Abstract
Resveratrol production in Saccharomyces cerevisiae was compared to that in Escherichia coli. In both systems, 4-coumarate:coenzyme A ligase from tobacco and stilbene synthase from grapes were expressed. When p-coumaric acid was used as the precursor, resveratrol accumulations in the culture medium were observed to be comparable in E. coli (16 mg/liter) and yeast (6 mg/liter).
Resveratrol (3,5,4′-transhydroxystilbene) is one of the most widely studied representatives of the plant-produced polyphenols. It is well-known for its presence in red wine. As a component of grape skin (up to 0.1%), it is extracted, together with the colored anthocyanins, into red wine during fermentation. The health effect of red wine is believed to result from its high concentration of polyphenols, including resveratrol. A number of studies (recently reviewed in reference 8) have described the multiple beneficial effects of this molecule in humans, including anticarcinogenic effects and effects on low-density lipoproteins. Therefore, we set out to produce it in microbial systems, as an alternative to extraction from plant or chemical synthesis.
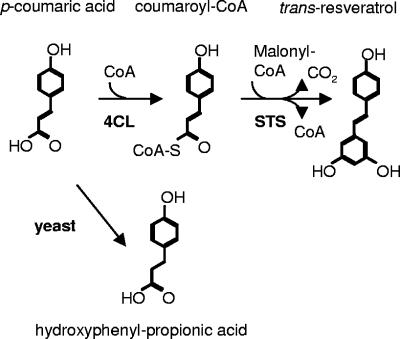
In plants, resveratrol is produced as a branch from the phenylpropanoid pathway (9). It is derived from p-coumaric acid, which is an intermediate in lignin production. By the action of 4-coumarate:coenzyme A (CoA) ligase (4CL) (EC 6.2.1.12), it is converted into coumaroyl-CoA. Coumaroyl-CoA and three units of malonyl-CoA are condensed into resveratrol by the action of stilbene synthase (STS) (EC 2.3.1.95) (Fig. 1).
FIG. 1.
Biosynthetic pathway of resveratrol. The biosynthesis of resveratrol starts by the coupling of p-coumaric acid to CoA by the 4CL enzyme. Subsequently, coumaroyl-CoA is converted into resveratrol by sequential addition of three malonyl-CoA units with the release of carbon dioxide.
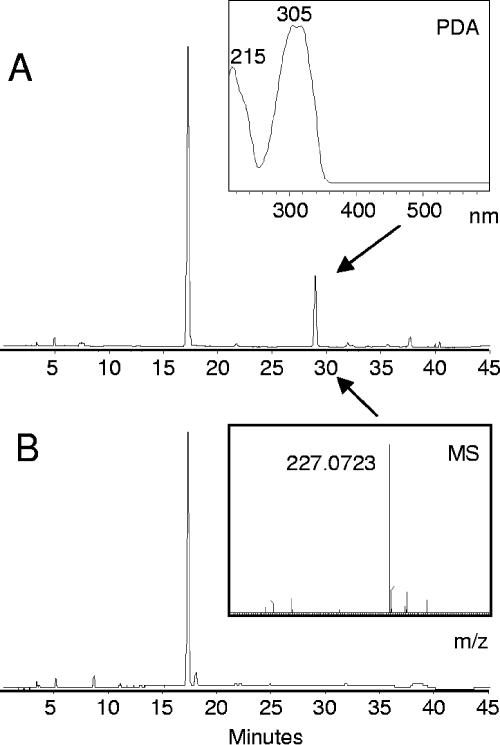
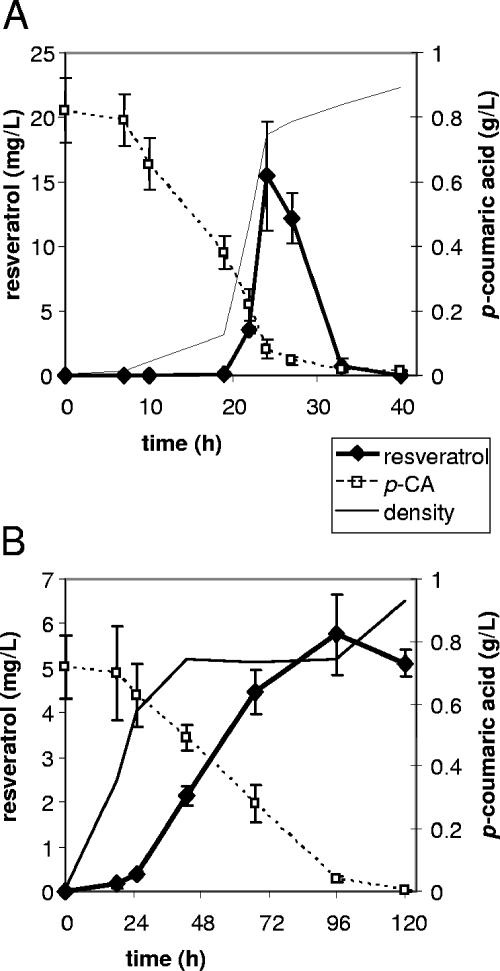
The 4CL2 gene (GenBank accession no. U50846) (5) was amplified from cDNA obtained from a young wounded leaf from Nicotiana tabacum cv. Samsun by use of oligonucleotides 5′-TATACCATGGAGAAAGATACAAAACAGG and 5′-GCGGCCGCTTAATTTGGAAGCCCAGCAG. The amplified fragment was cloned into vector pACYC-DUET1 (Novagen) by use of restriction enzymes NcoI and NotI. The