Abstract
Multiple reductive dehalogenase (RDase), hydrogenase (H2ase), and other respiration-associated (RA) oxidoreductase genes have been identified in cultured representatives of Dehalococcoides. Although their products are likely to play key roles in the environmentally important process of reductive dechlorination, very little information is available about their regulation and specific functions. Here we show increased expression and temporal variability in the expression of five RDase genes and in the expression of genes for a putative formate dehydrogenase (Fdh) and two H2ases, including a periplasmic [Ni/Fe] H2ase (Hup) and a cytoplasmic [Fe] H2ase (Vhu). mRNA transcripts extracted from tetrachloroethene-dechlorinating mixed cultures corresponding to Fdh, the H2ase Hup, and the RDase targets TceA and DET0162 were expressed most highly, with average levels 34 (± 7.5)-, 23 (± 6.7)-, 16 (± 3.3)-, and 13 (± 3.3)-fold higher, respectively, than that for RNA polymerase (RpoB). H2ase and RA transcripts reached their respective expression maxima within the first 2 h after feeding. RDase transcripts, however, were most highly expressed after 3 h and exhibited greater temporal variability than other transcripts. Comparison with D. ethenogenes strain 195 pure culture expression levels indicated that RDase DET1545 was more highly expressed in mixed cultures, where, on average, its transcript level was sixfold higher than that of RpoB. While the specific functions of several of these gene products remain elusive, the high expression levels and temporal variability reported here suggest that these groups of enzymes are metabolically important for the respiration of chlorinated ethenes in mixed cultures containing Dehalococcoides.
Chlorinated ethenes are common groundwater contaminants that are found at more than half of the sites on the EPA's National Priorities List (1). A mixed enrichment culture capable of reductively dechlorinating tetrachloroethene (PCE) successively to trichloroethene (TCE), cis-1,2-dichloroethene (cDCE), vinyl chloride (VC), and the harmless compound ethene was developed to study this microbially mediated process (4). This enrichment culture (D2) has been maintained for more than a decade and is currently the source of an extract required to grow pure cultures of Dehalococcoides ethenogenes strain 195, a member of the Chloroflexi phylum capable of complete anaerobic reductive dechlorination of PCE. D. ethenogenes uses H2 as its sole electron donor; in the D2 enrichment culture, H2 is formed from the anaerobic fermentation of butyric acid to acetate and H2 (3, 6). This organism, which was isolated from an earlier generation of the D2 enrichment culture, has been well characterized and its genome has been sequenced (14, 16, 18, 20). Characterization of additional Dehalococcoides strains indicates that although all representatives have multiple reductive dehalogenase (RDase) genes, both the number of RDase genes and the corresponding substrate ranges can vary by strain (8, 11, 22).
Of the 19 putative RDase genes identified in the genome of D. ethenogenes strain 195, only those corresponding to PceA and TceA, which are believed to catalyze the reductions of PCE to TCE (13) and of TCE to ethene (12), respectively, have been characterized. Very little is known about the specific functions of additional RDases and other putative respiratory enzymes in the D2 enrichment culture containing strain 195. Recent studies in both pure (J. Fung, personal communication) (17) and mixed (17) PCE-fed cultures containing strain 195 indicated that genes predicted to encode four RDases (TceA, DET0162, DET0318, and DET1559), a periplasmic [Ni/Fe] H2ase (Hup), and a putative formate dehydrogenase (Fdh) exhibited the highest overall expression levels. In another study, expression of multiple RDases in a functionally similar Dehalococcoides-containing mixed culture (KB1) was induced by a single chlorinated substrate, suggesting that several RDase enzymes might contribute to chloroethene dechlorination (22). Although RDase DET1545 did not show increased expression levels in PCE-grown strain 195 pure cultures, Waller and colleagues reported expression of a closely related homolog (22), and peptides matching DET1545 were detected during proteomic analyses of strain 195 (R. Morris, unpublished data).
