaques on all four E. coli O157:H7 strains (ATCC 43894, WSU180, WSU400, and WSU588) was selected for further study and designated SH1.
Large-scale high-titer stocks of KH1 and SH1 were prepared by the plate lysate method using large petri plates (150 mm by 15 mm), as previously described (34). To remove debris, the lysate and top agar were pooled and centrifuged at 10,000 × g for 10 min at 4°C. Due to the difficulty of filtering the suspension through a 0.45-μm-pore-size filter, the supernatant was heated at 60°C for 25 min to kill E. coli O157:H7. Chloroform was avoided at all preparation steps since phages were to be used in animals. The heated phage stock was titrated and stored at 4°C and the titer determined again, just prior to use.
A phage-free mock lysate to be used as a control for phage treatment was prepared as follows. Similar bacterial cultures were grown without phages and centrifuged at 9,000 × g for 10 min at 4°C. The cell pellets were mock lysed by grinding with liquid nitrogen and a prefrozen mortar and pestle. The same volume of supernatant (as used to suspend the phage pellet) was used to resuspend the lysed cells. Debris was removed by centrifugation; the supernatant was heated at 60°C for 25 min to kill viable bacteria, and the success of this step was confirmed by plating on LB agar plates and overnight incubation.
Phage SH1 and KH1 E. coli host range.
E. coli strain JM103, enterohemorrhagic E. coli O55:H6, O111:H12, O111:H21, O111:NM, and O113:H21, and 120 bovine non-O157 E. coli strains were tested for phage susceptibility. The non-O157 E. coli strains were isolated from cattle by plating rectoanal mucosal swab (RAMS) samples (32) from four E. coli O157 culture-negative cattle on violet-red bile agar (VRBA; BBL/Becton Dickinson, Detroit, Mich.) supplemented with 4-methylumbelliferyl-β-d-glucuronide (MUG). E. coli colonies were distinguished by a purple-red color and bluish fluorescence under long-wave UV light. The sensitivity of each bacterial strain to KH1 and SH1 was determined using the spot assay, as described above. The presence of a lytic zone was considered evidence of phage susceptibility; no lysis was considered evidence of phage resistance.
Determination of phage MOI in E. coli O157:H7.
The optimal multiplicity of infection (MOI) for E. coli O157:H7 strain ATCC 43894 was determined by mixing bacteria and phage SH1 at ratios ranging from 10−1 to 105 PFU/CFU in tubes containing 104 CFU of E. coli O157:H7/ml. Phage-free cultures (containing only bacteria) and cell-free cultures (containing only phage) were used as controls. The tubes were incubated at 37°C without aeration for 24 h, and the number of viable cells was determined in triplicate by plating on LB agar following concentration by centrifugation. MOI determinations were done in duplicate.
Phage infection in the presence of mucus.
Commercial porcine gastric mucin (PGM) (type III; Sigma, St. Louis, Mo.) was prepared to a final concentration of 10% (wt/vol) in 50 ml phosphate-buffered saline (PBS). Bovine mucus was collected from the RAJ of two 9-month-old steers as follows. The animals were held in a squeeze chute, feces in the terminal rectum were removed by rectal palpation, and the terminal rectum was rinsed with PBS. A soft rubber spatula was use to scrape mucus from the RAJ mucosa tissue. Approximately 3 ml of highly viscous mucus was obtained from each animal, pooled in a 50-ml conical tube, held on ice, and immediately transported to the laboratory. The bovine mucus was dissolved 1:1 (vol/vol) in PBS. Bovine mucus and PGM solutions were sterilized by autoclaving, a procedure known not to change the biological activity of these substances (4). One percent PGM, 1% bovine mucus, or PBS (200 μl) was mixed with 100 μl of an overnight culture of E. coli O157:H7 (ATCC 43894). The mixtures were incubated at 37°C for 25 min, added to 2.5 ml LB soft agar (0.75%) containing 102 PFU of phage SH1 or KH1, and overlaid onto an LB agar (1.5%) plate (21). Plates were incubated overnight at 37°C and plaques enumerated.
Experimental animals.
All animals were acclimated for at least 2 weeks prior to receiving E. coli O157:H7.
(i) Sheep.
Six 7-month-old Suffolk ewes were housed in a containment facility at Washington State University (Pullman). Two groups (n = 3) were separated in two different rooms without cross contamination. Ewes were fed pelleted alfalfa twice daily, with drinking water ad libitum. No supplements were included in their diets.
(ii) Mice.
Twelve 8-week-old female Swiss Webster mice were obtained from Simonsen Laboratories, Inc. (Gilroy, CA). Mice were randomly allocated to cages with three mice each and housed under standard day length, temperature, and humidity conditions. Water and pelleted food were offered ad libitum.
(iii) Steers.
Six-month-old Holstein steers were housed in a quarantined facility at the University of Idaho. Two groups (n = 5) were separated in different containment pens without contact between groups and without cross contamination for the phage treatment trial. Steers were fed alfalfa hay twice daily and grain pellets once daily and had drinking water ad libitum.
Bacterial challenge and phage treatment.
Six sheep were given a single oral dose of 3.5 × 1010 CFU E. coli O157:H7 ATCC 43894/animal on day −1. On days 0, 8, 9, and 10, three ewes were given oral doses of 1.3 × 1011 PFU KH1/animal/day (treatment group) and three ewes were given similar oral doses of medium without phage (control group).
Six mice were given a single oral dose of 108 CFU E. coli O157:H7 ATCC 43894/animal on day −1. On days 0, 1, and 2 after phage treatment, three mice were given 1010 PFU SH1 (phage 1) and three mice were given 100 μl mock lysate (control 1). Another six mice were given a single oral dose of 108 CFU of a four-strain mixture of E. coli O157:H7 containing strains ATCC 43894, WSU180, WSU400, and WSU588 (the number of CFU of each strain was confirmed prior to mixing by measuring the optical density at 600 nm). On days 0, 1, and 2 after phage treatment, three mice received an oral dose of 1010 PFU of both KH1 and SH1 (phage 2) or the mock lysate (control 2). To improve the palatability of the solutions, the bacterial cultures, mock lysate, and phage stocks were mixed with sucrose (final concentration of 20%) prior to oral administration. All oral doses were administered while the mice were held in a supine position with the head up, allowing the animals to suck the fluid from the tip of a micropipette.