STS gene from Vitis vinifera was amplified from plasmid HEAP25 (7) by use of oligonucleotides 5′-ATATAGATCTGGCTTCAGTCGAGGAAATTAGAAAC and 5′-ATATACTCGAGCTTAATTTGTAACCATAGGAATGC. The amplified fragment was introduced into pACYC-DUET1-4CL by use of BglII and XhoI restriction enzymes. The resulting plasmid, pAC-4CL-STS, was transferred to Escherichia coli BL21. A flask culture of this strain was grown in 2× YT medium (10 g/liter yeast extract, 16 g/liter tryptone, 5 g/liter NaCl) at 28°C and 250 rpm in 100 ml, supplemented with 5 mM p-coumaric acid (Sigma) and 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). After 24 h of growth, the culture was extracted with ethyl acetate, and high-pressure liquid chromatography (HPLC) analysis of the extract showed the accumulation of a compound with a λmax of 305 nm and elution at 28.9 min in the culture medium (Fig. 2). Both absorption spectrum and retention time of this compound corresponded to those of a standard of resveratrol (Sigma). Further identification using mass spectrometry revealed that this compound had an m/z value of 227.07 [M-H]−, thus confirming the identity of the product as resveratrol (Fig. 2). The formation of resveratrol was followed by HPLC analysis of 5-ml samples during the growth of the culture (Fig. 3A). During logarithmic cell growth (up to 24 h), the amount of resveratrol increased to 12 to 20 mg/liter, while the p-coumaric acid concentration dropped to 0.2 mM after 20 h. During the subsequent stationary phase, both the concentration of resveratrol and that of p-coumaric acid decreased to undetectable levels.
FIG. 2.
HPLC analysis of resveratrol produced by E. coli at 307 nm. The upper chromatogram (A) was derived from an extract of a culture of E. coli BL21 plus pAC-4CL-STS grown with p-coumaric acid for 20 h. Culture medium was separated by an HPLC setup as described in reference 2, and absorbance was recorded at 307 nm. The lower chromatogram (B) shows the same for control E. coli BL21 plus pACYC-DUET1. The peak at 17 min represents p-coumaric acid, and the peak at 28.9 min represents resveratrol. The absorption spectrum of the peak at 28.9 min is shown in the “PDA” inset, while the mass spectrum, recorded with a QTOF ULTIMA mass spectrometer (negative electrospray ionization mode) is shown in the “MS” inset.