It has recently been shown for Geobacter sulfurreducens that molecular parameters can serve as bioindicators of interesting metabolic processes and that levels of mRNA transcripts can be correlated with rates of substrate reduction (7). In the present study, we targeted several genes from D. ethenogenes strain 195 that may serve as potential bioindicators of reductive dechlorination and describe their expression profiles over the course of a PCE feeding cycle. Targets included eight RDase genes that showed increased expression during growth on PCE in pure culture, a gene whose RDase was identified by proteomic analyses (DET1545), five H2ase genes, and four additional respiration-associated (RA) transcripts (Table 1) . These data provide novel insights into the relative expression levels and temporal expression variability of key D. ethenogenes respiratory oxidoreductase genes in mixed cultures and suggest that they may serve as good bioindicators of PCE reductive dechlorination.
TABLE 1.
Genomic identification and description of qRT-PCR targets
| Locus taga | Gene or gene product description | Protein designationb | Gene category |
|---|---|---|---|
| 0603 | DNA-dependent RNA polymerase, beta subunit | RpoB | Housekeeper |
| 0187 | Formate dehydrogenase, alpha subunit, putative | Fdh | Other respiration-associated |
| 0926 | Proton-translocating NADH-quinone oxidoreductase, D subunit, putative | Nuo | |
| 0562 | ATP synthase, F1 alpha subunit | AtpA | |
| 0103 | Molybdopterin oxidoreductase, iron-sulfur binding subunit, putative | Mod | |
| 0110 | [Ni/Fe] hydrogenase, group 1, large subunit, putative | Hup | Hydrogenase |
| 0615 | Hydrogenase, group 3, VhuA subunit, putative | Vhu | |
| 0867 | Hydrogenase, group 4, EchE subunit, putative | Ech | |
| 1571 | Hydrogenase, group 4, HycE subunit, putative | Hyc | |
| 0147 | [Fe] hydrogenase, large subunit HymC, putative | Hym | |
| 0079 | Trichloroethene reductive dehalogenase (tceA) gene | TceA | Reductive dehalogenase |
| 0318 | Reductive dehalogenase, putative | PceAc | |
| 1545 | Reductive dehalogenase, putative | ||
| 0162 | Reductive dehalogenase, putative, auth pt mutationd | ||
| 1559 | Reductive dehalogenase, putative | ||
| 0173 | Reductive dehalogenase, putative | ||
| 0876 | Reductive dehalogenase, putative | ||
| 0306 | Reductive dehalogenase, putative | ||
| 1519 | Reductive dehalogenase, putative |
Open reading frame identification number from the D. ethenogenes strain 195 genome.
Designation of known or putative protein.
S. H. Zinder, personal communication.
auth pt mutation, authentic point mutation.
MATERIALS AND METHODS
Chemicals and stock solutions.
Butyric acid (99%; Acros Organics) and PCE (99%; Alfa Aesar) were used as culture substrates and, in the case of PCE, as analytical standards. TCE (99.5%; Fisher Scientific), cis-1,2-DCE (97%; Aldrich Chemical Co.), VC (99.5%; Matheson Gas Products), and ethene (Matheson Gas Products) were used for preparation of analytical standards. Yeast extract (Difco Laboratories) was used as a culture amendment.
Culture procedure.
A PCE-butyrate enrichment culture containing D. ethenogenes strain 195, designated D2, was maintained as described previously (5, 21). Ten percent of the culture was periodically wasted and replaced with fresh basal medium (3) to obtain an average hydraulic residence time of approximately 100 days. Expression studies were performed in triplicate 160-ml subculture serum bottles with a headspace-to-liquid ratio similar to that used for the D2 enrichment culture. The D2 enrichment culture and each subculture were fed PCE (110 μM) and butyric acid (440 μM) at a 2:1 ratio to PCE on an electron equivalent basis (with butyric acid defined as having 4 equivalents/mol based on its fermentation to 2 mol acetate and 2 mol H2 rather than its oxidation to CO2), a vitamin solution (15), and yeast extract to obtain a concentration of 20 mg yeast extract/liter of culture. A subculture lacking PCE was set up as a control to determine whether the activity of other organisms in the enrichment culture might contribute to expression levels. Strain 195 pure culture was grown on PCE and H2 as previously described (14, 16). In short, culture inoculum sizes were 2% (vol/vol) in 27-ml culture tubes containing 10 ml of growth medium. Basal salt medium was amended with 2 mM acetate, a vitamin solution containing 0.05 mg of vitamin B12 per liter, 10% (vol/vol) filter-sterilized anaerobic digester sludge supernatant, and 1% (vol/vol) D2 enrichment culture extract. Culture tubes were sealed with Teflon-coated butyl rubber stoppers and incubated at 35°C.