Ten Holstein steers were given a single rectal application of a mixture of 1010 CFU of each of four strains of E. coli O157:H7 (ATCC 43894, WSU180, WSU400, and WSU588, the same strains used in the mouse experiment above) on day −7. Rectal application of bacteria was carried out as described previously (38) with a slight modification as follows: instead of using a large cylindrical sponge, a sterile foam-tipped applicator (catalog no. 10812-022; VWR International, Buffalo Grove, Ill.) was saturated with E. coli O157:H7 overnight culture, and the swab was inserted into the terminal rectum and gently rubbed against the rectoanal junction mucosa. On days 0, 1, 2, and 4 after phage treatment, five steers were given phage rectally (treatment group). The anuses of the steers were digitally palpated to open, and feces were removed. The mixture of phages SH1 and KH1 (25 ml of 1010 PFU/ml) was delivered into the anus with a syringe and a 10-cm by 3.5-cm-diameter cylindrical sponge (Rubbermaid, St. Francis, WI) with a wooden handle. The sponge was saturated with 5 ml of the phage mixture and was inserted and gently swabbed against the wall of the RAJ mucosa. The tails of the treated steers were held down for 10 min immediately following withdrawal of the swab. This was done to prevent animals from defecating and presumably to allow the phage interactions without immediate passage of fecal material. Phages were also administered via drinking water by adding phage daily to a final concentration of 1.8 × 106 to 5.4 × 106 PFU/ml starting on day 0, and as animals drank the phage concentration was maintained at that level through the end of the experiment. Similarly, five steers were given the mock lysate rectally and in their drinking water (control group).
Sample collection for enumeration of E. coli O157 CFU and phage.
Sheep fecal samples were collected from each ewe by a previously described method on the days indicated in Table 1 (20). Briefly, fecal samples were collected aseptically, placed in Whirl-pak bags, and kept on ice until culture. Ten grams of feces was diluted into 50 ml Trypticase soy broth (TSB; BBL/Becton Dickinson, Detroit, Mich.) supplemented with cefixime (50 ng/ml; generously provided by D. D. Hancock, Washington State University), potassium tellurite (2.5 mg/liter; Sigma Chemical Co., St. Louis, Mo.), and vancomycin (40 mg/liter; Sigma) (referred to hereafter as TSB-CTV). Mouse fecal samples were collected daily as three freshly voided pellets from each mouse and placed into15-ml conical tubes containing 3 ml ice-cold TSB, suspended by vortexing, and kept on ice until the culture procedure. Cattle RAMS samples were collected by the previously described method (32). Briefly, RAMS samples from individual steers were placed into 15-ml conical tubes containing 3 ml ice-cold TSB, mixed by vortexing, and kept on ice until the culture procedure. All fecal and RAMS samples were processed within 3 h of collection. On day 0 after phage treatment and twice weekly thereafter, RAMS samples were taken from each individual steer for culture of E. coli O157:H7.
TABLE 1.
Duration and number of fecal E. coli O157:H7 CFU in sheep with and without phage treatment
| Day after phage treatmenta | No. of CFU/g of feces in animalb treated with:
|
|||||
|---|---|---|---|---|---|---|
| KH1 in treatment group:
|
Mock lysate in control group:
|
|||||
| P1 | P2 | P3 | C1 | C2 | C3 | |
| 0 | 2.55 × 106 | 1.15 × 106 | 5.25 × 106 | 2.50 × 105 | 1.50 × 106 | 5.10 × 106 |
| 2 | 6.00 × 104 | 1.05 × 105 | 5.00 × 103 | 1.50 × 103 | 5.00 × 103 | 1.00 × 102 |
| 4 | 1.35 × 103 | 5.00 × 103 | 3.00 × 101 | E+c | 5.00 × 104 | E+ |
| 6 | E+ | 0 | E+ | E+ | 4.50 × 104 | E+ |
| 8 | E+ | 0 | E+ | E+ | E+ | E+ |
| 9 | E+ | 0 | E+ | E+ | E+ | E+ |
| 10 | 0 | 0 | E+ | E+ | 0 | E+ |
| 11 | 0 | 0 | E+ | E+ | 0 | 0 |
| 12 | 0 | 0 | E+ | E+ | 0 | 0 |
| 14 | 0 | 0 | E+ | E+ | 0 | 0 |
| 21 | 0 | 0 | E+ | E+ | 0 | 0 |
On days 0, 8, 9, and 10, ewes were given an oral dose of 1.3 × 1011 PFU phage KH1/animal.
Animals were 7-month-old Suffolk ewes and were given a single oral dose of 3.5 × 1010 CFU E. coli O157:H7 (ATCC 43894)/animal on day −1.
E+, sample was positive by enrichment culture technique but not by direct culture technique, indicating that the number of E. coli O157:H7 CFU in the sample was ≤10 CFU/g.
Determination of bacterium and phage concentrations.
To remove free phage before bacterial enumeration, all samples in TSB or TSB-CTV were centrifuged at 10,000 × g for 5 min at 4°C to separate the supernatant from the solid particles and cells. Serial dilutions of supernatants were used in a standard soft-agar technique to determine the titer of phage in corresponding samples (34). The pellets were washed and resuspended into an equal volume of fresh TSB or TSB-CTV, correspondingly, for direct or enrichment procedures to determine the number of E. coli O157:H7 CFU.
Samples were cultured for E. coli O157:H7 by direct and enrichment procedures as previously described (32). Briefly, initial suspensions were made with 10-g fecal samples from sheep, three-pellet feces from mice, or RAMS from cattle. Direct cultures were prepared by plating serial dilutions made in sterile saline (0.15 M NaCl) onto sorbitol-MacConkey agar supplemented with MUG, cefixime, potassium tellurite, and vancomycin (SMAC-CTVM). Plates were incubated at 37°C overnight and observed for colonies not fermenting sorbitol (white colonies) and not hydrolyzing MUG (no florescence at 363 nm). Presumptive E. coli O157:H7 colonies were confirmed by latex agglutination (Pro-Lab Diagnostics, Toronto, Canada). Positive cultures from this direct-plating technique result in a quantitative measure of E. coli O157:H7 bacteria in CFU/gram feces for sheep, CFU/fecal pellet for mice, or CFU/swab for cattle. Cultures negative by these direct methods were further analyzed by an enrichment procedure. Mouse feces in TSB, RAMS in TSB, or sheep feces in TSB-CTV were incubated at 37°C with aeration for 18 h, serial dilutions were plated onto SMAC-CTVM, and presumptive E. coli O157:H7 colonies were confirmed by latex agglutination.