FIG. 3.
Production of resveratrol in E. coli (A) and S. cerevisiae (B) over time. Each graph displays the concentration of resveratrol in mg/liter in the culture medium as measured after extraction, the concentration of p-coumaric acid (p-CA) in g/liter, and the density of the culture (no absolute values indicated). The values shown are averages of two measurements of individual cultures.
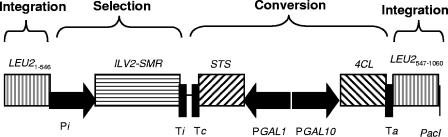
As a next step towards a food-grade production system, we expressed the same genes in Saccharomyces cerevisiae. For that purpose, the 4CL2 and STS genes were assembled into a construct that can be integrated into the yeast LEU2 locus (Fig. 4). In this construct, the 4CL gene is under the control of the GAL10 promoter, while the STS gene is under control of the GAL1 promoter. Upon transformation of yeast strain CEN.PK 113-3b (ura3 his3), integration events were selected for by using 25 mg/liter chlorsulfuron methyl (Supelco) (6) and were confirmed by PCR analysis. Flask cultures of the recombinant yeast colonies were grown in 50 ml yeast nitrogen base medium (28°C, 250 rpm), supplemented with 5 mM p-coumaric acid and 2% galactose to induce gene expression. Expression was continued over several days, while 5-ml samples were taken regularly and analyzed by HPLC. In STS-positive yeast clones, resveratrol was observed to accumulate over time (Fig. 3B), starting at 24 h, when the growth of the cell culture had reached a plateau. Unlike in E. coli, the amount of resveratrol increased during the stationary culture phase, to about 5.8 mg/liter (corresponding to 25 μM) at 96 h. While in E. coli no by-product formation was observed, in the yeast medium a significant additional HPLC peak (Rf = 15.1 min, λmax = 273 nm) was found to occur during the later phases of growth. This compound was identified as hydroxyphenyl-propionic acid (Fig. 1) by comparison with reference standards and mass spectrometry ([M-H]− = 165.0559 m/z). The concentration of this compound increased up to 3 mM. Its synthesis appeared to depend on the presence of the 4CL-STS expression construct, since this peak was absent in untransformed yeast cultures when incubated with p-coumaric acid.
FIG. 4.
Cassette for integration of STS and 4CL into the yeast genome. LEU21-546, LEU2 gene from nucleotides 1 to 546 of the coding region; Pi, promoter of the ILV2 gene; ILV2-SMR, sulfometuron methyl resistance gene; Ti, terminator of the ILV2 gene; Tc, terminator of the CYC gene; STS, coding region of the grape STS gene; PGAL1, promoter of the GAL1 gene; PGAL10, promoter of the GAL10 gene; 4CL, coding region of the 4CL2 gene from tobacco; Ta, terminator of the ADH1 gene; LEU2547-1060, LEU2 gene from nucleotides 547 to 1060 of the coding region; PacI, unique PacI restriction site.
Data from a recent paper (1) indicated that, in yeast, resveratrol was produced predominantly in the form of piceid (trans-resveratrol-3-O-β-glucoside), although in minute quantities. However, at the retention time of piceid no compound was observed in the medium or cells of our transgenic yeast strains. Incubation of culture medium or yeast cell lysate with almond β-glucosidase (Sigma) did not result in an increase in the amount of detectable resveratrol aglycone. Moreover, in our system, the levels of unglucosylated resveratrol were more than 3 orders of magnitude higher (5.8 mg/liter) than the level of piceid previously reported for yeast (1.5 μg/liter [1]).