Gas chromatographic methods.
Ethene and chlorinated ethenes were measured by taking 100-μl headspace samples via a gas-tight locking syringe, analyzed with a Perkin Elmer Autosystem gas chromatograph utilizing a 1/8-inch by 8-ft stainless steel column packed with 1% SP-1000 on 60/80 Carbopak B (Supelco, Inc.), and routed to a flame ionization detector as described previously (4, 21). Column temperature was held at 90°C for 2.5 min, subsequently ramped at 30 degrees per minute to 195°C, and then held isothermally for 8 min. The flame ionization detector was isothermally held at 90°C over the 14-minute run time. Standard curves for PCE, TCE, cDCE, VC, and ethene were created by adding known amounts of each pure compound to 160-ml serum bottles containing 100 ml of distilled H2O.
Sampling procedure and nucleic acid extraction.
Liquid culture samples were taken from the D2 enrichment culture and from each subculture prior to feeding (time zero) and at selected times following feeding. A sterile syringe was purged three times with a 70% N2-30% CO2 gas mixture and used to withdraw either 1 or 2 ml of liquid culture for DNA or RNA analyses, respectively. The samples were placed in centrifuge tubes and immediately pelleted at 21,000 × g for 2 min at 4°C. Supernatants were discarded and cell pellets were stored at −20°C or −80°C prior to DNA or RNA extraction, respectively. Pure culture cell pellets (3 ml) for RNA extractions were prepared by centrifugation at 4°C for 10 min at 21,000 × g. The supernatant was discarded and cell pellets were stored at −20°C.
DNA and RNA extractions were performed within 24 h using UltraClean microbial DNA isolation (Mo Bio Laboratories) and RNeasy Mini (QIAGEN) kits. To control for mRNA losses during sample preparation and inefficiencies in reverse transcription, a normalization protocol modified from that described in the work of Johnson et al. (9), in which 6 × 109 copies of luciferase control RNA (Promega) were added during the lysis step of each RNA extraction, was employed. DNA contamination was removed from RNA samples according to the optional on-column RNase-free DNase I (QIAGEN) digestion protocol. RNA was quantified using the RNA 6000 Nano assay on an Agilent 2100 bioanalyzer (Agilent Technologies). A second DNase treatment step lasting 30 min was performed using RQ1 RNase-free DNase (Fisher Scientific).
Quantitative reverse transcriptase PCR (qRT-PCR).
On average, about 1 μg of RNA was obtained per ml of culture collected. cDNA was synthesized from 0.2 μg of RNA using an iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer's instructions. Gene transcripts were quantified by amplification of cDNA with iQ SYBR green Supermix (Bio-Rad) and primers specific for D. ethenogenes strain 195 gene targets and for the luciferase control. H2ase primers were designed using the software package Beacon Designer 4 (Biosoft International) (17). Other D. ethenogenes-specific oligonucleotides were designed using PrimerQuest (19) and mFold software available at the IDT website (http://scitools.idtdna.com/Primerquest/) (J. Fung and S. H. Zinder, personal communication). Primer specificity was checked by BLAST analysis (2). Standard curves for D. ethenogenes targets and for the luciferase control target (log DNA concentration versus the cycle number at which fluorescence reaches an arbitrarily set cycle threshold value) were generated using serial dilutions of DNA of known concentration extracted from pure and mixed enrichment cultures (for D. ethenogenes targets) and luciferase control DNA (for the luciferase control target). Triplicate amplifications of all standards, unknowns, and controls were performed using an iCycler iQ multicolor real-time PCR detection system (Bio-Rad); 25-μl reaction volumes contained 1× iQ SYBR green Supermix, forward and reverse primer at a concentration of 700 nM, and approximately 3 ng of cDNA template. PCR conditions used for H2ase primer sets were as follows: 2 min at 50°C and 3 min at 95°C followed by 40 cycles of 1 min at 55°C and 1 min at 95°C. PCR conditions for all other primer sets were as follows: 2 min at 50°C and 10 min at 95°C followed by 40 cycles of 1 min at 60°C and 1 min at 95°C. Melt curve analyses were performed after all runs to check for purity of amplicons. A pure culture DNA sample of known quantity was analyzed with each primer set and yielded the same abundance value regardless of the primer used, supporting the suitability of the mixed-culture standard curves.