Analysis of colony morphology.
E. coli O157:H7 colony morphologies were analyzed visually from direct cultures of murine feces or bovine RAMS using SMAC-CTMV. To determine the consistency of colony morphologies, irregular colonies were picked, subcultured by restreaking onto SMAC-CTMV, and reanalyzed visually.
Statistical analysis.
The number of E. coli O157:H7 CFU recovered from individual samples was determined as the average of duplicate plate counts. Values from experiments were transformed to log10 CFU/pellet for the mouse experiments and log10 CFU/swab for the cattle experiments. Samples positive only by enrichment procedure were assigned a value of 5 CFU/pellet for the mice and 10 CFU/swab for the cattle. Significant differences between the phage-treated and control groups were determined using the statistical analysis's t test procedure (36).
RESULTS
All animals in this study were healthy throughout the trials and had no ill effects of carrying E. coli O157:H7 or phage. All animals were culture negative for E. coli O157:H7 prior to experimental challenge with the bacteria.
Oral phage treatment by KH1 did not reduce intestinal E. coli O157:H7 in sheep.
To test whether the previously characterized O157-specific lytic phage KH1 could eliminate E. coli O157:H7 in vivo, phage KH1 was applied to sheep given a single oral dose of E. coli O157:H7 (Table 1). All sheep had E. coli O157:H7 culture-positive feces 1 day after the bacterial dose. A single oral dose of phage KH1 was given to sheep in the phage treatment group on day 0. Although one ewe in the phage treatment group became culture negative for E. coli O157:H7 on day 6 after phage treatment and remained so for the duration of the experiment, the average number of fecal E. coli O157:H7 CFU for ewes in the phage treatment group was not significantly different from that for the ewes in the control group (P = 0.74 for the first 8 days after phage treatment) (Table 1). The numbers of fecal E. coli O157:H7 CFU and duration of carriage among both the control and phage-treated animals were typical for orally dosed ruminants. Thus, we can conclude that phage did not clear carriage of E. coli O157 in these sheep. Following the single oral dose of 1011 PFU of KH1 on day 0, there was a variable number of phage found in fecal samples, and on days 5 through 8 all phage-treated animals had between 105 and 106 PFU/g feces. The apparent ratios of E. coli O157:H7/KH1 ranged from <1 to >103 (data not shown). Three more oral doses of phage KH1 were administered on days 8, 9, and 10 to the animals in the phage treatment group. Although phage concentrations measured in fecal samples after these repeated treatments were as high as 106 PFU/g, the numbers of fecal E. coli O157:H7 CFU were similar to the untreated control group (Table 1 and data not shown).
Isolation, host range, and plaque-forming ability of phage SH1.
Despite the success of phage KH1 at lysing E. coli O157:H7 in vitro (21), repeated oral application of high titers of phage KH1 was not effective at eliminating intestinal E. coli O157:H7 from sheep (Table 1). For this reason, new phages were isolated and characterized. Phage SH1, isolated from a sewage sample, was highly lytic and formed large clear plaques in all 12 E. coli O157:H7 strains tested (four human isolates and eight bovine isolates; data not shown). In anticipation of its use in animals, bacterium-free phage preparations were made by heat treatment rather than chloroform extraction. The numbers of viable phage after heating were decreased from ∼6 ×1011 PFU/ml to ∼5.5 × 1010 PFU/ml. We compared the lytic activities of the phages KH1 and SH1 on the endogenous E. coli RAJ flora isolated from a group of four 6-month-old steers. Thirty random E. coli isolates from fecal cultures were selected per animal, and a total of 120 non-O157:H7 E. coli isolates were tested for sensitivity to the two phages in a plate lysis test. KH1 did not form plaques on any of the non-O157 E. coli isolates (data not shown). SH1 formed plaques on 18 of 120 non-O157:H7 E. coli isolates (data not shown). The result indicated that the host range of SH1 was broader than KH1, which is highly specific for E. coli O157:H7 (21). The theoretical host range of phage SH1 for 120 representative E. coli isolates recovered from RAMS samples was eight (6.7%). Neither SH1 nor KH1 formed plaques on E. coli JM103 or enterohemorrhagic E. coli O111:H12, O111:H21, O111:NM, or O113:H21. SH1 produced plaques on O55:H6 (data not shown). All four strains of E. coli O157:H7 (ATCC 43894, WSU180, WSU400, and WSU588) used to challenge animals in this study were sensitive to phages SH1 and KHI. The plaques produced by SH1 (2 mm) were larger than those produced by KH1 (<1 mm) on the all tested E. coli O157:H7 lawns (data not shown).
Phage SH1 at an MOI of ≥102 PFU/CFU eliminated E. coli O157:H7 ATCC 43894 in LB broth.
To further characterize SH1 and to prepare for its use in animal trials, the optimal MOI for the elimination of E. coli O157:H7 in broth was determined. There was no bacterial growth after phage infections with MOIs of ≥102 PFU/CFU. However, at an MOI of 10 PFU/CFU, an average of 9.0 × 103 CFU E. coli O157:H7/ml were recovered (data not shown). The bacteria that survived phage infection were all sensitive to phage SH1 in subsequent assays (data not shown). From these results, animal experiments with phage/E. coli O157:H7 ratios ≥102 were planned.
Mucin did not inhibit the lysis activities of phage SH1 and KH1.
The mucus layer in the gastrointestinal tract may provide a protective barrier shielding E. coli cells from phage lysis (31, 46). SH1 and KH1 lysis of E. coli O157:H7 was tested in medium containing porcine gastric mucin or bovine intestinal mucus. No differences were observed in broth lysis or plaque formation on the plates in terms of the number of cells killed or the number or size of the plaques when mucin or bovine mucus was present (data not shown).
Oral treatment with phage SH1 alone or mixtures of SH1 and KH1 eliminated intestinal E. coli O157:H7 in mice.