In the past few years, a number of results have been published on the production in microorganisms of plant-specific polyphenols, mainly naringenin, to different levels (0.45 mg/liter [3], 7 mg/liter [4], and 20 mg/liter [10]). During review of the manuscript, a report on the production of resveratrol in E. coli by use of a peanut STS gene appeared (11). Our data indicate that the recombinant yeast expression platform, using the same biosynthetic genes, is not much less efficient than E. coli. Thus, yeast is capable of producing high levels of resveratrol from inexpensive plant-derived precursors, contrary to previously published data (1). The advantage of yeast over E. coli is its food-grade status, which should, in principle, allow for applications in human nutrition. Interestingly, in both systems the desired product accumulated naturally in the medium rather than in the cells. This is particularly advantageous considering the envisaged commercial production system and its commercial viability. We conclude that economic resveratrol production is viable in a food-grade yeast.
Acknowledgments
This research was carried out as part of the EU Sixth Framework project FOOD-CT-2005-007130 “FLORA.”
The CEN.PK yeast strain was courtesy of Paul van Heusden, and the ILV2-SMR gene was courtesy of Brigitte Rønnow.
REFERENCES
- 1.Becker, J. V. W., G. O. Armstrong, M. J. Van der Merwe, M. G. Lambrechts, M. A. Vivier, and I. S. Pretorius. 2003. Metabolic engineering of Saccharomyces cerevisiae for the synthesis of the wine-related antioxidant resveratrol. FEMS Yeast Res. 4:79-85. [DOI] [PubMed] [Google Scholar]
- 2.Beekwilder, J., H. Jonker, P. Meesters, R. D. Hall, I. M. van der Meer, and C. H. R. de Vos. 2005. Antioxidants in raspberry: on-line analysis links antioxidant activity to a diversity of individual metabolites. J. Agric. Food Chem. 53:3313-3320. [DOI] [PubMed] [Google Scholar]
- 3.Hwang, E. I., M. Kaneko, Y. Ohnishi, and S. Horinouchi. 2003. Production of plant-specific flavanones by Escherichia coli containing an artificial gene cluster. Appl. Environ. Microbiol. 69:2699-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang, H. X., K. V. Wood, and J. A. Morgan. 2005. Metabolic engineering of the phenylpropanoid pathway in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 71:2962-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee, D., and C. J. Douglas. 1996. Two divergent members of a tobacco 4-coumarate:coenzyme A ligase (4CL) gene family—cDNA structure, gene inheritance and expression, and properties of recombinant proteins. Plant Physiol. 112:193-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronnow, B., L. Olsson, J. Nielsen, and J. D. Mikkelsen. 1999. Derepression of galactose metabolism in melibiase producing bakers' and distillers' yeast. J. Biotechnol. 72:213-228. [DOI] [PubMed] [Google Scholar]
- 7.Schijlen, E., C. H. R. de Vos, H. Jonker, H. van den Broeck, J. Molthoff, A. van Tunen, S. Martens, and A. Bovy. 2006. Pathway engineering for healthy phytochemicals leading to the production of novel flavonoids in tomato fruit. Plant Biotechnol. J., 4:433-444. [DOI] [PubMed]
- 8.Signorelli, P., and R. Ghidoni. 2005. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J. Nutr. Biochem. 16:449-466. [DOI] [PubMed] [Google Scholar]
- 9.Sparvoli, F., C. Martin, A. Scienza, G. Gavazzi, and C. Tonelli. 1994. Cloning and molecular analysis of structural genes involved in flavonoid and stilbene biosynthesis in grape (Vitis vinifera L.). Plant Mol. Biol. 24:743-755. [DOI] [PubMed] [Google Scholar]
- 10.Watts, K. T., P. C. Lee, and C. Schmidt-Dannert. 2004. Exploring recombinant flavonoid biosynthesis in metabolically engineered Escherichia coli. Chembiochem 5:500-507. [DOI] [PubMed] [Google Scholar]
- 11.Watts, K. T., P. C. Lee, and C. Schmidt-Dannert. 2006. Biosynthesis of plant-specific stilbene polyketides in metabolically engineered Escherichia coli. BMC Biotechnol. 6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]