RESULTS
Preliminary analysis of gene expression and target selection.
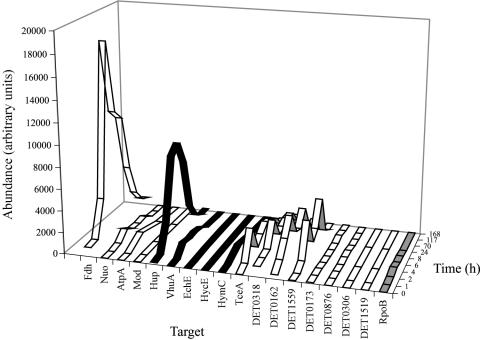
qRT-PCR data were taken from the D2 enrichment culture over the course of a PCE-butyrate feed to identify temporal trends in gene regulation and to select highly expressed targets for further analyses (Fig. 1; Table 1). Genes encoding four RDases (TceA, DET0162, DET0318, and DET1559), two H2ases (Hup and Vhu), and two RA targets (Fdh and AtpA) exhibited the highest overall expression levels and were greater than that of the gene encoding RpoB, which was chosen as a “housekeeper.” The RDase DET1545 gene was not targeted in the initial D2 expression study. Its product, however, was subsequently identified by liquid chromatography-tandem mass spectrometry proteomic approaches, and it was added to the list of potential bioindicators. A comparison of pure and mixed culture expression levels indicated that DET1545 expression was lower than RpoB expression in pure cultures but increased by nearly 5.6 (± 3.4)-fold relative to RpoB expression in mixed cultures. Genes encoding the H2ase Hym and the RA target NADH-ubiquinone oxidoreductase (Nuo) had expression levels similar to that for the RpoB gene, and the expression levels of additional RDase, H2ase, and RA targets, including the molybdopterin oxidoreductase (Mod) gene, were lower than that for the RpoB gene and were not included in additional expression studies reported here.
FIG. 1.
Expression profiles of potential bioindicator targets in the D2 enrichment culture. Transcripts corresponding to Fdh, the H2ase Hup, and the RDases TceA, DET0162, DET0318, and DET1559 are preferentially expressed.
Expression and temporal variability.
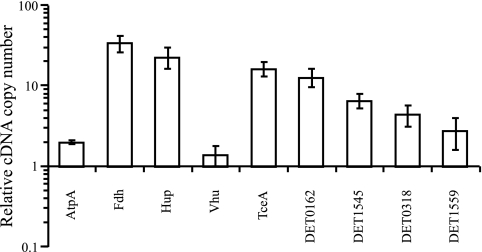
Preliminary qRT-PCR results (Fig. 1) suggested that expression of some targets increased within 1 h of feeding and reached maximum observed levels within 12 h, after which point expression tended to slowly decline until returning to the hour 0 state. As a result, high-resolution qRT-PCR measurements of a subset of highly expressed targets were taken from triplicate subcultures inoculated with D2 and incubated for 12 h. With the exception of DET1545 and DET0318, all targets studied showed at least an order of magnitude increase in expression between 0 and 1 h. The Fdh target had average expression levels 34 (± 7.5)-fold higher than that of the housekeeper RpoB and was followed by the H2ase Hup and the RDase targets TceA and DET0162, which had average expression levels 23 (± 6.7)-, 16 (± 3.3)-, and 13 (± 3.3)-fold higher, respectively, than that for RpoB (Fig. 2). Similarly high expression levels were observed for the Fdh target in a previous study, which indicated that the Fdh target was up-regulated in both batch-pure and mixed cultures (17); this result was surprising, given the evidence that D. ethenogenes strain 195 is not capable of using formate as an electron donor (X. Maymo-Gatell, Y. Chien, T. Anguish, J. M. Gossett, and S. H. Zinder, unpublished results). For all targets studied, average expression levels in the control subculture lacking PCE were approximately 2 orders of magnitude lower (2% on average) than in PCE-fed subcultures.
FIG. 2.