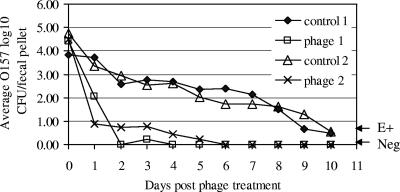
Phage SH1 and KH1 were tested in a murine model of E. coli O157:H7 intestinal carriage using a single strain (ATCC 43894) or a mixture of four strains (ATCC43894, WSU180, WSU400, and WSU588). After a single oral dose of 108 CFU E. coli O157: H7, the mice in both the control groups and phage treatment groups remained active and ostensibly normal over the period of experimentation. In the untreated control groups (control 1 and 2) fecal E. coli O157:H7 was cultured from most animals on all sample days (Fig. 1). The numbers of E. coli O157 CFU/fecal pellet were highest after the oral dose and declined slowly over the course of the experiment. The number of fecal E. coli O157 CFU was reduced by 1.7 log in phage treatment group 1 (phage 1) and 2.4 log in phage treatment group 2 (phage 2) within 24 h after phage treatment, compared to the number of fecal E. coli O157:H7 CFU in the respective control groups (Fig. 1). After the second and third phage treatments on days 1 and 2, the numbers of fecal E. coli O157:H7 CFU were reduced further and were either undetectable or detected only by enrichment culture. At this time, all untreated control mice continued to have >2 log E. coli O157 CFU/fecal pellet (Fig. 1). By day 6, all phage-treated mice were culture negative for E. coli O157:H7 (Fig. 1). Phage titers in the feces of treated mice were 10 6 PFU/fecal pellet on days 1, 2, and 3 after phage treatment and declined to 103 PFU/fecal pellet on day 5 (data not shown). Thus, the calculated ratio of phage/bacteria was ≥102 PFU/CFU at the beginning of the trial and throughout the experiment. The average log10 fecal E. coli O157 CFU among the phage treatment groups was significantly less at day 1 posttreatment and on all following days compared to the control groups (P < 0.01).
FIG. 1.
The effect of oral phage treatment on E. coli O157:H7 in mice. Two groups of three mice (control 1 and phage 1) received a single oral dose of 108 CFU E. coli O157:H7 ATCC 43894 on day −1. On days 0, 1, and 2, mice in phage treatment group 1 were given an oral dose of 1010 PFU of SH1 phage. Another two groups of three mice (control 2 and phage 2) received a single oral dose of 108 CFU E. coli O157:H7 containing equal numbers of four strains (ATCC 43894, WSU180, WSU400, and WSU 588) on day −1. On days 0, 1, and 2 the treated mice were given oral doses of 1010 PFU of both SH1 and KH1. Untreated control mice received 100 μl of a mock bacterial lysate without phage. Data points below E+ indicate samples positive for E. coli O157:H7 only by an enrichment procedure and with total numbers <10 CFU/fecal pellet. Standard errors ranged from 0.5 log to 1.2 logs. Neg indicates RAMS cultures that were negative by both direct and enrichment culture procedures.
Rectal application of phage reduced the number of E. coli O157:H7 CFU but did not clear the pathogen from steers.
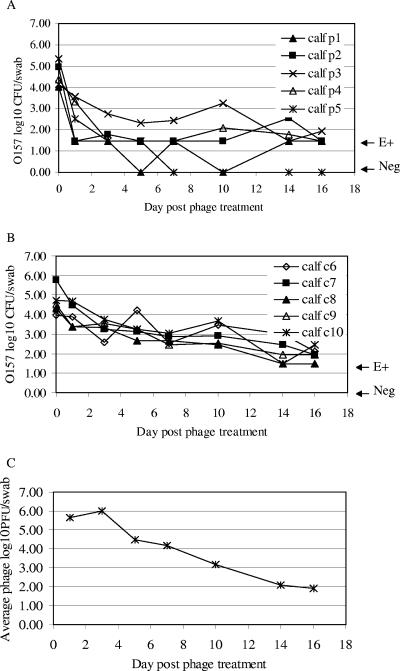
With the success of phage treatment in mice to clear intestinal E. coli O157:H7 (Fig. 1), the combination of SHI and KH1 seemed promising as a therapy for cattle. However, the failure of oral phage treatment in sheep (Table 1) and unsuccessful attempts by others to use oral phage therapy to clear intestinal E. coli O157:H7 from calves (D. D. Hancock [Washington State University, Pullman], personal communication) suggested that another approach to phage treatment might be needed to clear intestinal E. coli O157:H7 from ruminants. We postulated that the inability of phage therapy to clear intestinal E. coli O157 in ruminants may have been due to differences in the numbers of E. coli O157:H7 CFU surviving in the intestinal tract after oral dose, bacterium/phage ratios <102, the large volume of the bovine intestine, and/or differences between the environments of the monogastric and ruminant intestinal tracts. To address the first issues, we designed an experiment to insure that all control animals would carry the bacteria and that the ratio of the phage to bacteria could be maximized. Rectal application of E. coli O157:H7 was used to ensure the long-term carriage of bacteria. Ten steers (five controls and five phage treated) were individually dosed with 106 CFU of a four-strain mixture of E. coli O157 applied to the RAJ mucosa on day −7. Although the dose (106 CFU) was well below previous rectal application protocols that use ∼1010 CFU, long-term carriage of bacteria resulted in all the untreated control animals. All steers were culture positive for the bacteria with 4- to 5-log values of E. coli O157:H7 detected in RAMS samples on day 0 (Fig. 2A and B). All untreated control animals carried E. coli O157 for the duration of the experiment (Fig. 2B). Phages were applied at the RAJ mucosa of animals in the phage treatment group on days 0, 1, 2, and 4. The average number of E. coli O157:H7 CFU in RAMS samples of phage-treated steers declined sharply to 2.4 logs on day 1 after phage treatment, whereas the average number of E. coli O157 CFU/swab in the control group was 3.9 logs at this time. The number of E. coli O157 CFU cultured from the RAJ among the phage-treated group declined further to an average of 1.4 logs compared to 2.7 logs among the control group 7 days after phage treatment (Fig. 2A and B). Numbers of E. coli O157 CFU/swab among the phage-treated steers were significantly less (P < 0.05) than among the control group on day 1 and through day 10 posttreatment. Two steers were culture negative for E. coli O157 on days 5 and 7, and one remained negative for the pathogen throughout the study. However, the phage treatment did not clear E. coli O157 from four of five treated calves.