Expression level of each target in triplicate subcultures relative to that for the RpoB gene (data presented are averages of values from 1 to 12 h after PCE feeding). Targets are represented by their corresponding products. Error bars represent standard deviations.
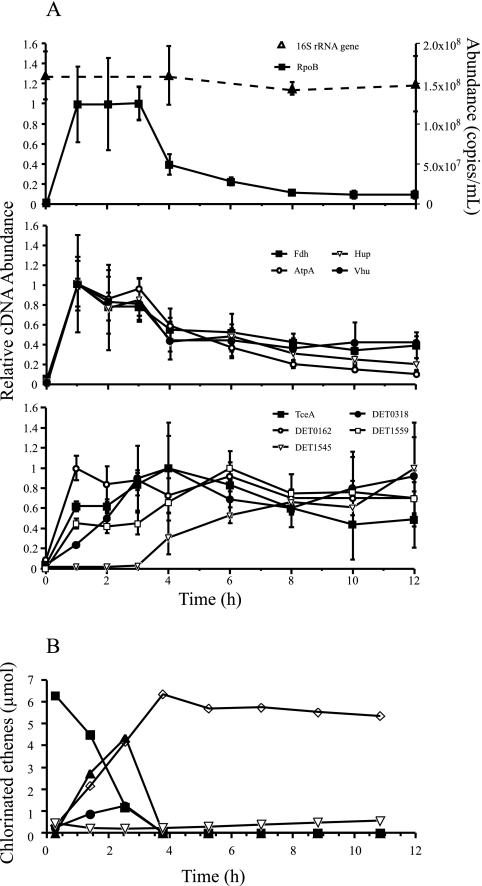
No significant increases were observed in D. ethenogenes 16S rRNA gene abundance during the first 12 h after PCE feeding (Fig. 3A). These data suggest that the population of D. ethenogenes stayed relatively constant throughout the experiment and that qRT-PCR trends reported here reflected differences in expression rather than cell numbers. Temporal variability in the expression profile of the RpoB gene, when plotted relative to its own expression maximum, indicates that the period of highest cellular activity is between 0 and 3 h (Fig. 3A). Statistical comparison of three gene groups—RpoB, H2ase/RAs (Hup, Vhu, Fdh, and AtpA), and RDases—indicate that expression profiles differ across the three groups [one-way analysis of variance showed significant interaction of time by gene group; F(8,2) = 4.612; P < 0.0001]. General trends in gene categories suggest that H2ase and RA transcript levels were highest early during dechlorination (0 to 3 h), while RDase transcript levels increased more slowly, reached their maxima after 3 h, and persisted for up to 12 h (Fig. 3A). RDase transcript levels were typically highest after TCE, cDCE, and VC daughter products had reached their maximum values (Fig. 3A and B). Reductive dechlorination profiles resembled those previously reported for the D2 enrichment culture (6). Daughter products were observed within 0.3 h, and added PCE was dechlorinated by near-zero-order kinetics to VC and ethene within 4 to 5 h. Between 2 and 3 h, TCE and cDCE accumulated to their respective maxima of approximately 10 and 40 μM. VC concentration increased for 3 to 4 h, at which point it was slowly converted to ethene for the duration of the experiment (Fig. 3B).
FIG. 3.
(A) 16S rRNA gene abundance (dashed line) and mRNA expression profiles (solid lines) of individual targets over time broken up into functional categories. Error bars represent standard deviations. Each mRNA target is plotted as a fraction of its maximum expression and is represented by its corresponding product. Expression of the RpoB gene, a central metabolic housekeeper, declines after 3 to 4 h, when a majority of respirable substrates are dechlorinated. The Fdh, AtpA, Hup, and Vhu genes are grouped as targets associated with hydrogen metabolism and experience maximum expression within the first 2 hours. RDase genes are grouped together and tend to experience maximum expression after 3 h. (B) Dechlorination profiles in triplicate subculture bottles. PCE (▪), TCE (•), and cDCE (▴) are fully dechlorinated within 4 to 5 h, followed by the slow conversion of VC (⋄) to ethene (▿).
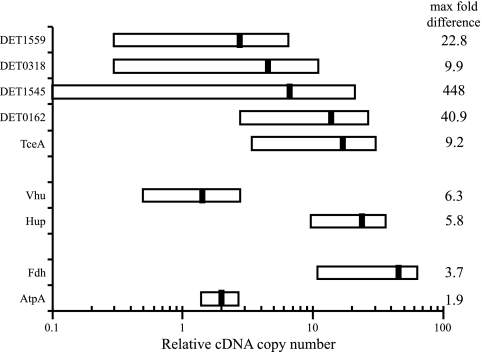
Previous studies have suggested that expression levels of Dehalococcoides targets may vary over time and that conclusions based on any single time point may be confounding (17). Figure 4 indicates that, between hours 1 and 12, some targets have a larger range of expression relative to that of the RpoB gene than others. The relative abundance of the TceA gene target at its maximum is 9.2 times higher than its lowest observed relative abundance during this time period. In contrast, the Hup gene target shows only a 5.8-fold difference in relative abundance between its highest and lowest expression levels. In general, group comparison indicates that RDase targets are expressed with greater variability than are H2ase or RA targets.
FIG. 4.
Temporal ranges of expression levels relative to that for the RpoB gene. Left and right ends of horizontal bars mark the levels of lowest and highest expression, respectively, for hours 1 through 12. Black hash marks within each bar indicate the average levels of relative expression during this time. The data column shows the difference (n-fold) between the highest and lowest expression level for each target; targets are represented by their corresponding products.
DISCUSSION
Genes encoding Fdh, the H2ase target Hup, and RDase targets TceA, DET0162, DET1545, DET0318 (tentatively identified as PceA [S.H. Zinder, personal communication]), and DET1559 were transcribed at levels above that for the housekeeping gene encoding RpoB, and their corresponding proteins, with the exception of DET0162, were identified in a previous study using proteomic approaches (17). The DET0162 target, although highly expressed, contains a point mutation, has a partially truncated anchor protein, and is unlikely to code for a functional RDase (20). RDase and H2ase targets tended to be expressed as groups of respiratory oxidoreductases that exhibited different patterns of temporal variability. Additionally, expression by gene category, particularly in the case of the RDase genes, varied significantly over time. It is likely that the products of these genes play important roles in reductive dechlorination. Further research into the relationship between expression levels and the catalytic functions of these enzymes is likely to provide novel insights into the signal(s) that controls their expression at field sites contaminated with chlorinated ethenes.
While increases in expression were observed within the first hour after PCE feeding, expression in no-PCE controls did not significantly increase, supporting the conclusion that expression levels are attributable to D. ethenogenes rather than to other members of the community, such as fermenters or methanogens. Furthermore, measurements of relative cell numbers did not indicate a significant change in the population size during the time frame of this study (Fig. 3A). Although some growth certainly occurred in these cultures, increases in cell numbers alone could not account for the order of magnitude increases in expression that were observed.
Two recent studies of Dehalococcoides have shown that expression levels of TceA and H2ase targets can vary with time (10, 17). Johnson et al. examined the RDase target TceA under a variety of conditions and found that its expression was independent of hydrogen concentration and chlorinated ethene concentration down to about 2 μM but that it varied according to the electron acceptor used (10). In general, they found that expression of the TceA target increased over time in response to growth-supporting substrates (TCE, cDCE, 1,1-DCE) as well as to trans-DCE, which does not support growth, but that PCE or VC did not lead to increased expression. Our results indicate that expression levels in batch cultures of many key respiratory targets, particularly RDases, depend on time of sampling and that individual target expression does not appear to correlate with the instantaneous dechlorination of specific chlorinated ethenes. Furthermore, results show distinct temporal patterns in the ways that RDases and H2ases are expressed: H2ases and RA targets (including Fdh and AtpA) tend to reach their maximum expression earlier in the feeding cycle, as does the housekeeper RpoB, while RDases tend to reach their maxima later. D. ethenogenes may maintain a relatively large “standing crop” of RDase enzymes which, though adequate for initial dechlorination, require augmenting as high concentrations of substrate persist. It is also possible that D. ethenogenes devotes its initial energy to the gathering of electrons, up-regulating RDase expression only after creating a sufficient pool of reducing energy (a high concentration of charged energy carriers). Finally, the expression of RDase and H2ase targets may be up-regulated as daughter products (such as TCE, cDCE, and VC) reach critical concentrations.