FIG. 2.
Effect of phage treatment on the number of E. coli O157:H7 CFU in steers. Five phage-treated steers (A) and five untreated control steers (B) received a single rectal application of E. coli O157:H7 (approximately 106 CFU) containing equal amounts of four strains (ATCC 43894, WSU180, WSU400, and WSU588) on day −7. On days 0, 1, 2, and 4, each steer in the phage treatment group was given rectal applications of 8.1 × 1010 PFU of phages containing equal amounts of SH1 and KH1 in a 30-ml solution. In addition, starting on day 0, drinking water contained SH1 and KH1 (106 PFU/ml). The control steers were given 30 ml mock lysate rectally on days 0, 1, 2, and 4, and the drinking water contained the mock lysate without phage. E+ indicates RAMS cultures positive for E. coli O157:H7 only by an enrichment procedure and thus contained <30 E. coli O157 CFU/swab. Neg indicates RAMS cultures that were negative by both direct and enrichment culture procedures. (C) Average numbers of total SH1 and KH1 phages in RAMS samples from phage-treated steers.
The titer of phages in the RAMS samples of treated steers was 4 to 6 logs/swab during the first 7 days after phage treatment and remained 2 logs/swab until the end of the study (Fig. 2C). The calculated ratios of phage/E. coli O157:H7 in the RAMS samples ranged from 1 to104 depending on the sample day and the individual steer.
Some E. coli O157 isolates recovered from phage-treated steers displayed unusual colony morphology, and all were sensitive to KH1 and/or SH1.
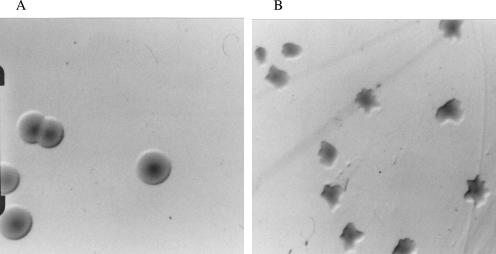
By pulsed-field gel electrophoresis (32), we identified all four E. coli O157:H7 strains in similar numbers among the isolates cultured from the phage-treated steers on days 7, 14, and 16. This indicated that all strains were able to survive the phage treatment (data not shown). However, these isolates were each sensitive to KH1 and/or SH1 (data not shown). Very often, every E. coli O157:H7 colony recovered from murine fecal or bovine RAMS samples from phage-treated animals differed in morphology from those recovered from control animals (occurring in about one of three cultured samples; data not shown). Colonies of E. coli O157:H7 recovered from some mice and steers in the phage treatment groups were relatively small and had irregular star-shaped edges on SMAC-CTVM plates (Fig. 3A). This was in contrast to E. coli O157:H7 recovered from control animals, not treated with phage, which had relatively larger colonies with rounded smooth edges (Fig. 3B). Restreaking these irregularly shaped colonies onto LB agar or SMAC-CTVM agar yielded colonies with normal morphology and cells that were susceptible to both KH1 and SH1 phage infections.
FIG. 3.
Digital photographs of representative E. coli O157 colonies from (A) control steers or mice and (B) phage-treated steers or mice. Colonies are on SMAC-CTVM plates incubated for 18 h at 37°C.
DISCUSSION
The most important finding of this study was that a combination of the previously described phage KH1 and a newly isolated and characterized phage SH1 reduced the number of E. coli O157:H7 CFU in cattle. This was achieved by applying the phage directly and repeatedly to the RAJ and by maintaining a continuous supply of phage in the drinking water. Although shedding was significantly reduced, the phage treatment did not clear E. coli O157 from the majority of steers. This was in contrast to pilot work done in a mouse model of intestinal E. coli O157 carriage, in which this phage combination or SH1 phage alone rapidly (4 to 6 days) cleared the bacteria from all test animals.
Ruminants, and especially cattle and sheep, are the major carriers of E. coli O157:H7. The recently described and unusual colonization site is the RAJ mucosa (26), and experimental carriage of E. coli O157:H7 can be achieved by application of the bacteria directly to this site (38). Since foods of bovine origins have been implicated in numerous outbreaks (1, 5, 17), control measures to reduce or eliminate E. coli O157:H7 from cattle would likely reduce food-borne infection but would also have a substantial impact on the presence of the pathogen in the farm/feedlot environments and could reduce waterborne and animal contact infections. Preharvest treatment of cattle with lytic phage is considered one option for the control of E. coli O157:H7 (42). A number of in vitro studies have been carried out in several laboratories to evaluate the abilities of a single phage or phage cocktails to eliminate E. coli O157:H7 (2, 21, 44). In beef or in E. coli O157:H7 culture-positive sheep or mice, phage treatments reduced the number of bacteria compared to untreated controls but did not eliminate the bacteria (2, 27, 45).
Phage KH1 treatment in sheep did not affect intestinal E. coli O157:H7, and the numbers detected by fecal culture among the phage-treated group were not statistically different from the untreated control group. The use of orally dosed sheep is consistent with the mode by which natural infections occur and addresses the survival of E. coli O157:H7 throughout the gastrointestinal tract, not just cells attached at the RAJ mucosa, when phage treatment is given. Our results were consistent with work by Bach et al. (2), in which the number of E. coli O157:H7 CFU shed by a phage DC22-treated sheep did not differ significantly from an untreated control group. Nonetheless, it was surprising that repeated large oral doses of phage KH1 that resulted in calculated ratio of phage/bacteria estimated as high as >103 did not affect E. coli O157:H7 carriage. This may have been due to specific conditions of the intestinal tract and/or the nature of the KH1 phage.
In an effort to enhance the phage treatment, a new phage, SH1, was isolated, characterized, and tested with KH1 in a mouse model of intestinal carriage of E. coli O157:H7. Mice, similar to cattle, do not become ill with E. coli O157:H7, and an oral dose of the bacteria results in transient intestinal carriage (12, 43). It was encouraging that treatment with phage SH1 alone or in combination with KH1 rapidly (within 2 to 6 days) cleared E. coli O157:H7 from mice.
Because the oral phage treatment in sheep reported here and previous oral phage trials in sheep and in cattle did not clear the intestinal bacteria and because the site of E. coli O157:H7 colonization (the RAJ mucosa) is accessible in live animals, we developed a phage treatment protocol that administered the KH1 and SH1 phages directly to this site. This treatment entailed repeated applications of high-titer phages and was successful in reducing the number of E. coli O157 CFU carried by animals but did not clear E. coli O157:H7 from most animals (four of five). This striking difference between the outcomes of phage therapy for the mice and steers could have been due to many factors including the numbers of E. coli O157:H7 CFU surviving in the intestinal tract after oral dose, the accessibility of E. coli O157:H7 attached to the RAJ mucosa, differences in actual phage/bacterium ratios, and/or differences between the environments of the monogastric and ruminant intestinal tracts.
The nature of E. coli O157:H7 carriage in the mouse intestine is different than in cattle and likely contributed to the effectiveness of the phage therapy. Although mice can carry E. coli O157:H7 for several weeks following a single oral dose, no specific site of colonization for this serotype has been identified in mice, and it may be that the bacteria persist primarily in the intestinal lumen digesta. Oral doses of E. coli O157:H7 to cattle result in brief carriage (<10 days, similar to the mice) or long-term carriage (>1 month), which most often means the animals are colonized at the RAJ mucosa (6, 8, 16, 26, 32). Application of E. coli O157:H7 directly to the RAJ mucosa in this study assured that all animals became colonized (38). Also, the timing of phage treatment could be an important factor. The phage treatment in the mice began 24 h after bacterial challenge, whereas the phage treatment in the steers began 7 days after E. coli O157:H7 application. This duration was chosen for the cattle trial so that E. coli O157:H7 had time to colonize at the RAJ mucosa. However, phage may more easily lyse E. coli O157:H7 cells that are free in the digesta and/or effective bacterial contact may be hampered when bacteria are attached to the intestinal mucosa.
The bovine RAJ mucosa is heavily coated with mucin, and this substance may provide a protective barrier for E. coli cells (31, 46). Although tests showed that mucin did not block the interaction between the phage and the bacteria, the complex in vivo conditions were not replicated in the laboratory. Studies to localize E. coli cells in the murine intestine using fluorescent oligonucleotide probes targeting rRNA in situ show that E. coli cells form microcolonies that are embedded in the mucosal material overlying the epithelial cells (31). It is possible that E. coli O157 was similarly embedded in the mucous gel layer at the RAJ and that this matrix reduced the efficiency of the phage-bacterium contact. If phage could not penetrate the mucus gel and accumulate to sufficiently high concentrations to result in appropriate phage/bacteria ratios, E. coli O157:H7 microcolonies would not be destroyed.
The emergence of E. coli O157:H7 insensitive or resistant to phage would also impair phage therapy. Although we did not identify the bacterial receptor for phage SH1, various bacterial outer membrane components have been identified for other phages and include lipopolysaccharide (LPS) and porin protein OmpF for phage K20 in E. coli K-12 (47); LPS and OmpC for phage AR1 (49), phage PP01 (25), and phage SP21-22 in E. coli O157:H7 (24, 44); and LPS for phage KH1 in E. coli O157:H7 (21). The loss of an outer membrane protein and/or alternation of LPS can result in bacteria that are resistant to a particular phage. In this study, we did not find evidence of phage resistance to either KH1 or SH1 among the E. coli O157:H7 isolates from animals receiving phage therapy. However, in some samples we did see unusual colony morphology with highly irregular edges that may have been due to cells resistant to phage, the presence of free phage in the sample, or phage attached to cells. Upon subculture to fresh medium, the colonies appeared normal. Phage, if present, may have been diluted, or resistant cells may have reverted by this restreaking procedure. Although the phenomenon of phage resistance and then reversion to phage sensitivity has been reported by others (27), in these studies, all isolates were sensitive to both KH1 and SH1 phages at every analysis.
Finally, although it is clear that reducing the number of E. coli O157:H7 CFU in cattle by phage treatment is possible, efforts to consistently clear E. coli O157:H7 from cattle with phage therapy may be unrealistic. The situations in which phage therapy is successful involve infections (3, 7, 23, 39, 41). Good examples of effective phage therapy for E. coli infections include treatment of calves and piglets for enteropathogenic E. coli diarrhea and treatment of chickens for E. coli septicemia and meningitis (3, 39). Similar to the effects of antibiotics, phage therapy reduces the number of bacteria so that the immune response can gain the upper hand and eliminate the infectious agent. However, natural or experimental carriage of E. coli O157:H7 is not an infection, and this serotype, as a member of the transient normal flora, does not illicit a strong immune response in cattle or sheep during its natural association with these animals. Thus, even when phage treatment is successful at reducing the number of bacteria, without a strong immune response the bacteria may not be eliminated.
All these factors make it difficult to predict the consequences of a particular phage treatment without empirical analysis. Payne and Jansen (30) use a mathematical model to illustrate that phage therapy is a kinetic process with density-dependent qualities for phage-bacterium interactions. Inoculum size, inoculum timing, phage burst size, appearance of phage-resistant or insensitive mutants, and/or host factors such as antiphage immune response (40) all play roles in this dynamic process. Our work showed that phage therapy would be effective at reducing the number of intestinal E. coli O157:H7 CFU in ruminants but highlighted the difficulties of developing an effective phage intervention to eliminate E. coli O157:H7.
Acknowledgments
This work was supported, in part, by the Idaho Agriculture Experiment Station; the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant numbers 99-35201-8539 and 04-04562; Public Health Service grants U54-AI-57141, P20-RR16454, NO1-HD-0-3309, and P20-RR15587 from the National Institutes of Health; and grants from the United Dairymen of Idaho and the Idaho Beef Council.
We thank Lonie Austin, Paula Austin, and Dara Gaskin for technical assistance and animal handling.