The variability in expression trends across functionally distinct gene categories (housekeeper, H2ase, RA, and RDase) suggest that transcriptional regulation is occurring at the group level. Housekeeper, H2ase, and RA target expression profiles share similar patterns of temporal variation that are different from those of RDase profiles (Fig. 3A). While RDases as a group are up-regulated later than H2ase and RA targets, the timing and extent of up-regulation varies for each RDase target. This suggests that unique regulatory pathways exist for each RDase gene and agrees with the prediction of two-component regulatory elements (histidine kinases and response regulators) flanking most of the RDase genes in the available Dehalococcoides genomes (11, 20). These regulatory elements may work in both cis and trans fashion to coordinate expression of multiple RDase genes.
Our goal was to identify possible bioindicators of reductive dechlorination and understand their expression over time in response to addition of growth-limiting substrates. A good bioindicator for ultimate field use should be specific (unique to the genes imparting the desired activity), accurate (correlated to the desired activity), and quantifiable (detectable and measurable). The housekeeper RpoB is an attractive option, since it is highly conserved among Dehalococcoides groups compared to more mobile and divergent targets such as the RDases. On the other hand, H2ases and RDases, once a better understanding of their expression under various conditions is obtained, may have the ability to yield more information about dechlorination potential and rate. Also, the higher expression levels of some H2ase and RDase targets may make them more attractive options at field sites where cell densities are low and detection limits are a major concern. Accurate and comprehensive documentation of in situ bioremediation at a field site will probably require a suite of bioindicators that includes highly conserved, more metabolically central targets, such as RpoB, and targets more specific to reductive dechlorination, such as Hup and TceA. Here we report novel respiratory oxidoreductase expression data from a mixed dechlorinating community containing D. ethenogenes, identify temporal patterns in gene regulation, and suggest potential bioindicators.
Acknowledgments
This work was supported by the U.S. Department of Energy Office of Cleanup Technologies and administered by the U.S. Department of Energy Savannah River Operations Office (contract no. DE-AC09-96SR18500) and is a product of the Monitored Natural Attenuation/Enhanced Attenuation for Chlorinated Solvents Technology Alternative Project.
We thank Jennifer Fung and Stephen Zinder for RDase primer sequences and valuable advice, Jennifer Zabinsky for assistance with the D2 enrichment culture, and Merri Rosen for help with statistical analyses. James Gossett also provided valuable advice on the manuscript.
REFERENCES
- 1.Agency for Toxic Substances and Disease Registry. 1996. ToxFAQs for chlorinated ethenes. [Online.] http://www.atsdr.cdc.gov/tfacts70.html.
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Meyers, and D. J. Lipman. 1990. Basic Local Alignment Search Tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.DiStefano, T., J. Gossett, and S. H. Zinder. 1992. Hydrogen as an electron donor for dechlorination of tetrachloroethene by an anaerobic mixed culture. Appl. Environ. Microbiol. 58:3622-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiStefano, T. D., J. M. Gossett, and S. H. Zinder. 1991. Reductive dechlorination of high concentrations of tetrachloroethene to ethene by an anaerobic enrichment culture in the absence of methanogenesis. Appl. Environ. Microbiol. 57:2287-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fennell, D. E. 1998. Ph.D. thesis. Cornell University, Ithaca, N.Y.