REFERENCES
- 1.Allerberger, F., M. Wagner, P. Schweiger, H. P. Rammer, A. Resch, M. P. Dierich, A. W. Friedrich, and H. Karch. 2001. Escherichia coli O157 infections and unpasteurised milk. Euro Surveill. 6:147-151. [DOI] [PubMed] [Google Scholar]
- 2.Bach, S. J., T. A. McAllister, D. M. Veira, V. P. J. Gannon, and R. A. Holley. 2003. Effect of bacteriophage DC22 on Escherichia coli O157:H7 in an artificial rumen system (Rusitec) and inoculated sheep. Anim. Res. 52:89-101. [Google Scholar]
- 3.Barrow, P., M. Lovell, and A. Berchieri, Jr. 1998. Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin. Diagn. Lab. Immunol. 5:294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beighton, D., K. Smith, D. A. Glenister, K. Salamon, and C. W. Keevil. 1988. Increased degradative enzyme production by dental plaque bacteria in mucin-limited continuous culture. Microb. Ecol. Health Dis. 1:85-94. [Google Scholar]
- 5.Bell, B. P., M. Goldoft, P. M. Griffin, M. A. Davis, D. C. Gordon, P. I. Tarr, C. A. Bartleson, J. H. Lewis, T. J. Barrett, J. G. Wells, et al. 1994. A multistate outbreak of Escherichia coli O157:H7-associated bloody diarrhea and hemolytic uremic syndrome from hamburgers. The Washington experience. JAMA 272:1349-1353. [PubMed] [Google Scholar]
- 6.Besser, T. E., D. D. Hancock, L. C. Pritchett, E. M. McRae, D. H. Rice, and P. I. Tarr. 1997. Duration of detection of fecal excretion of Escherichia coli O157:H7 in cattle. J. Infect. Dis. 175:726-729. [DOI] [PubMed] [Google Scholar]
- 7.Biswas, B., S. Adhya, P. Washart, B. Paul, A. N. Trostel, B. Powell, R. Carlton, and C. R. Merril. 2002. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect. Immun. 70:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, C. A., B. G. Harmon, T. Zhao, and M. P. Doyle. 1997. Experimental Escherichia coli O157:H7 carriage in calves. Appl. Environ. Microbiol. 63:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman, P. A., A. T. Cerdan Malo, M. Ellin, R. Ashton, and M. A. Harkin. 2001. Escherichia coli O157 in cattle and sheep at slaughter, on beef and lamb carcasses and in raw beef and lamb products in South Yorkshire, UK. Int. J. Food Microbiol. 64:139-150. [DOI] [PubMed] [Google Scholar]
- 10.Chapman, P. A., C. A. Siddons, A. T. Gerdan Malo, and M. A. Harkin. 1997. A 1-year study of Escherichia coli O157 in cattle, sheep, pigs and poultry. Epidemiol. Infect. 119:245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman, P. A., C. A. Siddons, and M. A. Harkin. 1996. Sheep as a potential source of verocytotoxin-producing Escherichia coli O157. Vet. Rec. 138:23-24. [PubMed] [Google Scholar]
- 12.Conlan, J. W., S. L. Bardy, R. KuoLee, A. Webb, and M. B. Perry. 2001. Ability of Escherichia coli O157:H7 isolates to colonize the intestinal tract of conventional adult CD1 mice is transient. Can. J. Microbiol. 47:91-95. [PubMed] [Google Scholar]
- 13.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan, S. H., C. J. Doherty, J. R. Govan, S. Neogrady, P. Galfi, and C. S. Stewart. 1999. Characteristics of sheep-rumen isolates of Pseudomonas aeruginosa inhibitory to the growth of Escherichia coli O157. FEMS Microbiol. Lett. 180:305-310. [DOI] [PubMed] [Google Scholar]
- 15.Fischer, C. R., M. Yoichi, H. Unno, and Y. Tanji. 2004. The coexistence of Escherichia coli serotype O157:H7 and its specific bacteriophage in continuous culture. FEMS Microbiol. Lett. 241:171-177. [DOI] [PubMed] [Google Scholar]
- 16.Grauke, L. J., I. T. Kudva, J. W. Yoon, C. W. Hunt, C. J. Williams, and C. J. Hovde. 2002. Gastrointestinal tract location of Escherichia coli O157:H7 in ruminants. Appl. Environ Microbiol. 68:2269-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimm, L. M., M. Goldoft, J. Kobayashi, J. H. Lewis, D. Alfi, A. M. Perdichizzi, P. I. Tarr, J. E. Ongerth, S. L. Moseley, and M. Samadpour. 1995. Molecular epidemiology of a fast-food restaurant-associated outbreak of Escherichia coli O157:H7 in Washington State. J. Clin. Microbiol. 33:2155-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hancock, D. D., T. E. Besser, M. L. Kinsel, P. I. Tarr, D. H. Rice, and M. G. Paros. 1994. The prevalence of Escherichia coli O157.H7 in dairy and beef cattle in Washington State. Epidemiol. Infect. 113:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock, D. D., T. E. Besser, D. H. Rice, D. E. Herriott, and P. I. Tarr. 1997. A longitudinal study of Escherichia coli O157 in fourteen cattle herds. Epidemiol. Infect. 118:193-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudva, I. T., P. G. Hatfield, and C. J. Hovde. 1995. Effect of diet on the shedding of Escherichia coli O157:H7 in a sheep model. Appl. Environ. Microbiol. 61:1363-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kudva, I. T., S. Jelacic, P. I. Tarr, P. Youderian, and C. J. Hovde. 1999. Biocontrol of Escherichia coli O157 with O157-specific bacteriophages. Appl. Environ. Microbiol. 65:3767-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lema, M., L. Williams, and D. R. Rao. 2001. Reduction of fecal shedding of enterohemorrhagic Escherichia coli O157:H7 in lambs by feeding microbial feed supplement. 39:31-39. [DOI] [PubMed]
- 23.Matsuzaki, S., M. Yasuda, H. Nishikawa, M. Kuroda, T. Ujihara, T. Shuin, Y. Shen, Z. Jin, S. Fujimoto, M. D. Nasimuzzaman, H. Wakiguchi, S. Sugihara, T. Sugiura, S. Koda, A. Muraoka, and S. Imai. 2003. Experimental protection of mice against lethal Staphylococcus aureus infection by novel bacteriophage phi MR11. J. Infect. Dis. 187:613-624. [DOI] [PubMed] [Google Scholar]
- 24.Mizoguchi, K., M. Morita, C. R. Fischer, M. Yoichi, Y. Tanji, and H. Unno. 2003. Coevolution of bacteriophage PP01 and Escherichia coli O157:H7 in continuous culture. Appl. Environ. Microbiol. 69:170-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morita, M., Y. Tanji, K. Mizoguchi, T. Akitsu, N. Kijima, and H. Unno. 2002. Characterization of a virulent bacteriophage specific for Escherichia coli O157:H7 and analysis of its cellular receptor and two tail fiber genes. FEMS Microbiol. Lett. 211:77-83. [DOI] [PubMed] [Google Scholar]
- 26.Naylor, S. W., J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. McKendrick, D. G. E. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Flynn, G., R. P. Ross, G. F. Fitzgerald, and A. Coffey. 2004. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Appl. Environ. Microbiol. 70:3417-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa, M., K. Shimizu, K. Nomoto, R. Tanaka, T. Hamabata, S. Yamasaki, T. Takeda, and Y. Takeda. 2001. Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157:H7 by probiotic Lactobacillus strains due to production of lactic acid. Int. J. Food Microbiol. 68:135-140. [DOI] [PubMed] [Google Scholar]
- 29.Ohya, T., T. Marubashi, and H. Ito. 2000. Significance of fecal volatile fatty acids in shedding of Escherichia coli O157 from calves: experimental infection and preliminary use of a probiotic product. J. Vet. Med. Sci. 62:1151-1155. [DOI] [PubMed] [Google Scholar]
- 30.Payne, R. J., and V. A. Jansen. 2001. Understanding bacteriophage therapy as a density-dependent kinetic process. J. Theor. Biol. 208:37-48. [DOI] [PubMed] [Google Scholar]
- 31.Poulsen, L. K., F. Lan, C. S. Kristensen, P. Hobolth, S. Molin, and K. A. Krogfelt. 1994. Spatial distribution of Escherichia coli in the mouse large intestine inferred from rRNA in situ hybridization. Infect. Immun. 62:5191-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice, D. H., H. Q. Sheng, S. A. Wynia, and C. J. Hovde. 2003. Rectoanal mucosal swab culture is more sensitive than fecal culture and distinguishes Escherichia coli O157:H7-colonized cattle and those transiently shedding the same organism. J. Clin. Microbiol. 41:4924-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Sanderson, M. W., T. E. Besser, J. M. Gay, C. C. Gay, and D. D. Hancock. 1999. Fecal Escherichia coli O157:H7 shedding patterns of orally inoculated calves. Vet. Microbiol. 69:199-205. [DOI] [PubMed] [Google Scholar]
- 36.SAS Institute. 1982. Statistical analysis system. SAS Institute, Inc., Cary, N.C.
- 37.Seeley, H. W., P. J. VanDemark, and J. J. Lee. 2001. Microbes in action: a laboratory manual of microbiology, 4th ed. W. H. Freeman and Company, New York, N.Y.
- 38.Sheng, H., M. A. Davis, H. J. Knecht, and C. J. Hovde. 2004. Rectal administration of Escherichia coli O157:H7: novel model for colonization of ruminants. Appl. Environ. Microbiol. 70:4588-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith, H. W., and M. B. Huggins. 1983. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J. Gen. Microbiol. 129:2659-2675. [DOI] [PubMed] [Google Scholar]
- 40.Smith, H. W., M. B. Huggins, and K. M. Shaw. 1987. Factors influencing the survival and multiplication of bacteriophages in calves and in their environment. J. Gen. Microbiol. 133:1127-1135. [DOI] [PubMed] [Google Scholar]
- 41.Soothill, J. S. 1992. Treatment of experimental infections of mice with bacteriophages. J. Med. Microbiol. 37:258-261. [DOI] [PubMed] [Google Scholar]
- 42.Stevens, M. P., P. M. van Diemen, F. Dziva, P. W. Jones, and T. S. Wallis. 2002. Options for the control of enterohaemorrhagic Escherichia coli in ruminants. Microbiology 148:3767-3778. [DOI] [PubMed] [Google Scholar]
- 43.Taguchi, H., M. Takahashi, H. Yamaguchi, T. Osaki, A. Komatsu, Y. Fujioka, and S. Kamiya. 2002. Experimental infection of germ-free mice with hyper-toxigenic enterohaemorrhagic Escherichia coli O157:H7, strain 6. J. Med. Microbiol. 51:336-343. [DOI] [PubMed] [Google Scholar]
- 44.Tanji, Y., T. Shimada, M. Yoichi, K. Miyanaga, K. Hori, and H. Unno. 2004. Toward rational control of Escherichia coli O157:H7 by a phage cocktail. Appl. Microbiol. Biotechnol. 64:270-274. [DOI] [PubMed] [Google Scholar]
- 45.Tanji, Y., T. Shimada, H. Fukudomi, K. Miyanaga, Y. Nakai, and H. Unno. 2005. Therapeutic use of phage cocktail for controlling Escherichia coli O157:H7 in gastrointestinal tract of mice. J. Biosci. Bioeng. 100:280-287. [DOI] [PubMed] [Google Scholar]
- 46.Tannock, G. W. 1996. Normal microbiota of the gastrointestinal tract of rodents, p. 187-206. In R. I. Mackie, R. A. White, and R. E. Isaacson (ed.), Gastrointestinal microbiology, vol. 2. Chapman & Hall, New York, N.Y. [Google Scholar]
- 47.Traurig, M., and R. Misra. 1999. Identification of bacteriophage K20 binding region of OmpF and lipopolysacchride in Escherichia coli K-12. FEMS Microbiol. Lett. 181:101-108. [DOI] [PubMed] [Google Scholar]
- 48.Wells, J. G., L. D. Shipman, K. D. Greene, E. G. Sowers, J. H. Green, D. N. Cameron, F. P. Downes, M. L. Martin, P. M. Griffin, and S. M. Ostroff. 1991. Isolation of Escherichia coli serotype O157:H7 and other Shiga-like-toxin-producing E. coli from dairy cattle. J. Clin. Microbiol. 29:985-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu, S. L., K. L. Ko, C. S. Chen, Y. C. Chang, and W. J. Syu. 2000. Characterization of the distal tail fiber locus and determination of the receptor for phage AR1, which specifically infects Escherichia coli O157:H7. J. Bacteriol. 182:5962-5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao, T., M. P. Doyle, B. G. Harmon, C. A. Brown, P. O. Mueller, and A. H. Parks. 1998. Reduction of carriage of enterohemorrhagic Escherichia coli O157:H7 in cattle by inoculation with probiotic bacteria. J. Clin. Microbiol. 36:641-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao, T., M. P. Doyle, J. Shere, and L. Garber. 1995. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl. Environ Microbiol. 61:1290-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]