- 6.Fennell, D. E., J. M. Gossett, and S. H. Zinder. 1997. Comparison of butyric acid, ethanol, lactic acid, and propionic acid as hydrogen donors for the reductive dechlorination of tetrachloroethene. Environ. Sci. Technol. 31:918-926. [Google Scholar]
- 7.Holmes, D. E., K. P. Nevin, R. A. O'Neil, J. E. Ward, L. A. Adams, T. L. Woodard, H. A. Vrionis, and D. R. Lovley. 2005. Potential for quantifying expression of the Geobacteraceae citrate synthase gene to assess the activity of Geobacteraceae in the subsurface and on current-harvesting electrodes. Appl. Environ. Microbiol. 71:6870-6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hölscher, T., R. Krajmalnik-Brown, K. M. Ritalahti, F. von Wintzingerode, H. Görisch, F. E. Löffler, and L. Adrian. 2004. Multiple nonidentical reductive-dehalogenase-homologous genes are common in Dehalococcoides. Appl. Environ. Microbiol. 70:5290-5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson, D. R., P. K. H. Lee, V. F. Holmes, and L. Alvarez-Cohen. 2005. An internal reference technique for accurately quantifying specific mRNAs by real-time PCR with application to the tceA reductive dehalogenase gene. Appl. Environ. Microbiol. 71:3866-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson, D. R., P. K. H. Lee, V. F. Holmes, A. C. Fortin, and L. Alvarez-Cohen. 2005. Transcriptional expression of the tceA gene in a Dehalococcoides-containing microbial enrichment. Appl. Environ. Microbiol. 71:7145-7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kube, M., A. Beck, S. H. Zinder, H. Kuhl, R. Reinhardt, and L. Adrian. 2005. Genome sequence of the chlorinated compound-respiring bacterium Dehalococcoides species strain CBDB1. Nature 23:1269-1273. [DOI] [PubMed] [Google Scholar]
- 12.Magnuson, J. K., M. F. Romine, D. R. Burris, and M. T. Kingsley. 2000. Trichloroethene reductive dehalogenase from Dehalococcoides ethenogenes: sequence of tceA and substrate range characterization. Appl. Environ. Microbiol. 66:5141-5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnuson, J. K., R. V. Stern, J. M. Gossett, S. H. Zinder, and D. R. Burris. 1998. Reductive dechlorination of tetrachloroethene to ethene by a two-component enzyme pathway. Appl. Environ. Microbiol. 64:1270-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maymo-Gatell, X., T. Anguish, and S. H. Zinder. 1999. Reductive dechlorination of chlorinated ethenes and 1, 2-dichloroethane by “Dehalococcoides ethenogenes” 195. Appl. Environ. Microbiol. 65:3108-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maymo-Gatell, X., V. Tandoi, J. M. Gossett, and S. H. Zinder. 1995. Characterization of an H2-utilizing enrichment culture that reductively dechlorinates tetrachloroethene to vinyl chloride and ethene in the absence of methanogenesis and acetogenesis. Appl. Environ. Microbiol. 61:3928-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maymo-Gatell, X., Y. Chien, J. M. Gossett, and S. H. Zinder. 1997. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276:1568-1571. [DOI] [PubMed] [Google Scholar]
- 17.Morris, R. M., S. Sowell, D. Barovsky, S. H. Zinder, and R. E. Richardson. Environ. Microbiol., in press. [DOI] [PubMed]
- 18.Nijenhuis, I., and S. H. Zinder. 2005. Characterization of hydrogenase and reductive dehalogenase activities of Dehalococcoides ethenogenes strain 195. Appl. Environ. Microbiol. 71:1664-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozen, S., and H. J. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132:365-386. [DOI] [PubMed] [Google Scholar]
- 20.Seshadri, R., L. Adrian, D. E. Fouts, J. A. Eisen, A. M. Phillippy, B. A. Methe, N. L. Ward, W. C. Nelson, R. T. Deboy, H. M. Khouri, J. F. Kolonay, R. J. Dodson, S. C. Daugherty, L. M. Brinkac, S. A. Sullivan, R. Madupu, K. E. Nelson, K. H. Kang, M. Impraim, K. Tran, J. M. Robinson, H. A. Forberger, C. M. Fraser, S. H. Zinder, and J. F. Heidelberg. 2005. Genome sequence of the PCE-dechlorinating bacterium Dehalococcoides ethenogenes. Science 307:105-108. [DOI] [PubMed] [Google Scholar]
- 21.Smatlak, C. R., J. M. Gossett, and S. H. Zinder. 1996. Comparative kinetics of hydrogen utilization for reductive dechlorination of tetrachloroethene and methanogenesis in an anaerobic enrichment culture. Environ. Sci. Technol. 30:2850-2858. [Google Scholar]
- 22.Waller, A. S., R. Krajmalnik-Brown, F. E. Loffler, and E. A. Edwards. 2005. Multiple reductive-dehalogenase-homologous genes are simultaneously transcribed during dechlorination by Dehalococcoides-containing cultures. Appl. Environ. Microbiol. 71:8257-8264. [DOI] [PMC free article] [PubMed] [Google Scholar]